Abstract
The energy deposition and the ozone generation in a shielded sliding discharge were compared with those of a simple surface discharge and a pulsed corona discharge in atmospheric pressure air and oxygen. All discharges were generated by applying 160 ns, high voltage pulses. Under the same conditions, the highest energy deposition per pulse was obtained with the shielded sliding discharge. The energy deposition was lower by a factor of four for the surface discharge plasma and by an order of magnitude for the pulsed corona discharge plasma. Replacing air with the more electronegative oxygen caused a decrease in the deposited energy due to electron attachment. The threshold voltage for plasma formation in oxygen in a shielded sliding discharge was approximately 5 kV, three times less than that of the surface discharge (≥15 kV) and four times less than that of the pulsed corona discharge (≥20 kV). A new finding of this study is that, whereas the decrease in energy in the pulsed corona discharge was ≥50 %, and that of the simple surface discharge ≥40 %, it was negligible in the shielded sliding discharge. Since the ozone generation scales with energy density, the results show that plasma reactors based on the nanosecond sliding discharge principle have major advantages in compactness, ignition voltage, and in the use of oxygen, rather than air, compared to surface discharges and corona discharges.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ozone synthesis using nonthermal plasmas is an important commercial process [1]. Traditionally ozone is produced by employing dielectric barrier discharge (DBD) [2]. Recently, other nonthermal plasmas, such as those generated in pulsed corona discharges (PCD) [3], pulsed glow discharges [4], surface-DBD (S-DBD) [5–9], shielded sliding discharge [10, 11], coupled sliding discharge [12] or plasma hybridized with a catalyst [13–15] are being explored with respect to their efficiency. Both air and oxygen are used as feed for ozone synthesis [1]. Although air is cheaper and commonly available, oxygen is preferred due to higher ozone concentration achieved using it. However, since oxygen is an electronegative gas, increasing oxygen from 20 to 100 % results in increased losses of free electrons by electron attachment reactions such as the following [16].
The loss of free electrons adversely affects the electron avalanche process in the electrical discharges resulting in a significant decrease in the discharge current and consequently in the energy density of the plasma. The same problem is also encountered in some other processes involving electronegative gases, e.g., halogenated hydrocarbons employed in semiconductor etching or in electrochemical lasers [16] or those involving atmospheric pressure water vapor where ready recombination of H3O+ species present in the water medium causes the electron loss [17].
Experimental
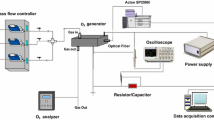
Power depositions for the discharges formed in three electrode assemblies shown in Fig. 1 are compared. The power dissipation in the shielded sliding discharge (Fig. 1c) using air or oxygen as working gas is compared with that of the surface discharge (Fig. 1b) and the pulsed corona discharge (Fig. 1a) [10, 18]. A Compact Pulsed Power Modulator MPC3000S-OP1 (Suematsu Electronics Co., Ltd., Japan) delivered high voltage of positive polarity (+HV) of ~160 ns duration (FWHM) and peak voltages of 5–30 kV at frequencies of 500 Hz. A Tektronix P6015A voltage probe, a Pearson Electronics Current Monitor, Model 110A and Tektronix TDS 3052 oscilloscope were used to measure the temporal development of voltage and current. The energy per pulse (Ep) is the time integral (∫VIdt where V and I are the discharge voltage and current, respectively) of the power.
Top view of electrode assemblies (top) and cross-section view of the discharge chambers through A–A line (bottom) that generate pulsed corona discharges (a), surface discharge (b), and shielded sliding discharge (c). 1 High voltage electrode (anode), 2 counter electrode at ground potential (cathode), 3 dielectric layer, 4 and 5 are spacers, and 6 is the plasma channel (streamers)
The electrodes were made of aluminum foil (ALF200L from Intertape Polymer Groups, USA) of 50 µm thickness that was glued to a dielectric frame using an acrylic adhesive (Fig. 1a). The width of the electrodes (#1 and #2 in Fig. 1a) was kept at 6 mm, the inter-electrode gap was 16 mm, and the effective length of the center electrode was 89 mm. The electrode assembly of Fig. 1a forms pulsed corona discharges in the inter-electrode gap upon application of the HV pulses. The same electrodes were glued to a dielectric sheet made of soda glass with dimensions 2.4 mm × 76 mm × 152 mm as shown in Fig. 1b to generate a surface discharge [18]. The electrode configuration for the shielded sliding discharge is shown in Fig. 1c [10, 18]. It was formed by connecting an aluminum foil to the ground electrode #2 and by wrapping it around the opposite side of the dielectric, covering the entire inter-electrode gap area.
The spacer (#4 in Fig. 1b, c) was made of silicon measuring 4 mm × 76 mm × 152 mm, from which a 4 mm × 51 mm × 127 mm block (defining the inside dimensions of the reactor) was cut from the center. The gas inlet and outlet were nylon tubes of 2.0 mm ID and 2.3 mm OD extending through opposite corners of the spacer and sealed using silicon glue. Spacer #5 in the case of Fig. 1a was of the same dimension as the spacer #4 described above except that it was made of acrylic sealed with silicon glue. Its thickness was 15 mm instead of 4 mm. The thickness of the spacer was increased to avoid surface plasma formation in the case of the pulsed corona discharge as explained earlier [10].
The experiments were carried out at atmospheric pressure and room temperature. All gas volumes are expressed under conditions of 25 °C and 1 atmospheric pressure with the exception of ozone concentration which is expressed in grams of ozone per normal cubic meter (g/Nm3), where normal stands for 1 atmospheric pressure and 0 °C. Dry air (UN1002 from Airgas USA) or oxygen (UN1072 from Airgas USA) were supplied from a pressurized gas cylinder at a flow rate (Q) of 1 liter per minute (L/min) in all the experiments. Specific input energy which is energy dissipated in reactor per unit volume of gas processed in joules per litter (J/L). When the feed gas was switched, it was allowed to run for about half an hour before experiment was started. Ozone was analyzed based on an UV absorption technique using an ozone analyzer (gFFOZ, IN USA, Inc., Needham, MA, USA) with an accuracy of 1 % of the reading and is recorded in grams of ozone per normal cubic meter (g/Nm3), where normal stands for 1 atmospheric pressure and 0 °C. The experimental procedures and statistical treatment of the data was the same as in our earlier study [11].
Results and Discussion
Figure 2 shows that the energy per pulse increased with the increase in peak applied voltage in all cases, but the plasma initiation threshold voltage and the increase in the energy deposition with increase in peak voltage strongly depended on the type of discharge. Pulsed corona discharges required the highest voltages, i.e., ≥15 kV in air and ≥20 kV in oxygen. Surface discharges required a lower voltage ≥15 kV, and the energy per pulse was more than double that of pulsed corona discharges at the same voltage. The shielded sliding discharge required a peak voltage of only about 5 kV, and the energy per pulse was about an order of magnitude higher compared to that of pulsed corona discharges at the same voltage which is in accordance with our earlier studies [10]. The new finding of this study is that when air was replaced with oxygen as a working gas, the energy per pulse decreased by ≥50 % in the case of pulsed corona discharge and ≥40 % in the case of the surface discharge, but almost no decrease was observed in the case of a shielded sliding discharge at the same applied voltage.
To the best of our knowledge negligible energy loss when the feed gas is changed from air to the more electronegative oxygen in the shielded sliding discharge is being reported for the first time. So we verified it by several alternate ways in the following experiments. When the thickness of the dielectric layer in the shielded sliding discharge assembly of Fig. 1c was decreased from 2.4 to 1 mm, the energy per pulse was increased by about a factor of two. The reduction in the energy per pulse when replacing air with oxygen again was not affected by this change in the reactor geometry. We have reported several versions of shielded sliding discharges, all of them scalable in a compact configuration with high gas throughput; these are coupled sliding discharge [12], surface-dielectric barrier discharge, and coupled surface-dielectric barrier discharge [9]. When the present experiments were repeated using those electrode assemblies with electrodes of the same or similar dimensions as in Fig. 1, the same trend was observed. That is, the decrease in energy per pulse was negligible upon replacing air with oxygen at the same applied voltage.
In the case of plasmas developing along a dielectric surface (Fig. 1b and 1c), the plasma draws additional electrons from the surfaces on which it is sliding [19]. The generation of these electrons is considered to compensate in part for the free electron capture by oxygen. Particularly, the anions like O− and O2 − may be adsorbed on the surface and ultimately may decay and release free electrons through reactions such as the following [16].
In the case of a shielded sliding discharge, the counter electrode overlapping the electrode at close proximity (2.4 mm in this case) significantly enhances electric fields at the edges of the electrode fostering initiation of more plasma channels [12]. Further, re-distribution of electric fields favors stronger attraction of the plasma to the surface favoring more electron emissions through surface mediated reactions. These factors explain the increased energy per pulse and the significantly less adverse effect of replacing air with more electronegative oxygen.
Ozone synthesis using air and oxygen as feed was used to compare the chemical efficiency of the reactors employed in this study (Fig. 3). The following is observed from the results.
-
1.
Ozone concentration was dependent on the feed gas composition; it is significantly higher in oxygen than in air (20 % oxygen), which is in agreement with earlier literature [1].
-
2.
The ozone concentration was found to be dependent on the deposited electrical energy and almost independent of the type of discharge, particularly under the condition of specific input energy <200 J/L. Deviations from the common curve were observed when specific input energy was >200 J/L.
The main reaction channel for ozone formation form oxygen is the following [20–23]:
where ‘e*’ represents a high-energy electron, ‘e’ represents a relatively low-energy electron, and ‘M’ is a third collision partner that can be O2, N2, a solid surface in contact with the plasma, etc. Ozone yield is generally lower in air than in oxygen because dissociation and/or excitation of nitrogen competes with the dissociation of oxygen, leading to synthesis of unwanted by-products like nitrogen oxides (NOx) in addition to ozone. An increase in specific input energy increases the rates of these reactions leading to high ozone concentration, but, at the same time, increases the local temperature in the plasma leading to destruction of some of the ozone formed.
Based on higher energy per pulse, one can expect strong negative effect of temperature in the case of shielded sliding discharge compared to the other two discharges. Further, the plasma tends to localize more around the high voltage electrode in a shielded sliding discharge compared to sliding discharge and pulsed corona discharge as illustrated by images of the plasmas shown in Fig. 4. It is a plausible explanation for the slightly better ozone generation efficiency by a sliding discharge than by a shielded sliding discharge, particularly when the specific input energy was more than 200 J/L.
Measured temperature of the exhaust gas was no more than a few degrees higher compared to initial temperature in ozone synthesis experiments of Fig. 3. One can expect more heating of gas in the case of shielded sliding discharge due to higher energy density in it. However, plasma in this study was in contact with dielectric surface which most likely dissipated major fraction of heat from the plasma channels.
The results in Fig. 3 show that for all discharge types with energy deposition and consequently increasing ozone concentration from 1 to 20 g/Nm3, the energy yield for the ozone generation decreases gradually from 250 to 150 g/kWh. These concentrations and energy yield values are comparable to ~17 g/Nm3 with an energy yield ~85 g/kWh in a surface-DBD formed in micro-channels on an aluminum substrate [8], 0.2 to 43 g/Nm3 ozone produced in a surface-DBD with energy yield 170 to 75 g/kWh [6], and ~100 g/Nm3 ozone and 180–200 g/kWh energy yield in surface-DBD with forced cooling of electrodes [7]. It should be mentioned here that this comparison of ozone efficiencies is a qualitative one because energy yield is dependent on multiple factors, including ozone concentration, power dissipation, and temperature of cooling liquid [24]. Nanosecond pulses as in this study are also known to be more efficient than the sinusoidal driving voltage [25]. Results of this study are valid for positive polarity pulses only. It needs to be mentioned because ozone and its precursor O are produced more efficiently by employing negative polarity pulses than positive polarity pulses when the feed gas is air [10, 11, 26]. However, this dependence of ozone efficiency on the polarity of the voltage pulse disappears in the case of oxygen feed [11].
The voltage pulses employed in this study had a duration of approximately 160 ns (full width at half maximum). Earlier studies indicate that the efficiency for ozone can be increased by employing voltage pulses of shorter duration. For example, the highest ozone energy yield known to us with oxygen feed is about 700 g/kWh reported for the case of short duration pulses of 5 ns, but at a much higher peak voltage of 60 kV at negative polarity [3]. There is a potential of further improvement by employing a dielectric layer made of materials known to catalyze ozone formation in electrical discharges, e.g., alumina [14, 15] and silica gel [13].
Conclusions
Although the energy cost for ozone synthesis under a constant flow rate of oxygen feed only depends on the deposited energy and is consequently almost the same for all three discharge types, the shielded sliding discharge has an advantage, due to its high energy deposition in a smaller volume and consequently its high energy density in the discharge plasma compared to pulsed corona discharge. Another advantage is its relatively low discharge voltage, allowing its use in very compact plasma reactors. In addition, it is found for the first time in this study that the decrease in energy density was the smallest in shielded sliding discharges when air is replaced by electronegative oxygen. This again shows that this type of discharge has an advantage for uses where electronegative gases like oxygen, halogens, halogenated compounds or water vapors need to be added in the composition of the process gas.
References
Wojtowicz JA (2005) Ozone in Kirk–Othmer encyclopedia of chemical technology. Wiley, New York. doi:10.1002/0471238961.1526151423151020.a01.pub2
Siemens WV (1857) Ueber die elektrostatische Induktion und die Verzögerung des Stroms in Flaschendrähten [About the electrostatic induction and the delay of the current in bottles wires]. Ann Phys 178(9):66–122
Takamura N, Matsumoto T, Wang D, Namihira T, Akiyama H (2011) Ozone generation using positive- and negative-nanoseconds pulsed discharges. In: IEEE pulsed power conference (PPC), pp 1300–1303
Buntat Z, Smith IR, Razali NA (2009) Ozone generation using atmospheric pressure glow discharge in air. J Phys D Appl Phys 42(23):235202
Masuda S, Akutsu K, Kuroda M, Awatsu Y, Shibuya Y (1988) A ceramic-based ozonizer using high-frequency discharge. IEEE Trans Ind Appl 24:223–231
Simek M, Pekarek S, Prukner V (2010) Influence of power modulation on ozone production using an AC surface dielectric barrier discharge in oxygen. Plasma Chem Plasma Process 30:607–617
Jodzis S (2012) Effective ozone generation in oxygen using a mesh electrode in an ozonizer with variable linear velocity. Ozone Sci Eng 34:378–386
Kim MH, Cho JH, Ban SB, Choi RY, Kwon EJ, Park SJ, Eden JG (2013) Efficient generation of ozone in arrays of microchannel plasmas. J Phys D Appl Phys 46:305201
Malik MA, Schoenbach KH, Heller R (2014) Coupled surface dielectric barrier discharge reactor-ozone synthesis and nitric oxide conversion from air. Chem Eng J 256:222–229
Malik MA, Schoenbach KH (2014) Nitric oxide conversion and ozone synthesis in a shielded sliding discharge reactor with positive and negative streamers. Plasma Chem Plasma Process 34:93–109
Malik MA (2014) Ozone synthesis using shielded sliding discharge: effect of oxygen content and positive versus negative streamer mode. Ind Eng Chem Res 53:12305–12311
Malik MA, Jiang C, Dhali SK, Heller R, Schoenbach KH (2014) Coupled sliding discharges: a scalable nonthermal plasma system utilizing positive and negative streamers on opposite sides of a dielectric layer. Plasma Chem Plasma Process 34:871–886
Jodzis S (2003) Effect of silica packing on ozone synthesis from oxygen–nitrogen mixtures. Ozone Sci Eng 25:63–72
Chen HL, Lee HM, Chang MB (2006) Enhancement of energy yield for ozone production via packed-bed reactors. Ozone Sci Eng 28:111–118
Huang W, Ren T, Xia W (2007) Ozone generation by hybrid discharge combined with catalysis. Ozone Sci Eng 2:107–112
Belevtsev AA (2013) Charge kinetics in weakly ionized plasma of electronegative gases. High Temp 51(4):435–442
Koo IG, Lee WM (2006) Hollow-cathode based electrical discharge in atmospheric pressure water vapor at wide range of temperature. Japn J Appl Phys 45(10R):7888–7893
Malik MA, Schoenbach KH (2012) New approach for sustaining energetic, efficient and scalable non-equilibrium plasma in water vapours at atmospheric pressure. J Phys D Appl Phys 45(13):132001
Bloshchitsyn V (2010) Review of surface discharge experiments. http://arxiv.org/pdf/1005.5044v1.pdf. Accessed 31 Sep 2013
Yagi S, Tanaka M (1979) Mechanism of ozone generation in air-fed ozonisers. J Phys D Appl Phys 12:1509–1520
Kitayama J, Kuzumoto M (1999) Analysis of ozone generation from air in silent discharge. J Phys D Appl Phys 32:3032–3040
Kogelschatz U, Eliasson B, Hirth M (1989) Ozone generation from oxygen and air: discharge physics and reaction mechanisms. Ozone Sci Eng 10:367–377
Kitayama J, Kuzumoto M (1997) Theoretical and experimental study on ozone generation characteristics of an oxygen-fed ozone generator in silent discharge. J Phys D Appl Phys 30:2453–2461
Jodzis S (2011) Application of technical kinetics for macroscopic analysis of ozone synthesis process. Ind Eng Chem Res 50:6053–6060
Williamson JM, Trump DD, Bletzinger P, Ganguly BN (2006) Comparison of high-voltage ac and pulsed operation of a surface dielectric barrier discharge. J Phys D Appl Phys 39:4400–4406
van Heesch EJM, Winands GJJ, Pemen AJM (2008) Evaluation of pulsed streamer corona experiments to determine the O* radical yield. J Phys D Appl Phys 41:234015
Acknowledgments
This work is supported by a Commonwealth Research Commercialization Fund Grant (MF13-019-Env.) from Virginia’s Center for Innovative Technology, ‘Frank Reidy Fellowship in Environmental Plasma Research’ and with internal funds of the Frank Reidy Research Center for Bioelectrics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Malik, M.A., Hughes, D., Heller, R. et al. Surface Plasmas Versus Volume Plasma: Energy Deposition and Ozone Generation in Air and Oxygen. Plasma Chem Plasma Process 35, 697–704 (2015). https://doi.org/10.1007/s11090-015-9611-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-015-9611-3