Abstract
Corrosion by molten phases leads to severe corrosion of heat exchangers in waste-to-energy plants. In addition, the presence of heavy metal chlorides in ash deposit increases degradation at low temperature due to the formation of highly corrosive molten phases. In this study, two heat exchanger materials, a low alloy steel (16Mo3) and a nickel based alloy (Inconel 625) were exposed in air to three different synthetic ashes, with various chloride contents, including ZnCl2 at isothermal temperatures of 450 and 650 °C in a muffle furnace. After the test, thickness and mass losses were evaluated on two separate samples, and metallographic cross sections of the specimens were characterized via SEM/EDX analyses. Both measurement results were in good agreement and showed that the corrosion observed on both materials was higher in the presence of zinc chloride in ash at 450 °C than in ashes without heavy metal chloride at 650 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In waste-to-energy (WtE) plants, the composition of ash deposits has a big influence on corrosion rate of superheater tubes [1] which is a major issue in those facilities. WtE’s deposits mainly contain calcium sulfates, oxides, alkali chlorides and sulfates and heavy metal chlorides such as PbCl2 and ZnCl2 [2–9]. Those heavy metal chlorides cause accelerated corrosion in the WtE environment [9, 10] due to the formation of salt mixtures with low temperature melting points. The influence of the alkali chloride content in ash mixtures representative of WtE deposits on the corrosion behavior of 16Mo3 steel and Inconel 625 alloy was studied at temperatures between 450 and 650 °C [11]. Two different ashes, one with 10 wt% and one with 40 wt% of chlorides were used for this previous study, which established that:
-
The appearance of molten phases in ashes was correlated with a change of corrosion mechanisms and kinetics,
-
Nickel based superalloy (Inconel 625) exhibited very low attack at low temperature [below each ash solidus temperature (Tsol)] in both ashes contrary to ferritic steel (16Mo3) which was corroded even at 450 °C,
-
At 450 °C, corrosion under deposit, described by GRABKE et al. [12] was the mechanism responsible of the attack,
-
Corrosion rate also increased with the chloride content of ashes. Corrosion increased for both alloys with temperature, and accelerated above Tsol of the ash, due to the appearance of molten phases,
-
Both evaluation methods, thickness loss and mass loss, were in good agreement and showed that increasing chloride content in ash lead to higher amounts of molten phases, which produced greater amounts of corrosion, especially for low alloy steel (16Mo3).
The present work is focused on the influence of 10 wt% zinc chloride in synthetic ashes on the corrosion kinetics in an air atmosphere. Results have been compared with those obtained in ashes without heavy metal chlorides [11]. Few studies have been made with heavy metal chlorides in representative ashes, and their precise effect remains not fully understood yet. Other studies are generally focused on testing deposits composed with one or two species [13–21]. First, three synthetic ashes with the same components as those typically found in WtE superheaters tubes and without oxide were prepared with different heavy metal chlorides content. Those three compositions were prepared in order to choose one of them based on their corrosivity. The low steel alloy (16Mo3) and the Ni-based superalloy (Inconel 625), were exposed to those ashes at 650 °C for 100 h in order to evaluate the most corrosive ash mixture. Tests in air lab, with alumina crucibles were performed in the same way as described in the previous study [11] in order to compare results, as well as to evaluate the influence of heavy metal content in ash on corrosion kinetics. Thickness and mass losses of the alloys were the methods of evaluating the amount of corrosion during this study. These methods are more relevant than measuring the mass gain or oxide layer thickness, in order to establish lifetime prediction. The residual thickness is the critical criterion for determining the lifetime of superheater tubes in WtE plants.
Experimental Procedures
Materials
In this work, the influence of heavy metal chlorides in deposits on corrosion resistance of two alloys was tested: one ferritic steel (16Mo3, Masteel) and one nickel-based superalloy (Inconel 625, Goodfellow). Table 1 presents the elemental compositions (wt%) given by Masteel and Goodfellow. Metallographic preparation, measurements of thickness and mass of samples were described in a previous study [11].
Physical Chemistry of Synthetic Ashes
Three different synthetic ashes containing 10 wt% of heavy metal chloride were prepared for preliminary tests: one with zinc chloride (# ZnCl2), one with lead chloride (# PbCl2) and one with both heavy metals in equally weighted proportions (# ZnCl2/PbCl2). Their compositions are given in Table 2. Those ashes were prepared with NaCl (99 %, SDS), KCl (99.5 %, Roth), ZnCl2 (98 %, Sigma Aldrich), PbCl2 (98 %, Sigma Aldrich), Na2SO4 (98 %, VWR), K2SO4 (99 %, Acros Organics) and CaSO4 (97 %, Sigma Aldrich) salts. The same preparation (grounding and mixing) was performed on ashes with zinc and lead chlorides as the ones made free of heavy metal chlorides. This procedure is described in [11]. Then, results of corrosion obtained after exposure in # ZnCl2 ash were compared to those obtained previously [11] in one chloride rich ash (# 40 % Cl) and one chloride poor ash (# 10 % Cl). Thermomechanical analyses (TMA, Setaram TMA92 16-18) were also performed on # ZnCl2 ash as well as # 10 % Cl and # 40 % Cl ashes in order to determine their solidus temperatures (Tsol). Differential Thermal Analysis (DTA, Setaram TGA92 16-18) measurements were also performed on # ZnCl2 salts mixtures. In addition, salt mixtures without CaSO4 but keeping proportions between chlorides and sulfates were also prepared and analyzed (Table 3). These three ashes have been prepared as comparative basis for understanding ash phase behavior. CaSO4 cannot be considered as its interactions with the other salts are not defined in the FactSage Thermodynamic database (FTsalt database [22]).
Corrosion Tests
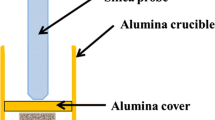
The procedure of corrosion tests was exactly the same as the one defined and described in [11]. Samples were immersed into an alumina crucible with ashes as described in Fig. 1, following the ISO/DIS 17248 standard [23]. In preliminary tests, 16Mo3 and Inconel 625 were exposed for 100 h to the three heavy metal chlorides ashes at 650 °C in ambient air in order to evaluate the corrosivity of those mixtures and choose one ash for the rest of the study. Then, the corrosion tests were performed in ambient air at two isothermal temperatures. The test temperatures have been chosen to evaluate the influence on corrosion of heavy metal chlorides in ash at low temperature (450 °C) and to compare them with ashes without them at higher temperature (650 °C). Exposure times were 100, 500 and 1000 h.
Post Corrosion Tests Analyses
After the exposure in ash, the specimens used to measure thickness loss were cut off in the middle and the metallographic cross-sections were characterized with SEM/EDX analyzers (JEOL J7600F, JEOL JSM-6010/LA). All metallographic preparations were done in dry conditions in order to avoid dissolution of hygroscopic Cl-containing compounds. Corrosion product scales (oxide, sulfide…) were removed from specimens used to evaluate the weight loss by chemical washing following ISO/DIS 17248 standard [23]. Inconel 625 samples were dipped in a heated (60 °C) solution containing 18 % of sodium hydroxide and 3 % of potassium permanganate for 20–30 min, then in a solution of ammonium citrate (10 %) in the same conditions. 16Mo3 samples were dipped in hydrochloric acid (20 %) doped with hexamethylene tetramine. The washed samples were weighed and the masses were compared to those before the test. Preliminary tests have shown that none of the two alloys were corroded in chemical washing solutions.
Results and Discussion
Preliminary Results
In order to study the influence of the presence of heavy metal chlorides on corrosion kinetics, three ash compositions have been considered based on # 10 % Cl containing alkali chloride ash with an addition of 10 wt% of heavy metal chloride (Table 2). The content of NaCl, KCl, Na2SO4 and K2SO4 is constant and the balance is done with CaSO4. Preliminary tests were performed to give a first estimation of the corrosivity of those synthetic ashes by measuring mass losses on 16Mo3 and Inconel 625 alloys after 100 h of exposure at 650 °C. Results of those tests are presented on Fig. 2. Mass losses are close in # 40 % Cl and # 10 % Cl ashes after 100 h of exposure. The three ashes containing heavy metal chlorides have led to high mass losses (up to 6 times bigger than those measured in heavy metal free ashes) which reached 315 mg/cm2 for 16Mo3 steel in # ZnCl2/PbCl2 ash and 100 mg/cm2 for Inconel 625 alloy in # ZnCl2 ash. Mass losses measured in # PbCl2 were the lowest of the three heavy metal chlorides ashes. Mass losses in the two other ashes (# ZnCl2 and # ZnCl2/PbCl2) are in the same range. The # ZnCl2 ash was chosen for the rest of the study because the melting point of zinc chloride is the lowest of all the ashes (320 °C, [24]), and as it was also the most corrosive ash (especially for Inconel 625). Furthermore, this choice allowed the addition of only one other species to the 5 component salt mixture.
Chemistry of Ashes
It has been shown [11] that large corrosion rates were correlated with the presence and proportion of molten phases, as well as with the solidus temperature. Furthermore, heavy metal chlorides have low melting point and their presence in ash is known to decrease the temperature of the melt and thus, increase the corrosion rate at lower temperature [9, 25]. Two complementary methods (DTA and TMA) have been combined to measure the solidus temperature of # ZnCl2 ash. Obtained results are reported in Table 3 and compared to those measured previously on # 10 % Cl and # 40 % Cl. Both measurement methods were in good agreement and showed that the presence of ZnCl2 in ash mixture decreased drastically Tsol from 510 to 355 °C. It was shown in our previous study that CaSO4 can be considered as a spectator species in the mixture and only plays an effective role on the melt fraction. The melt fraction was 20 wt% in # 10 % Cl ash and 50 wt% in # 40 % Cl at 650 °C. From the conclusions on zinc free chloride ashes, the maximum liquid fraction reached at 650 °C corresponds to the sum of the fraction of all the species without CaSO4 [11]. Thus, it can be considered that the fraction of melt in this mixture is 30 wt%. This is confirmed by macroscopic observations on samples: only a small part of the ash mixture is melted. The presence of molten phases at 450 °C in # ZnCl2 ash is supposed to induce higher corrosion compared to salts (# 10 % Cl and # 40 % Cl) without molten phases at the same temperature.
Corrosion tests
Mass loss Figs. 3 and 4 show mass losses of 16Mo3 and Inconel 625 at 450 °C in # ZnCl2 ash and at 650 °C in # 10 % Cl and # 40 % Cl ashes as a function of time. Mass losses of both alloys at 650 °C in zinc chlorides ash after 100 h are also represented on the graph (Figs. 3 and 4). They correspond to the preliminary tests discussed previously. Mass losses measurements provide an average metal loss but the measurements do not take into account localized corrosion that thickness loss measurements provide. It should be noted that the straight lines connecting the dots are not relevant and have been added to help readers. Molten fraction of each ash mixture is also indicated on the graph. In all tested conditions, Inconel 625 samples were less corroded than 16Mo3. Mass losses observed on samples exposed to # ZnCl2 ash at 450 °C were much more significant than those observed for ashes without ZnCl2 at 650 °C. As it has been shown [11], low mass losses were observed at 450 °C in salts without ZnCl2 (less than 10 mg/cm2 for 16Mo3 after 1000 h in both # 10 % Cl and # 40 % Cl ashes and close to 0 for Inconel 625), due to the absence of molten phases at this low temperature. In # ZnCl2 ash, the highest mass loss was observed on 16Mo3 (A, about 320 mg/cm2). In the same ash, the highest mass loss of Inconel 625 was observed after 1000 h of exposure (E, about 225 mg/cm2). At 650 °C in ashes without ZnCl2, the highest mass losses were observed after 1000 h of exposure in # 40 % Cl ash for both 16Mo3 (C, about 160 mg/cm2) and Inconel 625 (G, about 90 mg/cm2). The addition of only 10 wt% of ZnCl2 in the ash led to a corrosion rate five times greater than those without ZnCl2 after 1000 h for the nickel based alloy, and two times greater for the 16Mo3 steel. In addition, mass loss of 16Mo3 after 100 h at 650 °C in # ZnCl2 ash was about 280 mg/cm2 (D) and close to 100 mg/cm2 for Inconel 625 (H). The values observed after 100 h (D and H) were up to two times higher than those observed in # 10 % Cl ash (without ZnCl2) after 1000 h of exposure at 650 °C for 16Mo3 and up to three times higher for Inconel 625 (B and F). This plot displays the high corrosivity of ZnCl2 even at low temperature due to the presence of molten phases as demonstrated in the Chemistry of Salt Mixture section. It should also be noted that higher corrosion was observed in # ZnCl2 ash than in # 40 % Cl ash even though # 40 % Cl ash has an higher molten fraction. Few studies are presenting mass loss of samples in representative ashes with heavy metal chlorides and none in similar conditions to the present ones. Nevertheless, the high corrosivity of heavy metal chloride has already been studied [26] by measuring corrosion products thickness. Thicknesses of those corrosion products were much higher in presence of heavy metal chlorides in deposit.
Mass loss measured on 16Mo3 steel and on Inconel 625 alloy after exposure to # 10 % Cl and # ZnCl2 ashes versus time (h). The estimated molten phase fraction in ash mixture is indicated by (0.X). Doted and straight lines are guide for the eyes in order to emphasize the two kinetics below and above Tsol
Mass loss measured on 16Mo3 steel and on Inconel 625 alloy after exposure to # 40 % Cl and # ZnCl2 ashes versus time (h). The estimated molten phase fraction in ash mixture is indicated by (0.X). Doted and straight lines are guide for the eyes in order to emphasize the two kinetics below and above Tsol
Thickness Loss
Figures 5 and 6 show the thickness losses versus time of 16Mo3 and Inconel 625 after exposure to # ZnCl2 ash at 450 °C compared to # 10 % Cl (Fig. 5) and # 40 % Cl (Fig. 6) ashes at 650 °C. The maximum thickness loss observed for 16Mo3 alloy was about 615 µm in # ZnCl2 ash after 1000 h at 450 °C while for Inconel 625 alloy, the maximum was about 410 µm in the same conditions of corrosion. Those results were in good agreement with mass losses discussed in the previous part. As observed by mass loss measurements, the thickness loss in # ZnCl2 was two times higher than those observed on both alloys in salts without ZnCl2 (and up to four times higher than for 16Mo3 exposed to ash with and without ZnCl2 after 1000 h of exposure). Contrary to mass loss, thickness loss measurements give more information on localized corrosion. The thickness loss was an average of twenty measurements and the error bars provide a minimum and a maximum of thickness loss which reflected the localized corrosion. The standard deviations obtained for samples exposed to # ZnCl2 ash were up to four times larger than those in ash without ZnCl2 and underlined a less homogenous attack.
Thickness loss measured on 16Mo3 steel and Inconel 625 alloy after exposure to # 10 % Cl and # ZnCl2 ashes versus time (h). The estimated molten phase fraction in ash mixture is indicated by (0.X). Doted and straight lines are guide for the eyes in order to emphasize the two kinetics below and above Tsol
Thickness loss measured on 16Mo3 steel and Inconel 625 alloy after exposure to # 40 % Cl and # ZnCl2 ashes versus time (h). The estimated molten phase fraction in ash mixture is indicated by (0.X). Doted and straight lines are guide for the eyes in order to emphasize the two kinetics below and above Tsol
SEM/EDX Studies of the Corrosion Products in Zinc Chloride Ash
Figure 7 presents the SEM macroscopic view of 16Mo3 (a, b) and Inconel 625 (c, d) samples after 500 h at 650 °C in # 10 % Cl ash (a, c) and at 450 °C in # ZnCl2 ash (b, d). A stronger attack was observable and a high internal attack by pitting at grains boundaries was perceptible in # ZnCl2 ash, especially for the iron-based alloy. Inconel 625 sample exposed to # ZnCl2 ash also exhibited a non-uniform attack. The corrosion profiles for each sample are described below.
16Mo3
Figure 8 shows SEM images of 16Mo3 sample after 500 h of exposure to # ZnCl2 ash at 450 °C. Even though the test was performed at low temperature, severe corrosion was observed. The corrosion profile was very different than the one observed at the same temperature without ZnCl2 (described in [11]) and looked similar to a high temperature corrosion profile. Oxide layers were mixed with ash compounds along with strong internal attack by chlorine (pitting corrosion) was observed and seemed to propagate along the grain boundaries as shown on Fig. 9. Measured pits were about 80 µm deep. Reaction between chlorides and oxide seemed to be occurring here since salt elements (Ca, K, S) were detected into oxide layer as shown on X element maps (Fig. 8). Small amounts of zinc salt were detected in the entire oxide layer and not systematically associated with chlorine. The thickness of those oxide scales was up to 2000 µm, and mainly composed of iron oxide, zinc and iron mixed oxide and a few iron sulphides (according to EDS analysis).
Inconel 625
Figure 10 shows SEM images of Inconel 625 sample after 500 h of exposure to # ZnCl2 ash at 450 °C. It was also severely attacked at this low temperature. Thickness of corrosion products was about 500 µm. Internal pitting was also detected with a depth about 20 µm. Chromium oxide was the main corrosion product observed above metal/oxide interface but it is porous and mixed with salt mixture compounds (chloride and sulphates). The upper part of the oxide scale was composed of mixed oxides (Cr, Zn, Mo), chlorides and sulphates enriched in Ni. Zinc salt was detected in the entire oxide layer and not only associated with chlorine. Contrary to profiles observed at 650 °C without ZnCl2 (also described in [11]), no chromium free zone was observed at metal/oxide interface and no separate corrosion products scales (Cr2O3, NiS, NiO) were present.
Discussion
In presence of ZnCl2 in the salt mixtures, the corrosion profile observed for 16Mo3 samples at 450 °C is quite the same as the one observed at higher temperature (650 °C) without ZnCl2. However, the corrosion was more severe and exhibited an extremely high internal attack. The presence of ZnCl2 in the molten phase also containing alkali chlorine and sulfate is assumed to be responsible of such attack since its presence is the only difference between the ashes (particularly between # ZnCl2 and # 10 % Cl ash). The attack was assumed to be due to fluxing of the protective oxide following reactions proposed by BANKIEWICZ [26] and illustrated by the four steps in Fig. 11 in addition of reactions described without ZnCl2 in deposit [11]. In the two first steps, ZnCl2 contained in the salt liquid phase reacts with iron oxides and induces the formation of the spinel ZnFe2O4 and iron chlorine FeCl2 (g) [27]:
Gibbs free energies of each reaction versus temperature are presented in Fig. 12. ZnCl2 can also react directly with iron [27]:
ZnCl2 can also contribute to form Cl2 (g) by direct oxidation even if this reaction is less favored from thermodynamic point of view [27]:
The chlorine formed by reactions 1–4 can attack the iron at interface as described in [11], illustrated in the second step on Fig. 11 [27]:
Then (steps 2 and 3), as for corrosion under deposit [12], the volatilization of iron chlorine leads to (1) the delamination of the oxide scale, (2) the oxidation of iron chlorine with formation of non-continuous iron oxides and release of Cl2 (g). Reactions between ZnO and iron oxides can also occur in agreement with thermodynamic calculation (Fig. 12). In the last step, a cyclic phenomenon is considered to occur inducing the growth of an important oxide layer. The low viscosity liquid salt phase is supposed to be responsible of pitting at iron/oxide interface.
Contrary to 16Mo3 samples, corrosion profile observed on Inconel 625 samples in presence of ZnCl2 is very different from those observed at higher temperature (650 °C) in absence of ZnCl2 in ash. The attack is also severe (as for 16Mo3) but no stratification was observed contrary to profiles presented in [11] after exposure to # 10 % Cl and # 40 % Cl at 650 °C. All corrosion products are mixed, which is a clear sign of fluxing mechanism probably coupled with direct attack of metal by molten chloride. Formation of initial Cr2O3 layer is also probable considering the high Cr content in Inconel 625 (23 wt%) and the oxidizing condition assumed as the tests are performed in laboratory air. Nevertheless it is obvious that formed Cr2O3 was no longer protective (non continuous, non adhesive). Globally, the mechanism steps for Inconel 625 are quite similar to the one described for 16Mo3 (Fig. 11) and are schematically represented in Fig. 13. First (steps 1 and 2), zinc chloride present in the liquid salt phase attacks the alloy according to the following reactions [27]:
In the second and third step, the volatilization and oxidation of CrCl2 (g) induce the formation of a non-continuous chromium oxide layer and the release of Cl2 (g) which produces a cyclic attack at step 4.
ZnO from reaction 4 can also dissolve chromium oxide [27]:
The strong internal attack by pitting at grains boundaries observed on both 16Mo3 and Inconel 625 was presumed to be favored by the presence of a low viscosity (high wettability) molten species due to ZnCl2 addition. By comparing thickness and mass losses in ashes with and without ZnCl2 and molten fraction of each ash, it also appeared that the chloride specie was more important than the molten fraction. Indeed, thickness and mass losses observed were much more important in # ZnCl2 ash with a molten fraction of 0.3 than in # 40 % Cl ash which had a molten fraction of 0.5.
Conclusions
The purpose of this study was to evaluate the influence of zinc chloride content in synthetic ashes representative of waste-to-energy deposits on the relative resistance of two heat exchanger materials. After preliminary tests, a synthetic ash containing 10 wt% of ZnCl2 was chosen to evaluate the corrosion of metallic alloys (16Mo3 and Inconel 625). Corrosion tests were performed in air at 450 °C and compared to those run at 650 °C [11] (without ZnCl2). Temperatures of solidus of ashes were determined using two different methods: DTA, TMA.
-
The measured temperature indicated that molten phases were already present at 450 °C (Tsolidus = 320 °C), which is the temperature of corrosion tests, but is also representative of current metal tube operating conditions.
-
In presence of ZnCl2 in ash, both alloys exhibited high corrosion rate at 450 °C compared to very low attack without ZnCl2 at the same temperature. Corrosion observed with ZnCl2 at 450 °C was twice as significant than without ZnCl2 at 650 °C.
-
ZnCl2 induced the presence of highly corrosive molten phases at this temperature, and fluxing mechanisms occurred.
-
The strong internal attack by pitting (intergranular attack) at grains boundaries observed on both 16Mo3 and Inconel 625 was assumed to be due to a low viscosity molten phase induced by ZnCl2. Lower viscosity of the molten phase in presence of ZnCl2 should lead to higher wettability of the molten phases within oxide scale cracks and pores. Viscosity measurements should confirm this hypothesis.
-
Furthermore, it seems that the chloride species is more important than the molten fraction since corrosion observed in # 40 % Cl ash with a molten fraction of 0.5 was lower than in # ZnCl2 ash which has a molten fraction of 0.3.
-
Both evaluation methods, thickness loss and mass loss, were in good agreement and showed that presence of zinc chloride in ash led to high corrosion rate at low temperature and non uniform attack, due to the presence of molten phases and the highly corrosive character of ZnCl2 compared to alkali chlorides.
References
Y. Kawahara, Corrosion Science 44, 223 (2002).
A. J. Pedersen, F. J. Frandsen, C. Riber, T. Astrup, S. N. Thomsen, K. Lundtorp and L. F. Mortensen, Energy & Fuels 23, 3475 (2009).
M. Becidan, L. Sørum, F. Frandsen and A. J. Pedersen, Fuel 88, 595 (2009).
T. Talonen, Chemical equilibria of heavy metals in waste incineration: comparison of thermodynamic databases. Lic. Thesis. (Åbo Akademi University, Finland, 2008).
Y. L. Zhang and E. Kasai, ISIJ International 44, 1457 (2004).
M. Bøjer, P. A. Jensen, F. Frandsen, K. Dam-Johansen, O. H. Madsen and K. Lundtorp, Fuel Processing Technology 89, 528 (2008).
S. Osada, D. Kuchar and H. Matsuda, Journal of Material Cycles and Waste Management 11, 367 (2009).
C. Chan, C. Q. Jia, J. W. Graydon and D. W. Kirk, Journal of Hazardous Materials 50, 1 (1996).
M. Spiegel, Materials and Corrosion 50, 373 (1999).
Y. S. Li, Y. Niu and W. T. Wu, Materials Science and Engineering: A 345, 64 (2003).
E. Schaal, N. David, P. J. Panteix, C. Rapin, J. M. Brossard and F. Maad, Oxidation of Metals 84, 307 (2015).
H. J. Grabke, Materials at High Temperatures 11, 23 (1993).
J. Lehmusto, P. Yrjas, B. J. Skrifvars and M. Hupa, Fuel Processing Technology 104, 253 (2012).
J. Pettersson, N. Folkeson, L. G. Johansson and J. E. Svensson, Oxidation of metals 76, 93 (2011).
D. Bankiewicz, P. Yrjas, D. Lindberg and M. Hupa, Corrosion Science 66, 225 (2013).
T. Jonsson, N. Folkeson, J. E. Svensson, L. G. Johansson and M. Halvarsson, Corrosion Science 53, 2233 (2011).
T. Varis, D. Bankiewicz, P. Yrjas, M. Oksa, T. Suhonen, S. Tuurna, K. Ruusuvuori and S. Holmström, Surface & Coatings Technology 265, 235 (2015).
A. Ruh and M. Spiegel, Corrosion Science 48, 679 (2006).
Y. S. Li, M. Spiegel and S. Shimada, Materials Chemistry and Physics 93, 217 (2005).
J. Lehmusto, B. J. Skrifvars, P. Yrjas and M. Hupa, Corrosion Science 53, 3315 (2011).
S. Enestam, D. Bankiewicz, J. Tuiremo, K. Mäkelä and M. Hupa, Fuel 104, 294 (2013).
C. W. Bale, P. Chartrand, S. A. Degterov, G. Eriksson, K. Hack, R. B. Mahfoud, J. Melancon, A. A. Pelton and S. Petersen, Calphad 26, 189 (2002).
ISO/DIS 17248, AFNOR, (2014)
N. P. Lushnaya, N. N. Evseeva and I. P. Vereshchetina, Russian Journal of Inorganic Chemistry 1, 35 (1956).
M. Sánchez Pastén and M. Spiegel, Materials and Corrosion 57, 192 (2006).
D. Bankiewicz, S. Enestam, P. Yrjas and M. Hupa, Fuel Processing Technology 105, 89 (2013).
A. Roine. HSC Chemistry Version 4.1. (Outokumpu Research Organization, Pori, Finland, 1999).
Acknowledgments
This work has been supported by the French National Research Agency with project ANR SCAPAC 11-RMNP-0016 in partnership with, AIR LIQUIDE, SEDIS and CIRIMAT/ENSIACET. The authors thank L. ARANDA of Institut Jean Lamour, Nancy (France) for carrying out TMA and DTA analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schaal, E., David, N., Panteix, P.J. et al. Effect of Zinc Chloride in Ash in Oxidation Kinetics of Ni-Based and Fe-Based Alloys. Oxid Met 85, 547–563 (2016). https://doi.org/10.1007/s11085-016-9612-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-016-9612-5