Abstract
The oxidation behaviour of an austenitic stainless Fe–14.29Mn–5.57Si–8.23Cr–4.96Ni (wt%) shape memory alloy was investigated in air at 600 °C and 700 °C. The results showed that the oxidation process obeyed the parabolic rate law at both temperatures. At 700 °C, the final oxide scales were composed of an outer Mn2O3 layer, a middle Mn3O4 layer, and an inner MnCr2O4 layer. The scale consisted of only Mn2O3 at 600 °C. A composite structure of ferrite and austenite phases was obtained after oxidation at 600 °C and 700 °C because an oxidation-induced Mn-depleted layer formed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-manganese FeMnSiCrNi austenitic stainless steel shape memory alloys (SMAs) have attracted considerable attention in the past decade as possible substitutes for expensive Ti–Ni-based SMAs because of their low cost, good workability, machinability, and corrosion-resistant performance [1,2,3,4,5,6,7,8,9,10]. However, the Si content in these alloys is 4–6 wt%, which is much higher than that in conventional austenitic stainless steels. Paúl et al. [11] showed that the addition of 0.8% Si enhanced the oxidation resistance of AISI 304 at 1000 °C via the formation of protective SiO2 films. Adachi et al. [12] showed that the oxidation rate of iron decreased by more than two orders of magnitude with the addition of 5% Si due to the formation of a SiO2 film. When Si addition is greater than 10%, the oxidation rate of iron is even lower than that of Fe-26Cr, a typical Cr2O3-forming alloy [10]. Therefore, investigating the high-temperature oxidation behaviour of austenitic stainless high-manganese FeMnSiCrNi SMAs containing 4–6 wt% Si is important.
In a previous study, we investigated the oxidation behaviour of an Fe14.29Mn5.57Si8.23Cr4.96Ni alloy at 800 °C in air [13]. Although its oxidation behaviour obeyed parabolic law, its weight gain (2.88 mg/cm2) was much higher than that of 304 stainless steel (0.23 mg/cm2). However, oxidation remarkably weakens as the temperature decreases. At the same time, the Thermo-Calc® and TCFE7 database confirm that ferrites and G phases precipitate at 700 °C and 600 °C. Therefore, investigating the oxidation behaviour of FeMnSiCrNi SMAs at 700 °C and 600 °C is important. In the present work, the oxidation behaviours of Fe14.29Mn5.57Si8.23Cr4.96Ni alloy at 700 °C and 600 °C in air were investigated and compared with the oxidation behaviour at 800 °C.
Materials and Methods
The experimental alloy was prepared by induction melting under an argon atmosphere using commercially pure iron, manganese, silicon, chromium, and nickel. The chemical composition of the experimental alloy was as follows: 14.29% Mn, 5.57% Si, 8.23% Cr, 4.96% Ni, and balance Fe. After homogenization at 1100 °C for 15 h, the ingot was hot forged at 1100 °C into round bars 15 mm in diameter, and then, the bars were annealed at 1000 °C for 1 h, followed by quenching with water. Finally, the quenched bars were machined to specimens with a diameter of 13 mm and height of 5 mm. Before oxidation, the specimens were mechanically polished using 500-grit SiC paper and then cleaned in ethanol by an ultrasonic bath before the oxidation tests.
The oxidation tests were conducted for different periods in static air at 700 °C and 600 °C. The specimens were placed in individual annealed alumina crucibles with lids to collect the spalled oxide. After various periods of oxidation, the alumina crucibles and specimens were removed from the furnace and air-cooled to room temperature. The mass changes were measured using an electronic balance with an accuracy of 0.1 mg. Three replicates were used in the oxidation assays; each of the data was an average value.
The microstructures were analysed using an optical microscope (OM) and an FEI Inspect scanning electron microscope (SEM) equipped with an energy-dispersive spectrometer (EDS). The cross-sectional specimens were prepared by mounting the specimens in an epoxy mould. After mechanical polishing, the specimens were etched in a solution containing 1 g trinitrophenol, 1 mL hydrochloric acid, and 50 mL ethanol. The above etched specimens were further etched in a solution composed of 1.2 g K2S2O5, 0.5 g NH4HF2, and 100 mL distilled water to clearly distinguish the phases, which were determined using a Philips X′ Pert Pro MPD X-ray diffraction (XRD) apparatus with a Cu target. The surfaces of the oxidized specimens were also observed by SEM with EDS. The surfaces were ground to characterize the phases present at different depths. A micrometre was used to determine the thickness of the surface removed. A grazing angle X-ray diffractometer (GAXRD) was used to identify the phases of the oxide layer with a 0.02°2θ/step scanning rate at a grazing angle of 2°. To decrease the influence of the ε martensite phase due to grinding, the mechanically ground specimens were etched in a solution of 5 mL hydrofluoric acid, 25 mL hydrogen peroxide, and 100 mL distilled water.
Results
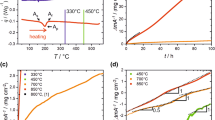
Figure 1a shows the cyclic oxidation data at different temperatures in air. The measured weight gains were 0.36 mg/cm2, 1.44 mg/cm2, and 2.88 mg/cm2 after oxidation at 600 °C, 700 °C, and 800 °C for 100 h, respectively. A very good positive linear relationship existed between the square of the weight gain and time (Fig. 1b), indicating that the oxidation behaviours of the alloy obeyed a parabolic law at 800 °C, 700 °C, and 600 °C. The corresponding calculated parabolic rate constants were 0.112 mg2/(cm4·h), 0.019 mg2/(cm4 h), and 1.39 × 10−3mg2/(cm4 h), respectively.
Weight gain curves (a) and square of the weight gain curves (b) of alloys oxidized at different temperatures in air. The data at 800 °C were reproduced from the literature [13]
Figure 2 shows the SEM images of the surface after oxidation for 100 h at different temperatures. The oxide scales on the surface of the specimen oxidized at 700 °C were non-compact, directional ridges arranged in strips (Fig. 2a), with a composition of 63.76%Mn, 6.67%Cr, and 29.57%O. In addition, no spallation appeared, but pores and cracks were clearly observed on the oxide scale. The enlarged oxide scales consisted of many nanoparticles. The oxide scales on the surface of the specimen oxidized at 600 °C were arranged in a directional streak (Fig. 2b) and contained 58.60%Mn, 9.14%Cr, and 32.26%O. In addition, some spalled areas consisting of many fuzzy shapes were observed. The composition of these areas was 38.78%Mn, 1.52%Si, 10.52%Cr, 19.25%Fe, 0.31%Ni, and 29.62%O.
Figure 3 shows the cross-sectional OM micrographs of the specimens oxidized for different periods. Regardless of the oxidation temperature, one distinct layer was present between the matrix and the oxidation layer in all the oxidized specimens, and the thicknesses of the layer increased with the increasing oxidation time (Fig. 4). Note that the thickness after oxidation at 800 °C was significantly larger than that after oxidation at 700 °C and 600 °C. The XRD results (Fig. 5) revealed that this layer was ferrite, with some chi (χ) phase.
Figures 6 and 7 show the cross-sectional SEM images of the specimens after 100 h of oxidation at 700 °C and 600 °C. The oxide scale well adhered to the ferrite layer. The interfaces between the oxide and ferrite were very irregular. The EDS analysis reveals that the Mn content in the ferrite layer was much lower than the average content in the specimen, i.e. 14.29% (Tables 1, 2). The second-phase particles that precipitated inside the ferrite layer were depleted in Mn but enriched in Si and Ni, while the particles inside the grain boundaries of the matrix were enriched in Mn and Si. These second-phase particles were similar to those obtained after oxidation at 800 °C [13].
The XRD patterns of the specimen surfaces after oxidation at 700 °C and 600 °C for different periods are shown in Fig. 8. After 100 h oxidation, the dominant oxide for both 700 °C and 600 °C was Mn2O3. The XRD patterns (Fig. 9) show that the precipitates inside the matrix after oxidation for 100 h at both 700 °C and 600 °C were chi (χ) phases [14].
The surfaces of different specimens oxidized at 700 °C were ground to remove the Mn2O3 layer to characterize the oxide scale and ferrite layer. The XRD patterns (Fig. 10) show that the main oxide inside was Mn2O3 scale after 5 h of oxidation (ICDD-PDF: 04-004-8966). When the oxidation time reached 40 h, several MnCr2O4 peaks appeared (ICDD-PDF: 00-054-0876), and their intensities increased as the exposure time increased to 100 h, indicating more MnCr2O4 formed. In addition, many areas with Mn3O4 formed close to the MnCr2O4 (Fig. 10b). In contrast, for oxidization at 600 °C, only Mn2O3 peaks were detected in addition to some SiO2 peaks (Fig. 11).
Discussion
Austenitic stainless steels have been used for chemical processing and energy productions, which both involve exposure to high temperatures ranging from 600 to 900 °C. At these operation temperatures, oxidation resistance is very important and should be considered. Oxidation resistance mainly depends on the oxide scale that forms on the surface. The main oxide scale that forms on conventional austenitic stainless steels is Cr2O3 due to their high content of Cr [15, 16]. However, for this tested Fe14.29Mn5.57Si8.23Cr4.96Ni alloy, the outer oxide is Mn2O3, the inner oxide is MnCr2O4, and the middle oxide is Mn3O4 at 700 °C and 800 °C [13]. However, the oxide scales that form at 600 °C are different from those that form at 700 °C and 800 °C. At 600 °C, the oxide scale only contains Mn2O3. In addition, the oxidation mechanism is also different. The detailed oxidation behaviour at 600 °C is discussed below.
Figure 12 shows a schematic diagram of the oxidation behaviour at 600 °C. In the initial oxidation stage, the Mn2O3 grains nucleate and grow more quickly within the voids, as shown in Fig. 12b. According to the DSC measurement in Fig. 6 in our previous paper [13], the Mn-depleted layer with compositions of Fe–4.02Mn–6.25Si–8.03Cr–6.5Ni after oxidation at 800 °C for 100 h was austenite at the corresponding oxidation temperature, and it transformed to ferrite during the cooling when the temperature was below 700 °C. For the present Mn-depleted layer with compositions of Fe–3.56Mn–5.86Si–6.92Cr–5.49Ni after oxidation at 600 °C for 100 h, the Mn-depleted layer should be the ferrite at 600 °C.
Schematic illustrations of the oxidation process of FeMnSiCrNi SMAs at 600 °C. a Prior to oxidation, the matrix is austenite; b in the initial oxidation stage, Mn2O3 scale with many voids quickly grows on the surface, resulting in the formation of a Mn-depleted layer, and some chi phase particles precipitate along the grain boundaries and the interface of the matrix and Mn-depleted layer; c the amount of Mn2O3 scale on the surface increases with increasing oxidation time; d eventually, some Si, Cr, and Fe layers form
In this ferrite layer, the diffusion rate of Mn is much higher than that in the austenitic matrix (Fig. 13 and Table 3). Therefore, the subsequent oxidation process is controlled by the diffusion of Mn in the matrix. Initially, due to the large concentration gradient, the rapid outward diffusion of Mn increases the thicknesses of the Mn2O3 scale and the ferrite layer. As the oxidation time increases, the thickness of the ferrite layer increases, the Mn concentration gradient decreases, and the oxygen partial pressure decreases remarkably. Therefore, the growth rate of the Mn2O3 scale decreases. In addition, minimal segregation of Fe, Si, and Cr exists at the interface between the oxide layer and the ferrite layer, and these elements react with oxygen to form their corresponding oxides (Fig. 12d), which is why these elements were detected by the EDS analysis of the spalled areas (Fig. 2).

[17]
Relationship between the Mn diffusion rate and temperature in the ferrite and austenitic phases.
Conclusions
-
1.
The oxidation behaviour of austenitic Fe–14.29Mn–5.57Si–8.23Cr–4.96Ni shape memory alloy followed a parabolic law at both 600 °C and 700 °C.
-
2.
After 100 h of oxidation at 700 °C, the final oxide scales contained an outer Mn2O3 layer, a middle Mn3O4 layer, and an inner MnCr2O4 layer. At 600 °C, only Mn2O3 and some SiO2 were detected.
-
3.
After oxidation at both 600 °C and 700 °C, a relatively large amount of chi (χ) phase precipitated inside the austenite matrix and the ferrite layer.
-
4.
Composite structures containing the ferrite and austenite phases can be obtained after oxidation at 600 °C and 700 °C.
References
A. Sato, E. Chishima, K. Soma and T. Mori, Acta Materialia 33, 1982 (1177).
H. Otsuka, H. Yamada, T. Maruyama, H. Tanahashi, S. Matsuda and M. Murakami, ISIJ International 30, 1990 (674).
N. Bergeon, S. Kajiwara and T. Kikuchi, Acta Materialia 48, 2000 (4053).
Y. H. Wen, W. Zhang, N. Li, H. B. Peng and L. R. Xiong, Acta Materialia 55, 2007 (6526–6534).
Y. H. Wen, H. B. Peng, P. P. Sun, G. Liu and N. Li, Scripta Materialia 62, 2010 (55–58).
H. B. Peng, Y. H. Wen, G. Liu, C. P. Wang and N. Li, Advanced Engineering Materials 13, 2011 (388–394).
C. A. D. Rovere, J. H. Alano, R. Silva, P. A. P. Nascente, J. Otubo and S. E. Kuri, Materials Chemistry and Physics 133, 2012 (668–673).
C. A. Della Rovere, J. H. Alano, R. Silva, P. A. P. Nascente, J. Otubo and S. E. Kuri, Corrosion Science 57, 2012 (154–161).
A. Paúl, S. Elmrabet, L. C. Alves, M. F. da Silva, J. C. Soares and J. A. Odriozola, Nuclear Instruments and Methods in Physics Research 181, 2001 (394–398).
T. Adachi and G. H. Meier, Oxidation of Metals 27, 1987 (347–366).
F. J. Pérez, M. J. Cristóbal, G. Arnau, M. P. Hierro and J. J. Saura, Oxidation of Metals 55, 2001 (105–118).
F. J. Pérez, M. J. Cristóbal and M. P. Hierro, Oxidation of Metals 55, 2001 (165–175).
Rui Ma, Huabei Peng, Yuhua Wen, Lijun Zhang and Kai Zhao, Corrosion Science 66, 2013 (269–277).
N. Stanford, D. P. Dunne and B. J. Monaghan, Journal of Alloys and Compounds 430, 2007 (107–115).
P. J. Maziasz, R. W. Swindeman, J. P. Shingledecker, K. L. More, B. A. Pint, E. Lara-Curzio and N. D. Evans, Proceedings of 6th International Charles Parsons Turbine Conference. in The Institute of Materials, Minerals, and Mining, eds. A. Strang, R. D. Conroy, W. M. Banks, M. Blackler, J. Leggett, G. M. McColvin, S. Simpson, M. Smith, F. Starr and R. W. Vanstone (Maney Publishing, London, 2003), pp. 1057–1073.
F. J. Pe´rez, M. J. Cristo´bal, G. Arnau, M. P. Hierro and J. J. Saura, Oxidation of Metals 55, 2001 (105–108).
Y. J. Liu, L. J. Zhang, Y. Du, et al., Calphad-Computer Coupling of Phase Diagrams and Thermochemistry 33, 2009 (614–623).
Acknowledgements
The authors would like to thank the National Nature Science Foundation of China (No. 51671138) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiao, Y., Zhang, H. & Wen, Y. Influence of Temperature on the Oxidation Behaviour of an Austenitic Stainless FeMnSiCrNi Shape Memory Alloy. Oxid Met 92, 109–121 (2019). https://doi.org/10.1007/s11085-019-09916-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-019-09916-y