Abstract
In this study, α-Ga2O3 nanorod arrays (NRAs) were grown using a simple and low-cost two-step hydrothermal method. Subsequently, a photoelectrochemical ultraviolet photodetector (PEC-UV PD) based on the α-Ga2O3 NRAs was fabricated, which can operate at zero bias and has a performance far superior to that of PEC-UV PDs based on the one-step hydrothermal method. Under 2 mW/cm2 illumination at 254 nm, the device achieved a responsivity of 26.59 mA/W, an external quantum efficiency of 12.98%, and a rise/decay time of 53 ms/7 ms without external bias. These results demonstrate outstanding detection performance compared to previously reported devices. The superior performance can be attributed to the better crystallinity which provides a larger absorption area for the device, the solution contact area, and a faster channel for carrier transport, the space charge region at the solid/liquid interface which also allows efficient separation of photogenerated carriers. Furthermore, it also benefits from the positive effects of moderate oxygen vacancy (VO) concentrations for NRAs. The α-Ga2O3 NRAs PEC-UV PD with two-step hydrothermal method is undoubtedly a promising candidate for future solar-blind detection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The solar spectrum can usually be divided into the visible, infrared, and ultraviolet bands (Hu et al. 2011, 2014; Chen et al. 2021; Guo et al. 2023; He et al. 2023). Within ultraviolet (UV) light, it can be meticulously divided into long-wave UV (UVA, low frequency, 400–315 nm), medium-wave UV (UVB, medium frequency, 315–280 nm), and short-wave UV (UVC, high frequency, 280–200 nm) according to the wavelength of the light (Pearton et al. 2018). UVC radiation from the sun is reflected and absorbed by ozone, water vapor, and other atmospheric molecules in the atmosphere, making it scarcely detectable at the surface of the Earth. Consequently, this wavelength range is often referred to as solar-blind UV. As it is not affected by the background noise of the sunlight, there are a wide range of potential applications for solar-blind light detection, such as biomedicine, UV monitoring, UV communications, solar-blind imaging, and missile tracking (Wu et al. 2017, 2021; Ahn et al. 2021; Wang et al. 2020a; Zhuo et al. 2018; Mahmoud 2016).
In recent years, many attempts have been made to construct UV photodetectors from suitable materials (Guo et al. 2023). At this stage, wide-bandgap materials commonly used for the preparation of solar-blind UV photodetectors include AlGaN, MgZnO, Ga2O3, and diamond (Tsao et al. 2017; Cicek et al. 2013; Hou et al. 2013; Kong et al. 2016; Liao et al. 2010). The bandgap of diamond is 5.5 eV, which is very inefficient for the solar-blind band (Gorokhov et al. 2011), while the bandgaps of silicon carbide (SiC, 3.3 eV) (Morkoç et al. 1994) and gallium nitride (GaN, 3.4 eV) (Lan 2021) are sufficient for the near-ultraviolet band but are no longer suitable for the solar blind band below 280 nm. In addition, the AlxGa1−xN material can be adjusted by the Al component to meet solar-blind detection. However, when the Al content is too heavy, a large number of defects appear in the thin film (Chi et al. 2022). Similarly, MgZnO undergoes severe phase separation as the proportion of Mg elements rises, which ultimately leads to a reduction in device performance. Ga2O3, a new wide-bandgap semiconductor material, is a natural deep ultraviolet probe material due to its bandgap between 4.4 and 5.3 eV (Akaiwa and Fujita 2012; Onuma et al. 2015; Fujita et al. 2016) and its response peak in the solar-blind band (Orita et al. 2000; Chen et al. 2016).
However, most current Ga2O3-based photodetectors are the metal–semiconductor-metal (MSM) type and need to be supported by an external power supply, which limits their use in a variety of extreme conditions. The Ga2O3-based electrochemical self-powered solar-blind photodetector has a promising future because it does not require an external power supply and has low manufacturing costs. Recently, Zhang et al. (2022a) have grown and fabricated α-Ga2O3 NRAs PEC PDs on FTO substrates by a one-step hydrothermal method, and the devices are capable of self-powered operation with a responsiveness of 3.87 mA under solar blind light illumination only. Zhang et al. (2018) and Atilgan et al. (2020) have successfully grown β-Ga2O3 nanowires with superior crystal morphology on Si substrates using a two-step hydrothermal method. They added anhydrous ethanol to the reaction solution during the first hydrothermal step, which eased the surface tension between the solution and the substrate, allowing more nucleation sites to appear on the substrate. The central idea of the method is to use solution effects to alter the interaction of solution clusters with the surface.
In this work, the α-Ga2O3 NRAs were synthesized on FTO glass substrates via a two-step hydrothermal method and fabricated the α-Ga2O3 NRAs PEC-UV PD. Compared to other reported Ga2O3-based UV photodetectors, the α-Ga2O3 NRAs PEC-UV PDs exhibited higher responsiveness, higher external quantum efficiency, and faster responsivity rates.
2 Experimental details
2.1 Synthesis and characterization of the α-Ga2O3 NRAs
Firstly, the FTO substrate was cleaned with acetone, alcohol, and deionized water (DIW) to remove impurities from the substrate surface. Secondly, the reaction solution for the two hydrothermal steps was prepared separately: the solution for the first hydrothermal step consisted of 10 ml anhydrous ethanol (99.5%), 30 ml DIW and 0.3 g gallium nitrate (Ga(NO3)3) solution (Ga 9–10% w/w, Alfa Aesar Co., Ltd); the solution for the second hydrothermal step consisted of 30 ml DIW and 0.3 g Ga(NO3)3 solution. Both hydrothermal solutions were stirred at room temperature for about 30 min to allow the components to mix thoroughly. The FTO substrate and the solution for the first hydrothermal step were then transferred to a stainless-steel reactor lined with PTFE, heated at 100 °C for 1 h, and allowed to cool naturally to room temperature. The reaction solution was then replaced with the second hydrothermal solution and heated at 150 °C for 10 h. Afterwards, the FTO glass with the GaOOH precursors was repeatedly rinsed with alcohol, DIW and dried in a desiccator at 70 °C for 2 h. Finally, the completely dried samples were placed in a muffle furnace set at 400 °C and annealed in air for 4 h to obtain α-Ga2O3 NRAs. We have labeled this two-step hydrothermal growth sample as Sample II. For a valid comparison, we have grown a similar sample using only the second hydrothermal step (30 ml DIW and 0.3 g Ga(NO3)3 solution), noted as Sample I, according to previous reports (Zhang et al. 2022a). Figure 1 shows the schematic diagrams of one-step hydrothermal and two-step hydrothermal synthesis of α-Ga2O3 NRAs. It is worth noting that there is a short nucleation phase during the crystal growth process, which is usually overlooked and will be discussed below. We subjected the one-step samples (Sample I) to an 11-h hydrothermal reaction as well as a 4-h annealing to ensure that both samples experienced consistent thermal reaction times.
To determine the morphological characteristics and material composition of the two samples, simple scanning electron microscope (SEM) and X-ray diffraction (XRD) characterization tests were carried out. The crystalline phase and structure of the samples were characterized using the XRD (Bruker AXS, D8 Advance) emitting Cu Kα radiation. SEM was used to observe the microstructure of the samples.
2.2 Fabrication of the electrochemical ultraviolet photodetector (PEC-UV PD)
The fabricated solar-blind photodetectors were tested for photovoltaic properties, etc., using a CHI 760E electrochemical workstation (CH Instruments, Chenhua Co., Shanghai). In the PEC setup, FTO glasses grown with α-Ga2O3 NRAs are held in place by platinum electrode clamps as photoanodes, with a platinum mesh electrode (1 × 1 cm2) and a saturated calomel electrode (SCE) as counter electrodes. The reaction solution for PEC was 0.5 M Na2SO4 aqueous solution. LED beads with wavelengths of 254 nm, 265 nm, 275 nm, 295 nm, 310 nm, and 365 nm were used as the UV light source. The effective light area of the device is approximately 1 cm2.
3 Results and discussion
The top SEM views of Sample I and Sample II are shown in Fig. 2a, b, respectively. In Sample I which has not experienced the first hydrothermal step, there are more incompletely grown nanorods (as shown in the circles in Fig. 2a). While in Sample II which has undergone two hydrothermal steps, there are only very few incompletely grown nanorods, most of which show approximately the same edge length (~ 240 nm). It can be clearly observed that the nanorods in Sample I are mostly tilted at an angle to the substrate, whereas the nanorods in Sample II grow almost perpendicular to the substrate. Figure 2c, d shows a side view of both samples, and it is clear that Sample II has a larger size and a more complete crystal morphology.
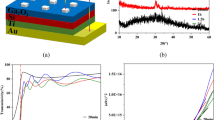
Figure 3 shows the XRD plots. The 2θ angles at 36.025°, 55.114°, and 64.777° correspond to the (110), (116) and (300) planes of α-Ga2O3 (PDF#06-0503) respectively. The remaining spurious peaks all originate from the SnO2 component of the FTO substrate, indicating that the sample contains no other impurities, ensuring satisfactory crystal growth. Furthermore, we can clearly observe that Sample II has a much higher peak level in the (110) plane than Sample I, indicating that the two-step hydrothermal method is more suggesting more directional rods towards (110) plane.
According to classical crystal growth theory (Fujihara et al. 2005; Lewis 1974), crystal growth in solution is usually divided into two basic steps: The first step is called the nucleation phase, where the solute is transported from the solution to the solution/substrate interface due to the gradient in solute concentration at the substrate and solution interface, and then adheres to the substrate to form a nucleus. The second step, known as the crystal growth phase, becomes the driving force for crystal growth due to the great difference in concentration gradients brought about by the nucleation phase in the previous step (Fujihara et al. 2005), which allows the crystal to start growing based on the nucleation sites. In general, the density of nucleation sites is proportional to the concentration of the solution, with higher precursor concentrations giving more nucleation sites to the substrate (Liang et al. 2017). When we consider the hydrophobicity of the hydrothermal solution (Ga3+/H2O) and the FTO surface, the Ga3+ ions are not readily adsorbed by the FTO substrate, so even with a higher concentration of solution it fails to bring about more nucleation sites, and the nanorods grow in clusters around these limited sites. At this point, several or even more nanorods grow on a nucleation site, and there is also competition between these nanopillars. According to Oswald's ripening theory (Reddy et al. 2015), the Gibbs free energy of the small particles is larger than that of the large particles, resulting in a higher concentration of parent phase components around the small particles than the large particles, and the concentration gradient creates diffusion, which in turn results in the small particles continuously delivering supersaturated components to the large particles, with the final nanorod exhibiting a high shape size difference.
According to the theory of R.W. Thomas with the hypernetted-chain approximation (Chan et al. 1978; Li et al. 2015), the solute distribution on the hydrophobic surface depends on the contact angle between the solution and the substrate (Li et al. 2015), and when ethanol is added to the solvent, the adhesion of the mixed solution is improved at this point, thus changing the contact angle between the solution and the substrate (Kokkoli et al. 1999). As shown in Fig. S1 (Supporting Information), we applied drops of DIW and an ethanol/DIW mixture to the FTO glass, respectively, and the ethanol-treated solution lay completely flat on the substrate. Because of the addition of ethanol, the surface tension of water is reduced, making it easier for the Ga3+ ions to adhere to the FTO substrate, thus increasing the nucleation site density. During the subsequent crystal growth process, more nucleation sites eased the competition for the nanopillars, which were eventually grown uniformly to form α-Ga2O3 NRAs with good morphology and excellent crystallinity.
We have tested the photovoltaic properties of α-Ga2O3 NRAs PEC-UV PDs in a quartz cell containing a Na2SO4 reaction solution in conjunction with an electrochemical workstation. The optical responsivity characteristics were first tested on two samples at 2 mW/cm2, 254 nm wavelength illumination, and 0 V bias voltage at 10 s On/Off intervals. As shown in Fig. 4a, the photocurrent density of Sample I is approximately 24.052 μA/cm2, while the photocurrent density of Sample II is approximately 51.08 μA/cm2. The Sample II exhibits a higher photocurrent density, due to the higher number of nucleation sites from the first hydrothermal step which guarantees a high crystallinity and a favorable array structure of the crystal. Larger cross-sectional areas and longer nanorod heights allow for a larger surface area for UV light absorption and easier device generation of photogenerated carriers. Similarly, this increases the contact area between the device and the electrolyte, and photogenerated carriers are more easily transferred from the α-Ga2O3 NRAs into the electrolyte (Lin et al. 2016; Chen et al. 2019; He et al. 2019).
a I-t curves of Sample I and II under 0 V bias voltage and 2 mW/cm2, 254 nm UV illumination. b Spectral responsivity patterns of Sample II under different wavelengths of UV illumination and the corresponding photocurrent density. c I-t curve of α-Ga2O3 NRAs (Sample II) PEC-UV PD under different light intensity irradiation at 0 V. d Relationship between photocurrent and light intensity. e Plot of responsiveness versus light intensity. f Plot of efficiency versus light intensity
In addition, to investigate the ability of the device to detect solar-blind light signals, we measured the photocurrent and the corresponding responsiveness under different wavelengths of UV illumination, as shown in Figs. 4b and S2 (Supporting Information). Both samples showed the largest photocurrent density at 254 nm and the highest responsivity (Sample I: 12 mA/W, Sample II: 26.59 mA/W). As the wavelength moves closer to the near-UV band, the photocurrent density as well as the responsiveness of the device decreases. Sample II achieved a photocurrent rejection ratio (I254 nm/I365 nm) of 1.274 × 105 in the solar blind band (254 nm) and the near ultraviolet band (365 nm), indicating that the α-Ga2O3 NRAs can detect the solar blind signal well without interference from other wavelengths of ultraviolet light. This is assisted by the ultra-wide bandgap of α-Ga2O3, which makes it extremely sensitive to solar blind signals without any energy band engineering. The UV–vis spectroscopy of the two samples is shown in Fig. S3 (Supporting Information).
Subsequently, the focus was on the light detection performance of α-Ga2O3 NRAs PEC-UV PDs under solar blind illumination (254 nm). The linear scanning voltammetry (LSV) characteristic curves of α-Ga2O3 NRAs PEC-UV PDs in the dark and under 254 nm light are shown in Figs. S4 and S5 (Supporting Information). The very weak dark currents exhibited by both samples at 0 V as well as the pronounced photocurrents demonstrate the self-powered capability of the α-Ga2O3 NRAs PEC-UV PD. The I-t curves of the α-Ga2O3 NRAs (Sample II) PEC-UV PD at 0 V, 0.5 ~ 2 mW/cm2 optical power are shown in Fig. 4c. The results show that the prepared detector can respond immediately and produce a relatively stable photocurrent at the Off/On transition, indicating that the device has achieved good sensitivity and stability. The effect of light intensity on PD performance is further discussed to visualize the relationship between optical power and photocurrent. Figure 4d shows the photocurrent density generated by the device at different light intensities. When the light intensity is gradually increased from 0.5 to 2 mW/cm2, the photocurrent density increases from 14.176 μA/cm2 to 51.08 μA/cm2, indicating that higher light intensity can stimulate more photogenerated electron–hole pairs, thus generating a larger photocurrent. In addition, the dark current density of the device at a light intensity of 2 mW/cm2 is merely 0.0289 μA/cm2, with a light-to-dark current ratio (Iphoto/Idark) of 1767.
We usually measure the performance of a photodetector in terms of responsivity (R) and external quantum efficiency (EQE). Responsiveness is defined as R = (Iphoto-Idark)/(S·P), where Iphoto is the photocurrent, Idark is the dark current, S is the effective light area and P is the light intensity (Li et al. 2019). The external quantum efficiency is usually defined as EQE = (Rhc)/eλ, where R is the responsivity, h is Planck's constant, c is the speed of light, e is the electron charge and λ is the wavelength of incident light (Zhang et al. 2019a). The responsivity curves and EQE curves for the two samples are shown in Fig. 4e, f. Under UV illumination at 254 nm, 2 mW/cm2, and 0 V, the responsivity and EQE of the Sample II were 26.59 mA/W and 12.98%, respectively. As the optical power increases, both R and EQE show a slight decrease, which we speculate may be due to the fact that higher light intensities excite more photogenerated carriers, but also increase the compounding of electron–hole in the nanorods, and the proportion of photogenerated carriers that can be used effectively decreases (Li et al. 2019; You et al. 2019; Yan et al. 2020). However, we found that the responsivity curves of Sample I did not follow a regular monotonic decrease, probably due to the sequence of tests and the fact that UV illumination changed the surface wettability. α-Ga2O3 NRAs converted from hydrophobic to hydrophilic would be more susceptible to redox reactions with Na2SO4 solutions, enhancing the photoresponsivity.
When irradiated with UV light (254 nm) at photon energies higher than the band gap of α-Ga2O3, electrons (e−) in the valence band will be excited to the conduction band, while the same number of holes (h+) will be generated in the valence band. Some of these holes will react with lattice oxygen to form surface oxygen vacancies (VO) and some of the electrons will react with lattice metal ions Ga3+ to form Ga2+ defects. Water and oxygen in solution will compete to dissociate and adsorb at these sites. Ga2+ tends to react with surface oxygen molecules, while at the same time water molecules will be coordinated to the VO, causing the dissociative adsorption of water. Thus, the wettability of α-Ga2O3 has changed from hydrophobic to hydrophilic. However, the time required for this hydrophilic transition varies in different nanomorphology. The shorter the hydrophilic transition time for nanostructures with larger contact areas, as reported by Khranovskyy et al. (2012). The order of increasing light intensity was used in our responsiveness tests. Therefore, we presume that the transition to wettability was completed at the time of the 0.5 mW/cm2 light intensity test when testing the Sample II because of the larger surface area. For Sample I, however, due to its smaller surface area, it took longer to generate sufficient water dissociation adsorption sites, so it only converted to hydrophilic at 1 mW/cm2 light intensity tested. This resulted in a higher responsivity for Sample I at 1 mW/cm2 than at 0.5 mW/cm2.
In addition to the effect of light intensity, the optical responsivity performance of the device is also affected by the applied bias voltage. Figure 5a shows the photocurrent density of the α-Ga2O3 NRAs (Sample II) PEC-UV PD at different bias voltages. As the bias voltage rises, the photocurrent generated by the device also follows. This is because the applied bias voltage accelerates the drift of the photogenerated carriers and increases the probability of separation of the electron–hole pairs (Ahmadi et al. 2017), so the device will be more responsive at higher bias voltages. To further evaluate the optical responsivity speed of the device, the rise time and decay time of the Samples I and II are shown in Fig. 5c,d. The rise time (τr) is defined as the time it takes for the photocurrent density to rise from 10 to 90% of its maximum value, and the decay time(τd) is the time used for the photocurrent density to fall from 90 to 10% of the maximum value. The rise time/decay time is 53 ms/7 ms for Sample II and 84 ms/9 ms for Sample I, with a particularly significant rise time improvement (31 ms). This enhancement is brought about by the high quality of the crystals. Longer and wider nanorods allow for more contact with the electrolyte solution and more light absorption area, while better crystallinity provides a faster channel for carrier transport, thus increasing the response times. In addition, we performed stability tests on the device for up to 1000 s (~ 50 cycles), as shown in Figs. 5b and S6 (Supporting Information). After 50 on/off cycles, the photocurrent density of the device remained at a promising level, indicating that the UV photodetectors have a good stability.
a Photocurrent responsivity of α-Ga2O3 NRAs (Sample II) PEC-UV PD at 0 V, 0.2 V, 0.4 V and 0.6 V under 254 nm, 2 mW/cm2 illumination. b Long-term stability test image of α-Ga2O3 NRAs (Sample II) PEC-UV PD under zero bias and 254 nm, 2 mW/cm2 illumination. c Rise time and decay time of Sample II. d Rise time and decay time of Sample I
To investigate in depth the self-powered characteristics and operating mechanism of the α-Ga2O3 NRAs PEC-UV PD, we have drawn a schematic diagram as shown in Fig. 6. When α-Ga2O3 NRAs are in contact with Na2SO4 electrolyte solution, the Fermi energy level (EF) of α-Ga2O3 is greater than the redox potential (EO/R) of Na2SO4 (He et al. 2019). To bring the Fermi energy level and redox potential back into dynamic equilibrium, electrons within the semiconductor are transferred to the electrolyte solution, which causes an upward bending of the energy band on the semiconductor surface and the creation of a space charge region (with a built-in electric field pointing from α-Ga2O3 to the Na2SO4 solution) (Wang et al. 2022). When the α-Ga2O3 NRAs are irradiated by UV light, photogenerated electron–hole pairs are produced and they are separated by the built-in electric field. The photogenerated electrons are separated and transferred to the Pt electrode via the conductive layer on the surface of the FTO substrate and the external circuit and undergo a reduction reaction with OH* in solution (e− + OH* → OH−). And photogenerated holes are driven to the solid/liquid interface to undergo oxidation with OH− (h+ + OH− → OH*) (Zhang et al. 2021; Bai and Zhang 2016; Li et al. 2013). As long as they are exposed to a constant stream of UV light, the photogenerated electron–hole pairs can be driven by the built-in electric field to continuously separate and react with the solution in a continuous redox reaction, thus achieving a self-powered effect. The reason for the better performance of Sample II is that there are enough nucleation sites in the crystal growth phase to allow the nanorods to be well formed.
In photodetectors based on Ga2O3 thin films, methods such as high oxygen annealing and oxygen plasma treatment are often used to reduce the oxygen vacancy (VO) concentration in the material (Zhang et al. 2022b; Wang et al. 2020b; Guo et al. 2014). This is because VO is generally thought to create trap states on the surface, leading to severe surface compounding and a reduction in photocurrent (Guo et al. 2014). In fact, the presence of VO during PEC increases the carrier concentration (when VO is generated in a semiconductor, the extra electrons compensate for the charge, leading to a boost in n-type semiconductor carrier concentration), enhances charge transfer, and inhibits in body complexation (Wang et al. 2019a). Therefore, a reasonable VO concentration is of extreme benefit for the performance enhancement of α-Ga2O3 NRAs PEC-UV PDs. As shown in Fig. 7a, we fitted the equivalent series resistance (Rs) and the interfacial charge transfer resistance (Rct) of two α-Ga2O3 NRAs PEC-UV PDs by electrochemical impedance spectra (EIS). Rs is positively proportional to VO concentration and Rct is inversely proportional to VO concentration. The smaller the Rs, the faster the photogenerated carrier transport, and the smaller the Rct, the faster the interfacial charge transfer, resulting in greater photocurrents (Qu et al. 2023). The Rs and Rct of our fitted Sample II are: 11.5 Ω and 9.994 Ω, while the two resistance values of Sample I are: 11.31 Ω and 117 Ω, respectively. After upgrading from the one-step to the two-step method, ΔRs is 0.19 Ω and ΔRct is -107.006 Ω. The trend of Rct is greater than Rs, so the optical responsivity in our α-Ga2O3 PEC-UV PDs is mainly modulated by Rct. Since the Sample II has a smaller Rct, it has a higher VO concentration and the surface will attract more OH− ions for reaction, accelerating the charge transfer at the interface and bringing a higher photoresponsivity.
As shown in Fig. 7b, we have compared this work with other work on Ga2O3-based photodetectors (Zhang et al. 2022a, 2021, 2019b; Chen et al. 2019; He et al. 2019; Wang et al. 2023, 2019b; Ni et al. 2022; Huang et al. 2021a, b). Our α-Ga2O3 NRAs PEC-UV PD exhibits higher responsiveness and faster response times than other Ga2O3-based PEC PDs. In addition, other performance parameters are comparable to or better than those previously reported. The advantages of the device are: (i) the preparation of α-Ga2O3 NRAs using a very low-cost hydrothermal method, (ii) better UV absorption and fast separation of carriers due to the larger surface area and excellent crystallinity of the NRAs, (iii) appropriate concentrations of VO increase the n-type carrier concentration and accelerate the interfacial charge transfer.
4 Conclusions
In this study, we have fabricated an α-Ga2O3 NRAs PEC-UV PD based on a two-step hydrothermal method. The built-in electric field formed at the solid/liquid interface allows the device to operate without an applied voltage and to exhibit favorable optoelectronic properties. In particular, the optical responsivity reached 26.59 mA/W, Iphoto/Idark reached 1767 and the rise and decay times of the optical responsivity were 53 ms and 7 ms at 254 nm, 2 mW/cm2, and zero bias. The excellent crystallinity, fast separation of photogenerated carriers, and better transport channels are responsible for the superior performance. Furthermore, the proper use of VO also accelerates the interfacial charge transfer for the device. These results demonstrate that the α-Ga2O3 NRAs PEC-UV PD, fabricated using the two-step hydrothermal method, emerges as a highly promising candidate for future solar-blind detection applications.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmadi, M., Wu, T., Hu, B.: A review on organic-inorganic halide perovskite photodetectors: device engineering and fundamental physics. Adv. Mater. (2017). https://doi.org/10.1002/adma.201605242
Ahn, J., Ma, J., Lee, D.: Ultrahigh deep-ultraviolet responsivity of a β-Ga2O3/MgO heterostructure-based phototransistor. ACS Photon. 8(2), 557–566 (2021). https://doi.org/10.1021/acsphotonics.0c01579
Akaiwa, K., Fujita, S.: Electrical conductive corundum-structured α-Ga2O3 thin films on sapphire with tin-doping grown by spray-assisted mist chemical vapor deposition. Jpn. J. Appl. Phys. (2012). https://doi.org/10.1143/jjap.51.070203
Atilgan, A., Yildiz, A., Harmanci, U.: β-Ga O nanoflakes/p-si heterojunction self-powered photodiodes. Mater. Today Commun. 24, 101105 (2020). https://doi.org/10.1016/j.mtcomm.2020.101105
Bai, Z., Zhang, Y.: Self-powered UV–visible photodetectors based on ZnO/Cu2O nanowire/electrolyte heterojunctions. J. Alloys Compd. 675, 325–330 (2016). https://doi.org/10.1016/j.jallcom.2016.03.051
Chan, D.Y.C., Mitchell, D.J., Ninham, B.W.: Short-range interactions mediated by a solvent with surface adhesion. Mol. Phys. 35(6), 1669–1679 (1978). https://doi.org/10.1080/00268977800101251
Chen, X., Liu, K., Zhang, Z.: Self-powered solar-blind photodetector with fast response based on Au/beta-Ga2O3 nanowires array film Schottky junction. ACS Appl. Mater. Inter. 8(6), 4185–4191 (2016). https://doi.org/10.1021/acsami.5b11956
Chen, K., Wang, S., He, C.: Photoelectrochemical self-powered solar-blind photodetectors based on Ga2O3 nanorod array/electrolyte solid/liquid heterojunctions with a large separation interface of photogenerated carriers. ACS Appl. Nano Mater. 2(10), 6169–6177 (2019). https://doi.org/10.1021/acsanm.9b00992
Chen, Y., Wang, Y., Wang, Z.: Unipolar barrier photodetectors based on van der Waals heterostructures. Nat. Electron. 4(5), 357–363 (2021). https://doi.org/10.1038/s41928-021-00586-w
Chi, P.-F., Lin, F.-W., Lee, M.-L.: High-responsivity solar-blind photodetectors formed by Ga2O3/p-GaN bipolar heterojunctions. ACS Photon. 9(3), 1002–1007 (2022). https://doi.org/10.1021/acsphotonics.1c01892
Cicek, E., McClintock, R., Cho, C.Y.: Alxga1-xn-based back-illuminated solar-blind photodetectors with external quantum efficiency of 89%. Appl. Phys. Lett. 103(19), 191108 (2013). https://doi.org/10.1063/1.4829065
Fujihara, S., Shibata, Y., Hosono, E.: Chemical deposition of rodlike GaOOH and β-Ga2O3 films using simple aqueous solutions. J. Electrochem. Soc. (2005). https://doi.org/10.1149/1.2060627
Fujita, S., Oda, M., Kaneko, K.: Evolution of corundum-structured III-Oxide semiconductors: Growth, properties, and devices. Jpn. J. Appl. Phys. (2016). https://doi.org/10.7567/jjap.55.1202a3
Gorokhov, E.V., Magunov, A.N., Feshchenko, V.S.: Solar-blind UV flame detector based on natural diamond. Instrum. Exp. Technol. 51(2), 280–283 (2011). https://doi.org/10.1134/s002044120802022x
Guo, D.Y., Wu, Z.P., An, Y.H.: Oxygen vacancy tuned Ohmic-schottky conversion for enhanced performance in β-Ga2O3 solar-blind ultraviolet photodetectors. Appl. Phys. Lett. (2014). https://doi.org/10.1063/1.4890524
Guo, J., Gu, Y., Liu, Y.: Polarization assisted interdigital AlGaN/GaN heterostructure ultraviolet photodetectors. IEEE Trans. Electron Devices 70(5), 2352–2357 (2023). https://doi.org/10.1109/ted.2023.3258920
He, C., Guo, D., Chen, K.: α-Ga2O3 nanorod array-Cu2O microsphere p–n junctions for self-powered spectrum-distinguishable photodetectors. ACS Appl. Nano Mater. 2(7), 4095–4103 (2019). https://doi.org/10.1021/acsanm.9b00527
He, T., Ma, H., Wang, Z.: On-chip optoelectronic logic gates operating in the telecom band. Nat. Photonics 18(1), 60–67 (2023). https://doi.org/10.1038/s41566-023-01309-7
Hou, Y.N., Mei, Z.X., Liang, H.L.: Dual-band MgZnO ultraviolet photodetector integrated with si. Appl. Phys. Lett. (2013). https://doi.org/10.1063/1.4802486
Hu, W.D., Chen, X.S., Ye, Z.H., Lu, W.: A hybrid surface passivation on HgCdTe long wave infrared detector with in-situ CdTe deposition and high-density hydrogen plasma modification. Appl. Phys. Lett. 99(9), 091101 (2011). https://doi.org/10.1063/1.3633103
Hu, W., Ye, Z., Liao, L.: 128×128 long-wavelength/mid-wavelength two-color HgCdTe infrared focal plane array detector with ultralow spectral cross talk. Opt. Lett. 39(17), 5184–5187 (2014). https://doi.org/10.1364/ol.39.005184
Huang, L., Hu, Z., Zhang, H.: A simple, repeatable and highly stable self-powered solar-blind photoelectrochemical-type photodetector using amorphous Ga2O3 films grown on 3D carbon fiber paper. J. Mater. Chem. C 9(32), 10354–10360 (2021a). https://doi.org/10.1039/d1tc02471j
Huang, L., Hu, Z., He, X.: Self-powered solar-blind ultraviolet photodetector based on α-Ga2O3 nanorod arrays fabricated by the water bath method. Opt. Mater. Express (2021b). https://doi.org/10.1364/ome.431377
Khranovskyy, V., Ekblad, T., Yakimova, R.Hultman, L.: Surface morphology effects on the light-controlled wettability of ZnO nanostructures. Appl. Surf. Sci. 258(20), 8146–8152 (2012). https://doi.org/10.1016/j.apsusc.2012.05.011
Kokkoli, E., Zukoski, C.F.: Effect of solvents on interactions between hydrophobic self-assembled monolayers. J. Colloid Interface Sci. 209, 60–65 (1999)
Kong, W.Y., Wu, G.A., Wang, K.Y.: Graphene-beta-Ga2O3 heterojunction for highly sensitive deep UV photodetector application. Adv. Mater. 28(48), 10725–10731 (2016). https://doi.org/10.1002/adma.201604049
Lan, Y.-P.: The influence of the preheating temperature of the (-201) β-Ga2O3 substrates on c-plane GaN epitaxial growth. Coatings (2021). https://doi.org/10.3390/coatings11070824
Lewis, B.: The growth of crystals of low supersaturation. J. Cryst. Growth 21, 29–39 (1974)
Li, Q., Wei, L., Xie, Y.: ZnO nanoneedle/H2O solid–liquid heterojunction-based self-powered ultraviolet detector. Nanoscale Res. Lett. 8, 415 (2013)
Li, H., Jiao, S., Li, H.: Tunable growth of PbS quantum Dot-ZnO heterostructure and mechanism analysis. Cryst. Eng. Commun. 17, 4722–4728 (2015)
Li, S., Guo, D., Li, P.: Ultrasensitive, super high signal-to-noise ratio, self-powered solar-blind photodetector based on n-Ga2O3/p-CuSCN core-shell microwire heterojunction. ACS Appl. Mater. Interfaces 11(38), 35105–35114 (2019). https://doi.org/10.1021/acsami.9b11012
Liang, H., Meng, F., Lamb, B.K.: Solution growth of screw dislocation driven α-GaOOH nanorod arrays and their conversion to porous ZnGa2O4 nanotubes. Chem. Mater. 29(17), 7278–7287 (2017). https://doi.org/10.1021/acs.chemmater.7b01930
Liao, M., Wang, X., Teraji, T.: Light intensity dependence of photocurrent gain in single-crystal diamond detectors. Phys. Rev. B (2010). https://doi.org/10.1103/PhysRevB.81.033304
Lin, H., Wei, L., Wu, C.: High-performance self-powered photodetectors based on ZnO/ZnS core-shell nanorod arrays. Nanoscale Res. Lett. 11(1), 420 (2016). https://doi.org/10.1186/s11671-016-1639-7
Mahmoud, W.E.: Solar blind avalanche photodetector based on the cation exchange growth of β-Ga2O3/SnO2 bilayer heterostructure thin film. Sol. Energ. Mater. Sol. C. 152, 65–72 (2016). https://doi.org/10.1016/j.solmat.2016.03.015
Morkoç, H., Strite, S., Gao, G.B.: Large-band-gap SiC, III–V nitride, and II–VI ZnSe-based semiconductor device technologies. J. Appl. Phys. 76(3), 1363–1398 (1994). https://doi.org/10.1063/1.358463
Ni, D., Wang, Y., Li, A.: Ald oxygen vacancy-rich amorphous Ga2O3 on three-dimensional urchin-like ZnO arrays for high-performance self-powered solar-blind photodetectors. Nanoscale 14(8), 3159–3165 (2022). https://doi.org/10.1039/d1nr08262k
Onuma, T., Saito, S., Sasaki, K.: Valence band ordering in β-Ga2O3 studied by polarized transmittance and reflectance spectroscopy. Jpn. J. Appl. Phys. (2015). https://doi.org/10.7567/jjap.54.112601
Orita, M., Ohta, H., Hirano, M.: Deep-ultraviolet transparent conductive β-Ga2O3 thin films. Appl. Phys. Lett. 77(25), 4166–4168 (2000). https://doi.org/10.1063/1.1330559
Pearton, S.J., Yang, J., Cary, P.H.: A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 5(1), 011301 (2018). https://doi.org/10.1063/1.5006941
Qu, L., Ji, J., Liu, X.: Oxygen-vacancy-dependent high-performance Alpha-Ga2O3 nanorods photoelectrochemical deep UV photodetectors. Nanotechnology 34(22), 225203 (2023). https://doi.org/10.1088/1361-6528/acbfbd
Reddy, L.S., Ko, Y.H., Yu, J.S.: Hydrothermal synthesis and photocatalytic property of beta-Ga2O3 nanorods. Nanoscale Res. Lett. 10(1), 364 (2015). https://doi.org/10.1186/s11671-015-1070-5
Tsao, J.Y., Chowdhury, S., Hollis, M.A.: Ultrawide-bandgap semiconductors: research opportunities and challenges. Adv. Electron. Mater. 4(1), 1600501 (2017). https://doi.org/10.1002/aelm.201600501
Wang, Z., Mao, X., Chen, P.: Understanding the roles of oxygen vacancies in hematite-based photoelectrochemical processes. Angew. Chem. Int. Ed. Engl. 58(4), 1030–1034 (2019a). https://doi.org/10.1002/anie.201810583
Wang, S., Chen, K., Zhao, H.: Beta-Ga2O3 nanorod arrays with high light-to-electron conversion for solar-blind deep ultraviolet photodetection. RSC Adv. 9(11), 6064–6069 (2019b). https://doi.org/10.1039/c8ra10371b
Wang, Y., Wu, C., Guo, D.: All-Oxide NiO/Ga2O3 p–n junction for self-powered uv photodetector. ACS Appl. Electron. Mater. 2(7), 2032–2038 (2020a). https://doi.org/10.1021/acsaelm.0c00301
Wang, Y., Shi, X., Oshikiri, T.: Plasmon-induced electron injection into the large negative potential conduction band of Ga2O3 for coupling with water oxidation. Nanoscale 12(44), 22674–22679 (2020b). https://doi.org/10.1039/d0nr06319c
Wang, G., Bai, Z., Wu, H.: A wire-shaped and high-sensitivity photoelectrochemical ultraviolet photodetector based on TiO2 nanotube arrays. Appl. Phys. Lett. (2022). https://doi.org/10.1063/5.0102834
Wang, G., Zhang, X., Li, J.: A wire-shaped photoanode of the solar-blind photoelectrochemical-type photodetector based on an α-Ga2O3 nanorods/electrolyte solid/liquid heterojunction. Appl. Phys. Lett. (2023). https://doi.org/10.1063/5.0134093
Wu, Z., Jiao, L., Wang, X.: A self-powered deep-ultraviolet photodetector based on an epitaxial Ga2O3/Ga: ZnO heterojunction. J. Mater. Chem. C 5(34), 8688–8693 (2017). https://doi.org/10.1039/c7tc01741c
Wu, C., Qiu, L., Li, S.: High sensitive and stable self-powered solar-blind photodetector based on solution-processed all inorganic CuMO2/Ga2O3 pn heterojunction. Mat. Today Phys. 17, 100335 (2021). https://doi.org/10.1016/j.mtphys.2020.100335
Yan, Z., Li, S., Liu, Z.: High sensitivity and fast response self-powered solar-blind ultraviolet photodetector with a β-Ga2O3/spiro-MeOTAD p–n heterojunction. J. Mater. Chem. C 8(13), 4502–4509 (2020). https://doi.org/10.1039/c9tc06767a
You, D., Xu, C., Zhao, J.: Vertically aligned ZnO/Ga2O3 core/shell nanowire arrays as self-driven superior sensitivity solar-blind photodetectors. J. Mater. Chem. C 7(10), 3056–3063 (2019). https://doi.org/10.1039/c9tc00134d
Zhang, D., Zheng, W., Lin, R.: Ultrahigh EQE (15%) solar-blind uv photovoltaic detector with organic–inorganic heterojunction via dual built-in fields enhanced photogenerated carrier separation efficiency mechanism. Adv. Funct. Mater. (2019a). https://doi.org/10.1002/adfm.201900935
Zhang, J., Jiao, S., Wang, D.: Solar-blind ultraviolet photodetection of an α-Ga2O3 nanorod array based on photoelectrochemical self-powered detectors with a simple, newly-designed structure. J. Mater. Chem. C 7(23), 6867–6871 (2019b). https://doi.org/10.1039/c9tc01417a
Zhang, J., Jiao, S., Wang, D.: Nano tree-like branched structure with α-Ga2O3 covered by γ-Al2O3 for highly efficient detection of solar–blind ultraviolet light using self-powered photoelectrochemical method. Appl. Surf. Sci. (2021). https://doi.org/10.1016/j.apsusc.2020.148380
Zhang, B., Wu, H., Feng, C.: Self-powered solar-blind photodetectors based on α-Ga2O3 nanorod arrays. ACS Appl. Nano Mater. 5(8), 11956–11963 (2022a). https://doi.org/10.1021/acsanm.2c03015
Zhang, C., Liu, K., Ai, Q.: Performance enhancement of Ga2O3 solar-blind UV photodetector by the combination of oxygen annealing and plasma treatment. J. Phys. Chem. C 126(51), 21839–21846 (2022b). https://doi.org/10.1021/acs.jpcc.2c07141
Zhang, J., Jiao, S., Wan, Y.: A well-grown β-Ga O microrod array formed from GaOOH on a Si (100) substrate and growth mechanism study. Cryst. Eng. Comm. 20(30), 4329–4335 (2018). https://doi.org/10.1039/c8ce00658j
Zhuo, R., Wu, D., Wang, Y.: A self-powered solar-blind photodetector based on a MoS2/β-Ga2O3 heterojunction. J. Mater. Chem. C 6(41), 10982–10986 (2018). https://doi.org/10.1039/c8tc04258f
Acknowledgements
This work was funded by the National Key R&D Program of China (Grant No. 2022YFB3605403), the National Natural Science Foundation of China (Grant Nos. 62374094, 62104110, 61974062, 62004104), the Natural Science Foundation of Jiangsu Province (Grant Nos. BK20200094, BK20210577), the Project funded by China Postdoctoral Science Foundation (2023T160332), the open research fund of the National and Local Joint Engineering Laboratory of RF Integration and Micro-Assembly Technology.
Author information
Authors and Affiliations
Contributions
Junjun Xue: Conceptualization, Data curation, Supervision, Writing-original draft, Writing-review & editing; Kaicheng Xu: Conceptualization, Methodology, Data curation, Writing-original draft; Jiaming Tong: Investigation, Validation, Visualization, Formal analysis; Zhouyu Chen: Investigation, Data curation, Validation; Haoyu Fang: Investigation, Validation, Formal analysis; Saisai Wang: Data curation, Validation, Visualization, Writing-review & editing; Jin Wang: Conceptualization, Methodology, Supervision, Writing-review & editing; Ting Zhi: Investigation, Methodology, Supervision, Writing-review & editing;
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xue, J., Xu, K., Tong, J. et al. Solar-blind UV photodetectors based on α-Ga2O3 prepared by a two-step hydrothermal method. Opt Quant Electron 56, 1247 (2024). https://doi.org/10.1007/s11082-024-07174-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-024-07174-0