Abstract
A comparison of oxide films formed on the stainless steel surface during laser and furnace heating is presented. Obtained samples were examined by optical and scanning electron microscopy. In order to characterize the optical properties, reflection spectra within the wavelength range 190–900 nm were measured with a spectrophotometer equipped with the integrating sphere for incidence angles from 0° to 60°. The topology of obtained oxide films was characterized by scanning probe microscopy. Due to light interference in produced films, the coloration of treated area is observed. It was found that there is no change in the characteristic appearance of reflectance spectra at different light incidence angles, but a blue-shift occurs especially for the case of laser-induced films, which results in a visible change of surface color. This effect is associated with an interference character of originating color and features of surface relief under an oxide film.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The oxidation of some metals is widely used in industry for decoration (Duprez and Cavani 2014) as well as for increasing the corrosive resistance (Łekecka et al. 2016). Various techniques of metal surface oxidation, such as thermal (Tepluhin and Gropyanov 2011), thermochemical (Yun et al. 2012) and electrochemical (Schneider et al. 2011; Simka et al. 2011) methods are known. The color of the surface is dictated by the thickness of an oxide layer, in which the light interference occurs (Arzuov et al. 1979). An application that can be separated is localized laser oxidation, which allows, among other things, the formation of high-definition color images and leads to the formation of microstructures on the surface (Veiko et al. 2016). Obtaining an exact color depends on the temperature and an effective interaction duration (Veiko et al. 2014), which are possible to regulate by changing the intensity I and the number of pulses per spot in a double-axis system N x and N y . According to thermodynamic calculations, the phase-chemical composition of films formed during the interaction of laser light with a steel surface in air (with heating up to 1500 K) is multilayered coatings of a complex composition: lower layer consists of FeCr2O4, upper layer—Fe2O3, and NiO (Veiko et al. 2013). This composition correlates well with experimental results (Jervis et al. 1990; Kozakov and Yares’ko 2011; Li et al. 2009). The color of the surface appears because of interference effects in the upper oxide layer of a multicomponent film and the intrinsic color of the lower oxide film. However, the difference of this method lies not only in it being localized because of the laser resolution but also in the possibility of obtaining a variety of colors for metals by optimizing the regimes (Veiko et al. 2016). For instance, the green color is possible to derive for stainless steel, which is not possible using other technologies. Despite the fact that in both cases the color is connected to the interference effects, the specific shape of reflectance spectra and the properties of the samples are going to be different. As shown in our previous work (Veiko et al. 2016), the color of the oxidized stainless steel plate surface after laser action can vary significantly when changing its viewing angle, whereas the films obtained by heating in the furnace show the change almost invisible to the naked eye. In this paper, the analysis of the effect of the visual color change of the samples oxidized by laser action of nanosecond pulse duration with an example of stainless steel in comparison with furnace oxidizing is conducted for the first time. This effect offers a great practical interest because creating color-changing identifying markings on metal products can be used for its protection against falsification.
2 Experimental
The targets consisting of stainless steel AISI 304 (50 × 50 × 0.5 mm) with the roughness of about 0.6 μm were cleaned with isopropanol.
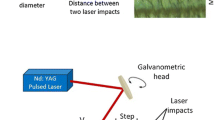
The laser oxidation was performed with a ytterbium fiber laser system (λ = 1.064 μm) generating pulses with 100 ns duration at 60 kHz (Fig. 1) and average power was in the range of 2–5 W. The beam was focused on the target in a nearly Gaussian spot with a diameter of approximately 50 µm leading to laser intensity from 0.85 to 5 × 107 W/cm2. The samples were irradiated in the air by line by line scanning of the surface with parallel traces with various overlapping at controlled scan velocity thus, to provide a different number of pulses per spot (N x , N y ).
Several stainless steel plates were oxidized in the air during the heating by the muffle furnace PM-10. To obtain various colors samples were placed to the preliminarily heated to 500 °C furnace for different periods of time. Consequently, oxide films of various thicknesses were produced, in which case it is possible to say that the oxidation process takes place under the isothermal conditions.
Obtained samples were examined by optical (Zeiss Axio Imager A1 M) and scanning electron microscopy (SEM) (JEOL JSM 7001F). To characterize the optical properties, reflection spectra within the wavelength range 190–900 nm were recorded with a spectrophotometer Lambda Perkin 1050 with the integrating sphere. Spectra were measured for incidence angles from 0° to 60°. The surface topography of obtained oxide films was characterized by scanning probe microscopy (SPM) (Veeco Dimension 3100), and Raman spectra were obtained with Raman spectrometers SENTERRA (Bruker) and T64000 (Horiba).
3 Results and discussion
3.1 Surface morphology
Figure 2 shows macrophotographs taken at the viewing angles 0° and 60° and corresponding microphotographs of the surface of samples oxidized by two methods described above. For furnace oxidation (Fig. 2a) the visually observable surface color is sufficiently uniform and does not change at viewing angle variation. SEM (Fig. 3a) and SPM (Fig. 3c) image of the sample demonstrates the absence of large level differences (Ra = 0.02 µm).
During laser line-by-line scanning a regular relief is formed on the surface of steel plate. This relief significantly enhances a visually observable color change at the variation of viewing angle (Fig. 2b). It can be observed in microscopy image that surface color primarily changes in the direction transversal to the line generated as a result of laser beam passing. The corresponding relief and SEM image is demonstrated in Fig. 3b (Ra = 0.27 µm) and Fig. 3d. Since laser intensity was higher than material melting threshold, localized melting and reverse solidification of the melt occur. As a result, inclined groove covered with an oxide film of variable thickness appeared. Its geometry is a function of recoil vapor pressure and cooling rate. Formed relief together with a variable thickness of the film provides a modification of interference conditions for incident light and a corresponding change of a surface color throughout the length of the groove. This is because light beams striking upon different surface areas in the transversal direction to the groove after passing through the oxide film will have not arbitrary, but periodic modifications of interference conditions along the groove.
3.2 Chemical composition of surface
For composition analysis of coatings formed on the surface of stainless steel after furnace and laser oxidation Raman spectra were taken (Fig. 4). The solid-state laser operated at 532 nm wavelength and provided an output of about 1 mW was used for Raman-scattering spectroscopy. Repeated reproducibility of obtained results confirms that laser exposure at Raman measurements has no effect on the sample.
Raman spectra of the sample heated in the furnace (Fig. 4a) demonstrate strong peaks of hematite (Fe2O3): 225, 245, 293, 410, 500, 610 and 1320 cm−1 (Thibeau et al. 1978).
For identification purposes, bands in Raman spectra of laser-oxidized sample (Fig. 4b) were fitted using 6 Lorentzian peaks. Peaks at 538, 632, 674 cm−1 are in good agreement with phonon modes for FeCr2O4. The presence of FeCr2O4 is additionally supported by chemical thermodynamics modeling presented in our previous paper (Veiko et al. 2013). It is possible that a minor displacement of peaks is related to the presence of thermomechanical stresses occurred as a result of fast material heating and cooling at laser exposure. According to (Chourpa et al. 2005) peaks at 290, 330, 390 and 712 cm−1 correspond to maghemite or maghemite-rich region. The content of Fe2O3 in the surface layer of stainless steel after laser processing was demonstrated in (Veiko et al. 2013) as well. At the same time, a broad maghemite band at 480–500 cm−1 region can merge with 538 cm−1 band of FeCr2O4, which results in a broadening of the last one during a fitting.
3.3 Optical properties
Samples oxidized in the muffle furnace have a single apparent extremum (minimum) in a visible region (Fig. 5a). With an increase of light incidence angle, a characteristic appearance of spectra does not change. Reflectivity successively grows over the range of 0.1 a.u.
The found maximum displacement of spectrum minimum position Δλ = 20 ± 5 nm for stainless steel after furnace oxidation was compared to the value calculated from the known theoretical relation. It is well-known that in single-layered thin films reflection interference takes place, where beams are reflected from the oxide film surface and substrate-film boundary. The interference maximum of reflected beam can be found if the following condition is met: \({\text{m}}\uplambda = 2{\text{d}}\sqrt {{\text{n}}^{2} - { \sin }^{2}\upalpha}\), where m—interference order, d—oxide film thickness, n—film refraction index, and α—light incidence angle. The following values were used for calculation: m = 1, thickness of the film of puce color on the steel surface d ≈ 75 nm (Somervuori et al. 2004), n = 2.55, \(\upalpha_{1} = 10^\circ\), and \(\upalpha_{2} = 60^\circ\). Finally, it can be found for the steel \(\Delta\uplambda = 22\) nm, which is in good agreement with experimental value (Δλ = 20 ± 5 nm).
Stainless steel reflectance spectra after laser oxidation (Fig. 5b) have two extremums and save the characteristic appearance at different light incidence angles. Reflectivity changes in the range of 0.05 a.u. With an increase of light incidence, angle peaks shift toward the short-wave region. The displacement Δλ = 40 ± 6 nm, which results in a visible hue shift of the surface.
Larger displacement of extremum position in reflectance spectra of laser oxidized samples toward shorter wavelength in comparison with furnace oxidized can be found in Fig. 4. It seems it is associated with the fact that both laser-induced relief and variable oxide thickness across the width of groove influence on the change of color. Nonuniform heating due to Gaussian distribution of laser intensity is a main reason for the abovementioned effect.
4 Conclusion
A comparison of oxide films formed on the stainless steel surface during laser and furnace heating was made in the paper. Laser-induced films change its color significantly at different viewing angles, namely the displacement of spectrum minimum position Δλ is about 40 nm when viewing angle ranges from 10° to 60°. At the same time for oxide films, produced by heat treatment in a furnace Δλ ≈ 20 nm, i.e. there is no visible change of color. This effect is associated with an interference character of originating color and features of surface relief under an oxide film. The relief features are defined by grooves and beads formation due to melt outflow under the action of recoil vapor pressure at laser action.
The reported angular dependence of surface color could be used for development of an additional security feature for protection against counterfeit metal products while using a technology of color laser marking.
References
Arzuov, M.I., Barchukov, A.I., Bunkin, F.V., Kirichenko, N.A., Konov, V.I., Luk’yanchuk, B.S.: Influence of interference effects in oxide films on the kinetics of laser heating of metals. Sov. J. Quantum Electron. 9, 281–284 (1979)
Chourpa, I., Douziech-Eyrolles, L., Ngaboni-Okassa, L., Fouquenet, J.-F., Cohen-Jonathan, S., Soucé, M., Marchais, H., Dubois, P.: Molecular composition of iron oxide nanoparticles, precursors for magnetic drug targeting, as characterized by confocal Raman microspectroscopy. Analyst 130, 1395–1403 (2005)
Duprez, D., Cavani, F.: Handbook of Advanced Methods and Processes in Oxidation Catalysis: From Laboratory to Industry. World Scientific Publishing Co. Pte. Ltd., Singapore (2014)
Jervis, T.R., Williamson, D.L., Hirvonen, J.-P., Zocco, T.G.: Characterization of the surface oxide formed by excimer laser surface processing of AISI 304 stainless steel. Mater. Lett. 9, 379–383 (1990)
Kozakov, A.T., Yares’ko, S.I.: Using auger electron spectroscopy for studying the composition of the surface of multicomponent alloys under the effect of pulsed laser irradiation. Inorg. Mater. Appl. Res. 2, 254–260 (2011)
Łekecka, K.M., Antończak, A.J., Szubzda, B., Wójcik, M.R., Stekepak, B.D., Szymczyk, P., Trzciński, M., Ozimek, M., Abramski, K.M.: Effects of laser-induced oxidation on the corrosion resistance of AISI 304 stainless steel. J. Laser Appl. 28, 032009 (2016)
Li, Z.L., Zheng, H.Y., Teh, K.M., Liu, Y.C., Lim, G.C., Seng, H.L., Yakovlev, N.L.: Analysis of oxide formation induced by UV laser coloration of stainless steel. Appl. Surf. Sci. 256, 1582–1588 (2009)
Schneider, M., Langklotz, U., Michaelis, A.: Thickness determination of thin anodic titanium oxide films—a comparison between coulometry and reflectometry. Surf. Interface Anal. 43, 1471–1479 (2011)
Simka, W., Sadkowski, A., Warczak, M., Iwaniak, A., Dercz, G., Michalska, J., Maciej, A.: Characterization of passive films formed on titanium during anodic oxidation. Electrochim. Acta 56, 8962–8968 (2011)
Somervuori, M., Johansson, L.-S., Heinonen, M.H., Van Hoecke, D.H.D., Akdut, N., Hänninen, H.E.: Characterisation and corrosion of spot welds of austenitic stainless steels. Mater. Corros. 55, 421–436 (2004)
Tepluhin, G.N., Gropyanov, A.V.: Metallovedenie i termicheskaya obrabotka (Metallurgical Science and Heat Treatment). SPbGTU RP, St. Petersburg (2011)
Thibeau, R.J., Brown, C.W., Heidersbach, R.H.: Raman spectra of possible corrosion products of iron. Appl. Spectrosc. 32, 532–535 (1978)
Veiko, V., Odintsova, G., Ageev, E., Karlagina, Y., Loginov, A., Skuratova, A., Gorbunova, E.: Controlled oxide films formation by nanosecond laser pulses for color marking. Opt. Express 22, 24342–24347 (2014)
Veiko, V., Odintsova, G., Gorbunova, E., Ageev, E., Shimko, A., Karlagina, Y., Andreeva, Y.: Development of complete color palette based on spectrophotometric measurements of steel oxidation results for enhancement of color laser marking technology. Mater. Des. 89, 684–688 (2016)
Veiko, V.P., Slobodov, A.A., Odintsova, G.V.: Availability of methods of chemical thermodynamics and kinetics for the analysis of chemical transformations on metal surfaces under pulsed laser action. Laser Phys. 23, 66001 (2013)
Yun, H.-G., Kim, M., You, I.-K.: Tuned optical reflection characteristics of chemically-treated Ti substrates. ETRI J. 34, 954–957 (2012)
Acknowledgements
SEM and SPM characterization was made on the equipment of the Federal Joint Research Center “Material science and characterization in advanced technology” (Ioffe Institute, Saint-Petersburg, Russia). Raman and reflectance spectra were performed at the Center for Optical and Laser Research of Research Park of St. Petersburg State University. The reported study was partially supported by Grant for International scientific laboratory with state support of leading universities of the Russian Federation # 074-U01 and RSF Agreement No 14-12-00351.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Fundamentals of Laser Assisted Micro- and Nanotechnologies.
Guest edited by Eugene Avrutin, Vadim Veiko, Tigran Vartanyan and Andrey Belikov.
Rights and permissions
About this article
Cite this article
Ageev, E.I., Andreeva, Y.M., Brunkov, P.N. et al. Influence of light incident angle on reflectance spectra of metals processed by color laser marking technology. Opt Quant Electron 49, 50 (2017). https://doi.org/10.1007/s11082-016-0876-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-016-0876-4