Abstract
Epilepsy is a group of chronic neurological disorders characterized with recurrent and hypersynchronous discharge. In the epileptic brain, epileptogenic zone (EZ) with abnormal firing patterns is considered as the culprit of hyperexcitable neuronal behaviors, increasingly manifested as alterations in dynamics. Meanwhile, the brain networks of epileptic patients show greater seizure susceptibility than those from healthy controls, referred as epileptogenic networks. There is a growing recognition of an intimate, but also complex, relationship between the self-organization of epileptogenic networks and abnormal dynamics of the EZ. In this review, we discussed the short- and long-term effects of recurrent epileptiform discharge on neural circuits, involving the regulation of connection weights and network topology. In addition, progressive abnormalities associated with secondary epileptogenesis imply that the excitability of regions connected with EZ may be enhanced, leading to frequent transitions from normal to epileptic states. From the perspective of network dynamics, EZ plays a dynamical pacemaker role in the evolution of epileptogenic networks as well as the activities generated by these networks. It may provide novel insights into the mechanism of epileptogenesis and inspire new therapeutic strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Epilepsy is a common chronic neurological disease characterized with intermittent functional disorder and progressive cognitive impairments. Since the early twentieth century, the idea of epileptic brain being susceptive to perturbations has guided pharmacological regulation and surgical treatment [1]. Substantial evidences suggested that epileptogenic zone (EZ) with abnormal firing patterns was responsible for seizure-prone peculiarity [2, 3]. Hyperexcitable neuronal behaviors, attributing to the imbalance between excitation and inhibition [4], were increasingly manifested as dynamical transitions due to qualitative changes in physiological variables [5, 6]. However, the rather high rate of surgical failure in removing a restricted region highlighted the existence of network disorder [7, 8]. Hence, distinctive seizure-related connectivity patterns were revealed [9], and the difference between patients and healthy controls were also fully examined [10, 11]. Furthermore, computational models incorporated with data-inferred networks were used to understand the mechanism behind spontaneous transition and large-scale synchronization in the epileptic brain [12,13,14]. Particularly, the normal and epileptic states were represented by stable equilibrium point and limit cycle attractor, respectively. Increased seizure susceptibility of epileptogenic networks was associated with bifurcation behaviors in a bistable dynamical system [15, 16].
Epileptogenic networks are considered as indispensable support for the initiation and spread of epileptic activities, although the formation of pathological connectivity patterns remains unclear. Over the past years, great attention has been paid to self-organization of epileptogenic networks especially with the involvement of synaptic plasticity [17,18,19]. Through short- or long-term effects on neural circuits, partial regions even spatially distant from seizure focus can be recruited, contributing to widespread epileptic activities across the brain [20]. The fact that the ongoing epileptic activities led to temporary or even permanent functional changes in synapses was fully confirmed in animal experiments [21, 22]. Recurrent epileptiform discharge might drive the transition of synaptic strength from baseline to high level [23], resulting in attractor state of the network with sustained firing. To some extent, EZ promotes the adjustment of synaptic efficacy, acting like a pacemaker in the evolution of epileptogenic network. Furthermore, progressive structural abnormalities (e.g., reduction in subcortical volume and cortical thickness) could be found beyond clinically defined EZ [24, 25], and in most cases the severity was associated with the duration of epilepsy [26]. Part of these seizure-induced abnormal regions even showed dynamics similar to that in the EZ, and involved secondary epileptogenesis, referred as the extension of epileptogenicity [27].
Synaptic dynamics, the response properties of networks to perturbations and the patterns of activity generated by those networks are profoundly altered by the involvement of hyperexcitable neurons. The following sections will depict the possible dynamical processes orchestrated by EZ.
2 Spatiotemporal dynamics in the epileptic processes
2.1 Nontrivial dynamical characteristics of epileptogenic zone
The etiology of epilepsy varies in a wide range, such as tumors, brain injuries, parasites [28]. These pathophysiological alterations may induce spontaneous seizures and progressive cognitive deterioration. Moreover, nearly 40% of cases have an unknown etiology, and nonlesional areas involved in the onset of epileptic activities are found [29, 30]. Part of these imaging negative areas are even associated with pathological high-frequency oscillations (80–500 Hz) [31], increasingly recognized as a promising biomarker of epileptogenicity [32,33,34]. From functional mapping with cortical electrical stimulation, Leung et al. confirmed that ictal high-frequency oscillations were affiliated to cortical hyperexcitability [35]. There was also robust evidence for the existence of faster high-frequency oscillation (500–1000 Hz), which was primarily associated with seizure activities [36, 37]. A recent meta-analysis showed that residual high-frequency oscillations in the postoperative recordings had the potential to predict poor surgical outcomes [38]. Moreover, neurons in the EZ intermittently produces synchronized bursts embedded with traces of determinism [39]. Intrinsic hyperexcitability is increasingly considered as a manifestation of qualitative changes in dynamics. Therefore, EZ represents functional abnormality as well as dynamical disorder rather than simple structural lesion. It is significant to understand the mechanism of epileptogenesis by revealing the nontrivial dynamical characteristics of the EZ.
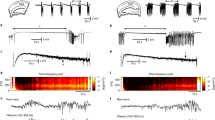
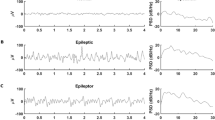
A general concept is to extract the characteristics of dynamical systems from the evolution of state variables. In the neurons, ionic current flows result in voltage fluctuations which can be captured by multiple extracranial electrodes [40], referred as Electroencephalograph (EEG) (Fig. 1a). For patients with epilepsy, it remains an essential clinical tool in the diagnostic workup [41, 42]. Repeated routine recordings can assist in treatment choices and the evaluation of therapeutic outcomes [43]. Despite the fact that time-consuming visual inspections still prevail in clinical practice, different signal processing methods have been proposed to automatically identify the EZ, such as Granger causality [44, 45], Epileptogenicity Index [46]. Particularly, dynamical analysis always attracts much attention, and provides mechanistic insights into epileptogenesis. Correlation dimension analysis, a quantitative description of complexity, was applied to intracranially recordings of 20 patients with unilateral temporal lobe epilepsy [47]. The lowest dimension was found in the EZ during the ictal state. In other brain areas recruited by epileptiform discharge, the dimension also decreased. Compared with physiological brain activities, epileptic events were accompanied with a loss of complexity. Furthermore, to investigate whether the complex behaviors in the epileptic processes originated from nonlinear deterministic or linear stochastic dynamics, a measure combining the methods of coarse-grained flow average and improved surrogates was applied to intracranial recordings in the interictal stage [48]. The recordings in the EZ showed unambiguous nonlinear determinism, while the behaviors in the normal region resembled linear stochastic dynamics. Andrzejak et al. also combined surrogates with a nonlinear prediction error to derive tests for randomness [49]. Compared with the normal regions, the signals from the EZ were less random (Fig. 1b). At the same time, the nonlinear dependence tests further suggested that recordings within the EZ showed robust dependence. In phase space, their nearly synchronized trajectories also implied homogeneous dynamics with deterministic hyperexcitability [50, 51], which provided a conceivable explanation for their first outbreak in the ictal activities.
Spatiotemporal dynamical characteristics inferred from recordings. Bistable dynamics was used to interpret the potential mechanism behind these significant dynamical differences. a EEG signals collected from patients with epilepsy. Particularly, EZ was characterized with abnormal epileptiform discharge. b A schematic diagram on significant spatiotemporal characteristics. Compared with non-EZ, recordings from the EZ showed lower complexity, higher determinism and more robust dependence. Meanwhile, signals in the ictal stage demonstrated deterministic and regular dynamical behaviors rather than randomness in the interictal stage. c Interictal and ictal states were represented by stable equilibrium point and limit cycle attractor, respectively. Two dynamical scenarios were assumed on the transition from norm to epileptic states. One was that ictal activities could be triggered for the existence of hyperexcitable oscillators sensitive to perturbations. The other was that perturbations were likely to cause the deformation of attractors. Seizures could emerge after a cascade of evolving electrophysiological events
2.2 Dynamical changes during the epileptic transition
Suffczynski et al. [52] reported that the distributions of durations of interictal and ictal epochs were fitted with a gamma distribution. A vast mass of recordings from animal models of absence epilepsy, human epilepsies and in vitro models were analyzed. By estimating the fitted shape parameter of the distribution, they found that the dynamics of ictal epochs differed from those in the interictal states (Fig. 1b). Specifically, the transition from normal to paroxysmal state could be accounted for by a random walk, while deterministic time-dependent mechanism probably existed in the ictal state. Panahi et al. [53, 54] proposed a chaos-based mathematical model and interpreted abrupt seizures as the transition from chaotic to periodic state. This behavioral model provided additional insights into the development of state-dependent control strategy. Furthermore, maximum entropy ratio, a symbolic method for the detection of recurrence domains of dynamical systems, was used to investigate dynamical characteristics of epileptic phenomena [55]. Yan et al. found that the maximum entropy ratio was significantly lower in the ictal state than that in the interictal state. Similarly, Yang et al. [50] applied time-variant recurrence plot to demonstrate the evolution of the trajectories in the phase space. A large number of isolated dots were found in the recurrence plots during the interictal state, which represented lack of regular trajectory. In the ictal state, the recurrence plots consisted of diagonal structure, indicating relatively deterministic and regular behaviors. In addition, selected recurrence quantification parameters were extracted from these plots and applied to automatically classify the EEG signals [56, 57].
The differences of dynamical characteristics between interictal and ictal states have been widely investigated. However, whether there are specific dynamical warning minutes or seconds before seizures remains unanswered, despite of enhanced neuronal activities or characteristic EEG signals in advance of seizure onset [46]. Due to extensive individual difference, many electrophysiological phenomena are not universal or necessary in all epilepsies, which makes the prediction of epilepsy controversial [58, 59]. Nevertheless, great efforts have been paid to predict the time of seizure occurrence from the interictal recordings [60]. Meanwhile, either open-loop or close-loop (mainly based on automatic seizure detection) stimulation paradigms have been developed to prevent the predicted ictal state [61]. However, because of the intrinsic randomness of interictal activities as well as complex pathology and chronic development of the disease, stimulation therapy is still not popular in clinical practice, even as auxiliary means in most cases. It is significant to reveal more detailed temporal evolution of dynamical characteristics, especially those related to seizure onset, which may enlighten new prediction techniques as well as novel stimulation strategies [62].
2.3 Bistable dynamics and timescale separation
Seizures are characterized with paroxysmal hypersynchronous discharge in relatively large neuronal networks. They may be triggered by external stimulus or internal fluctuation [63]. In a normal brain, such changes would not cause more than a transient and uninjurious modification of brain activities, while an epileptic brain seems to be more vulnerable [64]. Based on the transition from normal to epileptic states, Lopes da Silva et al. considered epilepsy as a dynamical disease, and presented a theoretical framework on the potential dynamical mechanism [65]. In the epileptic brain, there are at least two states: the interictal state with seemingly normal and random activities and the ictal state with relatively deterministic synchronous oscillations. The former could be abstracted as vibrations near a stable equilibrium point in a stochastic environment, while the latter might be considered as oscillations around the limit cycle (Fig. 1c). In the phase space, the basins of the two attractors were separated by what is called a “separatrix”.
From the dynamical perspective, the brain can be viewed as a network of interconnected oscillators [66]. The nontrivial dynamical behaviors of EZ means that at least the corresponding oscillators are significantly abnormal. Based on bistable theory, those changes in dynamics might be related to the susceptibility of transition between attractors. Therefore, Lopes da Silva et al. proposed two possible dynamical routes to epileptic seizures [65]. First, the distance between attractors within the EZ is much smaller in contrast to that of normal brain tissue (Fig. 1c). In this case, external stimulus or internal fluctuation, which could be tolerable for healthy brain, might lead to the paroxysmal state in the epileptic brain [67]. Kalitzin et al. [68] used intermittent pulse stimulation in the frequency range 10–20 Hz to investigate the spatial distribution of excitability in patients with temporal lobe epilepsy. Relative phase clustering index was introduced to quantify the spectral phase demodulation of evoked intracranial signals. The findings supported that the EZ were more sensitive and more responsive than normal areas. Moreover, voltage clamp recordings from temporal lobe epilepsy rats revealed pro-excitatory shifts in sodium channel activity of subiculum neurons, which facilitated action potential bursts and spontaneous epileptiform discharge [69]. Second, the attractors can deform either progressively or abruptly under the influence of noisy perturbations (Fig. 1c). In this case, the two attractors started to approach each other and the separatrix tended to zero. Consequently, those perturbations, which were commonly not capable of triggering a seizure, might result in paroxysmal oscillations later. Highly localized subclinical seizure-like activities and prolonged bursts might be observed both inside and outside the EZ before observable clinical sign emerged [67, 70, 71]. Meanwhile, two types of interictal discharge with opposite roles, slow GABAergic discharge and fast activities, were found, and they were thought to promote or control the occurrence of ictal activities, respectively [72,73,74]. Truccolo et al. [70] believed that progressive dynamical modification might be required for the abrupt transition from normal to epileptic states.
These two dynamical scenarios seem to hold true, and are supported by corresponding physiological observations. Notably, the transitions are neither mundane nor uniform, but involve complex spatiotemporal diversity, especially the time-scale separation behavior [75]. Despite different pathologies or conditions, two major categories of activities are consistently recorded in experimental animal models and human epilepsy: fast oscillations and spikes with or without waves [76, 77]. Interestingly, such behaviors could be well modelled by the fast and slow neural populations, which were coupled via signaling through a slow permittivity variable replicating extracellular effects [78, 79]. Permittivity coupling across brain regions were believed to perform a critical role in onset, recruitment and even termination in partial epilepsy [80]. Similarly, a common ultraslow variable interacting reciprocally with neural populations in the epileptogenic networks was introduced to interpret spontaneous seizures with complex patterns [81, 82]. In these slow-fast systems, the slow and fast timescales compete to determine the collective behaviors. Due to the complex etiology of epilepsy, the priority of the two effects are not necessarily the same. As a result, seizures may appear abruptly or begin with a cascade of evolving electrophysiological events.
3 Synaptic plasticity in the epileptogenic networks
3.1 Evolving epileptogenic networks
Epilepsy is increasingly considered as the result of network disorder. A number of well described alterations of anatomical or functional networks related with recurrent epileptic activities were reported [83]. Whelan et al. formed the ENIGMA-Epilepsy consortium to investigate structural abnormalities in the common epilepsies, pooling data from 24 research centers in 14 countries [84]. Compared to healthy controls, patients with epilepsy showed profound volume reduction in the ipsilateral subcortical structures and lower thickness in partial cortical regions. Typically, diffusion tensor imaging studies revealed that the absence of white matter connectivity associated with the EZ possibly resulted from recurrent epileptic activities [85]. Hutchings et al. applied a computational model to evaluate the seizure likelihood of individual anatomical network between patients and healthy controls [12]. As expected, patients tended to transit from normal to epileptic states more frequently in the model. van Mierlo et al. investigated the connectivity patterns during the first 20 s of ictal activities, and found that clinically defined EZ showed maximal out-degree [86]. Furthermore, EZ seemed to be the hub of epileptogenic networks not only during the ictal state, but also during interictal spike periods in some cases [87, 88].
In animal models, healthy rats begin to develop behavioral seizures 2–4 months after injecting kainic acid [89]. Progressive electrophysiological and anatomical abnormalities are found during the development of epileptogenesis. In human epilepsy, there is also a prolonged interval between an initial insult and the first emergence of spontaneous seizures, referred as silent period [25]. Moreover, seizures are often accompanied with developmental symptoms, it is reasonable to believe that epileptogenic networks are not innate but progressively shaped. There is a growing recognition of an intimate, but also complex, relationship between the evolution of epileptogenic networks and synaptic plasticity [90]. Wiemann et al. [91] found that epileptic neurons could induce augmenting synaptic depolarizations in non-epileptic neurons. Interactions between areas inferred from physiological recordings changed on various timescales [92]. Especially, connection strength between the EZ and parts of normal regions significantly increased with the occurrence of seizures [50]. Meanwhile, substantial model-based studies showed that synaptic plasticity was involved in spontaneous transition from desynchronous spikes to synchronous bursts [93]. Importantly, plasticity has been extended to account for structural self-organization of epileptogenic networks [18, 94]. Epileptogenic neurons are understood as the pacemaker of hypersynchronous behaviors in neuronal networks [95,96,97], and synaptic weights are regulated to facilitate the emergence of epileptic phenomena.
3.2 Bistable short-term plasticity
Experimental studies found that repetitive presynaptic firing could elicit either significant short-term decrease (depression) or increase (facilitation) in the amplitude of excitatory postsynaptic potential [98]. It was noticed that synapses might act like pass filters, and thus had preferred frequencies at which the efficacy of transmission was optimized [99]. Moreover, experimental evidence showed that the synaptic strength might be significantly enhanced after a high-frequency spike train [100]. Interestingly, the level of strength could maintain at least for a certain amount of time even for future low frequency spike trains [23]. González-Burgos et al. examined the effects of repetitive presynaptic stimulation on different types of neurons, including regular spiking pyramidal cells, fast spiking interneurons and adapting non-pyramidal cells [101]. The cell class-specific differences in response to constant-frequency stimulation were observed, and the modification of firing patterns might lead to the differential activation of distinct neuronal populations. Therefore, epileptogenic neurons characterized with abnormal firing patterns probably recruited neurons with high-frequency preference, which might result in imbalance between excitability and inhibition and emergence of pathological oscillations, such as fast onset activity [102]. Theoretical studies also proposed that short-term changes in synaptic efficacy could drive the network into attractor state with sustained firing [103].
The transition of synaptic strength from baseline to high level could be viewed as a bifurcation in a bistable system [104]. Alamir et al. [90] proposed a systematic framework to reproduce the above behaviors. The synaptic strength was governed by:
where \(\gamma_{{{\text{eq}}}} , \gamma_{{{\text{th}}}} \;(\gamma_{{{\text{eq}}}} < \gamma_{{{\text{th}}}} < 1)\) represented the low efficacy attractor and threshold, respectively. \(u_{{{\text{in}}}}\) could be considered as presynaptic input or external stimulus. \(a, b\) were positive coefficients. In the absence of input (\(u_{{{\text{in}}}} = 0\)), the dynamics admitted two stable attractors, one at \(\gamma = \gamma_{{{\text{eq}}}}\) and the other one at \(\gamma = 1\) (representing high efficacy). Excitatory input would increase the low efficacy attractor to \(\gamma_{{{\text{eq}}}}^{\prime }\), and reduce the threshold to \(\gamma_{{{\text{th}}}}^{\prime }\). Furthermore, transition between attractors was fired under repetitive high-frequency spike trains but not low frequency input (Fig. 2a). In a neuronal population network with heterogeneous excitability, normal neural ensembles could be recruited by hyperexcitable epileptogenic population due to short-term enhancement of synaptic strength [104]. Such enhancement might push the brain network to a pathological state characterized by increased susceptibility to endogenous or exogenous perturbations [105], leading to the initiation and propagation of epileptiform discharge. Noteworthily, the recruitment process may contain complex excitatory and inhibitory regulation beyond these phenomena at the macrolevel. Both within-frequency and cross-frequency directional interactions between the EZ and surrounding regions suggested that the secondary generalization of focal seizures might be constrained by multiple oscillatory push–pull antagonisms [106].
Through short- and long-term synaptic plasticity, EZ with abnormal firing patterns had a profound effect on the structure of evolving epileptogenic networks as well as the activities generated by these networks. a Bistable short-term plasticity governed by Eq. (1). Excitatory input could shift the equilibrium points of synaptic system, resulting in increased synaptic efficacy and decreased threshold. Furthermore, synaptic strength could be transferred to high level beyond threshold after a high-frequency spike train. b Repetitive interictal spike activities might promote local enhancement of synaptic strength related with the EZ, contributing to the transition to ictal state. Meanwhile, recurrent seizures might cause irreversible alterations in neural circuits (e.g., the increase or disappearance of connections), pushing the formation of epileptogenic networks with the EZ as a hub
3.3 Long-term effects on neural circuits
Short-term changes of synaptic efficacy play a critical role in the transition between interictal and ictal states (Fig. 2b). Furthermore, structural and functional imaging from both clinical and experimental studies demonstrated that permanent changes in the brain networks existed and contributed to recurrent seizures [84, 86].
Wasling et al. [107] applied paired-pulse afferent stimulation to the hippocampal CA1 area in young (1–2 week) rats. Notable long-term depression was induced within ten such low frequency stimuli. Lippman-Bell et al. [108] further found that the expression of long-term depression was associated with calcium response. Higher response in brain slices from hypoxia-induced neonatal epileptic rats showed that early-life seizures could alter synaptic calcium-permeable receptor function. Meanwhile, experimental evidence suggested that synaptic thresholds in the adult hippocampus were heterogeneous, which resulted in new timing rules for long-term potentiation [109]. The behavior of recurrent epileptic discharge causing large fluctuations of synaptic thresholds may disrupt circuit homeostasis [101, 110]. Under repeated actions, these alterations in neural circuits become irreversible (Fig. 2b), which may be involved in the formation of epileptogenic networks [111].
Recently, there has been a growing trend of epilepsy treatment and research strategies from the “molecular” level to the “circuit” level [112,113,114]. Technological advances, such as deep brain stimulation, optogenetics and chemogenetics, have also inspired novel strategies toward precise circuit therapy [115,116,117]. Operational targets are not limited to epileptogenic focus, but also include the regions that have intensive connections with epileptogenic focus, such as piriform cortex and hippocampal CA3 [118, 119]. Furthermore, Neal et al. found that the magnitude of network disconnection after surgery was associated with postoperative outcomes [120]. It was shown that relatively improved neural circuits could disintegrate epileptogenic networks to reduce or even suppress the generation of recurrent seizures.
4 The extension of epileptogenicity
4.1 Abnormalities beyond epileptogenic zone
Epilepsy is a common acquired chronic neurological disorder. Although progressive cognitive decline and behavioral impairments in the epilepsies remains largely unexplained, the relationships between structural abnormalities and the duration of epilepsy have been widely studied. Bernasconi et al. collected volumetric MRI of the hippocampus, amygdale and entorhinal cortex from 86 consecutive patients with temporal lobe epilepsy and 44 age- and sex-matched healthy controls [24]. Linear regression analysis showed that duration of epilepsy was significantly related to the atrophy of these structures ipsilateral to seizure focus. Whelan et al. further claimed that progressive reduction in subcortical volume and cortical thickness could be widely observed in the all epilepsies [84]. Furthermore, similar structural abnormalities, remote from clinically defined epileptogenic zone [121, 122], were found. Interestingly, in the group of patients whose hippocampus was spatially distant from seizure focus, increased \(T_{2}\) relaxation time indicated the occurrence of hippocampal sclerosis [123]. Experimental evidence from animal models showed that single or repetitive seizures might cause irreversible injury in brain structure (such as selective neuronal loss) and chronic changes in corresponding function [21, 124, 125].
In human epilepsy, these seizure-induced abnormalities were further found to be associated with secondary epileptogenesis [46]. Hypothalamic hamartoma was a developmental malformation often characterized by gelastic seizures [126]. Intracranial recordings suggested the hamartoma itself was intrinsically epileptogenic [127]. Unfortunately, the resection of hamartoma proved to be inefficient to achieve seizure free in up to 50% of cases despite partial improvements [128]. The poor operative outcomes might be explained by “hypothalamic plus” epileptogenicity. Scholly et al. reported two rare cases that the patients with gelastic seizures underwent two-step surgery: first a tailored resection of hamartoma, followed by temporal lobectomy [129]. Both patients became seizure free only after a complete two-step operation, which demonstrated that temporal lobe structures were likely to acquire epileptogenicity and even became secondary seizure focus. In addition, Goddard found that repeated electrical stimulation on hippocampus or subcortical nuclei could induce seizures artificially [130], referred as kindling effect. In the epileptic brain, partial normal regions might go through a similar process under continuous pacemaker-like action potential firing from primary EZ, and then become epileptogenic. Based on numerous electrophysiological findings, the concept of “the extension of epileptogenicity” was proposed [27], and the corresponding alterations in network dynamics were further explored. Theoretical results were used to provide novel insights into clinical findings, such as progressive behaviors, unsatisfactory long-term operative outcomes.
4.2 Seizure likelihood and synchronization patterns
Theoretically, Goodfellow et al. proposed a framework for discrete-time modelling of large-scale connected brain networks with distributed abnormalities, in which the spatial differences of intrinsic parameter were considered [131]. The differences in surrounding tissue excitability could offer a simple explanation for multiple onset patterns as well as previously conflicting clinical findings [132]. Moreover, Wang et al. found that independent patches of localized epileptiform activity would slowly invade the surrounding tissue and increase the excitability [133]. The extension of epileptogenicity is the manifestation of spatial heterogeneity progressively orchestrated by EZ. In order to investigate the possibility of transition in systems consisting of large number of interconnected heterogeneous neural populations (Fig. 3a), Kalitzin et al. proposed an analytical computational model which did not directly correspond to realistic neuronal processes (ion concentration regulation, synaptic interactions) [134, 135]. Despite numerous computational models even maintaining a certain degree of biological realism at the micro- or macroscales, such as Hodgkin–Huxley neuron model as well as simplified neuron models with less computational cost [136,137,138], kinds of neural mass model [139, 140], this analytical model could capture the general dynamics emerging in the epileptic process. The output of a realistic neuronal population consisting of excitatory and inhibitory subsets can be interpreted as the real and imaginary parts of a single complex variable Z. And seizure initiation can be well assumed to be a transition from stable fixed point to limit cycle attractor [52]. The dynamics of the complex variable Z was governed by:
where \(\omega\) determined the frequency of oscillation, \((a,b)\) were real constant coefficients and \(c\) represented the balance between synaptic inhibition and excitation inside the neural population. \(U\) denoted a complex Wiener process and \(\alpha\) determined the noise level. In the absence of noise, for \(- 1 < c < 0\;{\text{and}}\;a = - 1,\;b = 2\), the fixed point and limit cycle attractor coexisted, separated by an unstable limit cycle (Fig. 3b). In the bistable regime, an increase in parameter \(c\) indicated the enhancement of local excitability. Therefore, heterogeneous parameters could be used to model different levels of excitability between epileptogenic and non-epileptogenic regions. To account for network-level epileptic phenomena, the dynamics of brain network was represented by:
where \(N\) is the total number of nodes and \(G\) is the symmetric adjacency matrix, representing functional network. Escape time was introduced to quantify the likelihood of each node to develop into epileptic state [135]. Hutchings et al. validated the model by examining patient-control differences in seizure likelihood of brain networks [12]. Furthermore, the resection of those regions with higher seizure likelihood could contribute to satisfactory operative outcomes [13].
Alterations of network dynamics with the extension of epileptogenicity. a A network with each node represented by a bistable computational model (Eq. (3)) was established. Heterogeneous excitability were incorporated to distinguish the EZ and normal nodes. Moreover, part of nodes connected with EZ had the same excitability as the EZ, indicating the emergence of secondary epileptogenicity. b In the absence of noise with \(a = - 1,b = 2\), bifurcation diagram in parameter \(c\) was shown. In the bistable regime, \(c_{N} ,c_{S}\) represented the level of excitability of normal node and EZ, respectively. c The emergence of secondary EZ could significantly increase the overall seizure likelihood of network. d With the emergence of secondary epileptogenicity, connections between EZ and secondary EZ are significantly enhanced. Meanwhile, synchronization between secondary EZ and normal region may be progressively altered
Contrary to the surgical procedure, the extension of epileptogenicity represents that part of normal areas even spatially distant from seizure focus may be “infected” with high seizure likelihood. Yang et al. established a small-world network with heterogeneous excitability, in which part of non-epileptogenic nodes connected with epileptogenic ones could be transformed into secondary epileptogenic nodes [27]. Simulations under different noise realizations suggested that the extension of epileptogenicity would significantly increase the overall seizure likelihood of network (Fig. 3c). Clinically, patients with multiple lesions often have higher seizure frequency. At the same time, it would require more surgical effort to achieve seizure free, and at the cost of greater physiological impairment. Model-based surgical resections, removing several nodes from initial networks, also demonstrated the necessity to wipe out those nodes with high seizure likelihood [12, 13].
Since epilepsy was understood as the result of network disorder, distinctive seizure-related connectivity patterns have been revealed. Wang et al. found that highly clustered with modular structures could suppress the emergence of explosive synchronization [141]. The alterations of cluster structure contributed to the onset and propagation of epileptic discharge. Meanwhile, self-initiation and self-termination of epileptic events were associated with the regulation of network topology for regularity and randomness [142]. Particularly, epileptogenic focus arises as the hub of functional networks, and synchronization between areas involved in the epileptic events are remarkable at all stages [9]. However, whether these pathological patterns are the basis or by-product of epileptic activities remains unanswered [27]. From the perspective of network dynamics, the occurrence of secondary epileptogenicity represents the alteration in the dynamics of individual nodes or oscillators. In simulated multiunit system, synchronization between primary EZ and areas that were initially normal but later epileptogenic was greatly enhanced (Fig. 3d), indicating that the alterations in functional connectivity followed the extension of epileptogenicity. Intracranial recordings also demonstrated that those areas with frequent interictal epileptiform discharge showed robust dependence with each other [47, 50]. It probably meant that epileptogenic focus would drive the self-organization of epileptogenic networks through kindling-like effect, in addition to synaptic plasticity. As a result, epileptogenic networks are not just characterized with pathological topologies that are prone to synchronous behaviors, but also consist of epileptogenic nodes represented by abnormal oscillators, which are likely to play a pacemaker role in the epileptic process. The extension of epileptogenicity further increases the seizure susceptibility, which may provide additional understanding into progressive epileptic condition and cognitive impairment.
4.3 Possible prospect for localizing the EZ
Accurate localization of the EZ is the basis of successful resection operation, however postoperative recurrence in 30–50% of patients highlights that it is still an unsolved problem. We think that the extension of epileptogenicity may result in difficulty in determining the boundary and number of the EZ, accounting for the errors of localization. Based on clinical secondary epileptogenesis, seizures may be ignited by the pacemaker-like discharge from potential EZ (Fig. 4a). However, most of the existing methods tend to identify emerging EZ without uncovering potential effects [143]. Furthermore, when multiple EZs are calculated, it is ambiguous which should be chosen as surgical target (Fig. 4b). On the one hand, the extension of epileptogenicity can provide a reasonable explanation for the appearance of multiple epileptogenic tissues. On the other hand, it highlights the importance of revealing the dynamical characteristics of different nodes in the epileptogenic network. If the dynamical response of different regions can be accurately measured and mapped, tailored resection and excellent long-term operative outcomes may be realized.
Possible scenarios where localization errors often occur. a The extension of epileptogenicity may result in multiple EZs. One appears as the hub of epileptogenic network, while the others may act as potential pacemakers. b Multiple areas show epileptogenicity, but it is uncertain which should be surgical target
5 Conclusion
In this review, we summarized the current findings on the epileptic phenomena and corresponding dynamical explanations. There is no doubt that epilepsy especially focal epilepsy is characterized with locally initiated, network-supported hypersynchronous activities. In the epileptic brain, EZ shows relatively deterministic dynamical behaviors, which differs from the randomness in normal regions, and emerges as the hub of epileptogenic networks. But more than that, recurrent epileptic activities originating from EZ are likely to produce short- and long-term effects on neural circuits, involved in the self-organization of epileptogenic networks. Furthermore, spatially adjacent or distant regions from EZ may exhibit secondary epileptogenicity on account of kindling-like effect. From the perspective of network dynamics, epilepsy is a dynamical disorder orchestrated by EZ, which changes the topology of brain networks, the weights of connections and the excitability of partial normal regions. As a result, brain networks gradually evolves into epileptogenic networks labelled with great seizure susceptibility, which may be responsible for progressive impairments. These dynamical scenarios can help understand the mechanisms underlying epileptic processes, and aid the development of circuit therapy and tailored resection to reach satisfactory long-term outcomes.
References
Moraes, M.F.D., de Castro Medeiros, D., Mourao, F.A.G., Cancado, S.A.V., Cota, V.R.: Epilepsy as a dynamical system, a most needed paradigm shift in epileptology. Epilepsy Behav. 106838 (2019)
Connors, B.W., Gutnick, M.J., Prince, D.A.: Electrophysiological properties of neocortical neurons in vitro. J. Neurophysiol. 48, 1302 (1982)
Young, J.C., Nasser, H.M., Casillas-Espinosa, P.M., O’Brien, T.J., Jackson, G.D., Paolini, A.G.: Multiunit cluster firing patterns of piriform cortex and mediodorsal thalamus in absence epilepsy. Epilepsy Behav. 97, 229–243 (2019)
da Silva, F.H.L., Harding, G.F.A.: Transition to seizure in photosensitive epilepsy. Epilepsy Res. 97, 278–282 (2011)
Milton, J.G.: Epilepsy as a dynamic disease: a tutorial of the past with an eye to the future. Epilepsy Behav. 18, 33–44 (2010)
Stefanescu, R.A., Shivakeshavan, R.G., Talathi, S.S.: Computational models of epilepsy. Seizure 21, 748–759 (2012)
Ryzí, M., Brázdil, M., Novák, Z., Hemza, J., Chrastina, J., Ošlejšková, H., Rektor, I., Kuba, R.: Long-term outcomes in patients after epilepsy surgery failure. Epilepsy Res. 110, 71–77 (2015)
Woldman, W., Cook, M.J., Terry, J.R.: Evolving dynamic networks: an underlying mechanism of drug resistance in epilepsy? Epilepsy Behav. 94, 264–268 (2019)
Panzica, F., Varotto, G., Rotondi, F., Spreafico, R., Franceschetti, S.: Identification of the epileptogenic zone from stereo-EEG signals: a connectivity-graph theory approach. Front. Neurol. 4, 175 (2013)
Bernasconi, N., Duchesne, S., Janke, A., Lerch, J., Collins, D.L., Bernasconi, A.: Whole-brain voxel-based statistical analysis of gray matter and white matter in temporal lobe epilepsy. Neuroimage 23, 717–723 (2004)
Taylor, P.N., Han, C.E., Schoene-Bake, J.-C., Weber, B., Kaiser, M.: Structural connectivity changes in temporal lobe epilepsy: Spatial features contribute more than topological measures. NeuroImage Clin 8, 322–328 (2015)
Hutchings, F., Han, C.E., Keller, S.S., Weber, B., Taylor, P.N., Kaiser, M.: Predicting surgery targets in temporal lobe epilepsy through structural connectome based simulations. PLoS Comput. Biol. 11, e1004642 (2015)
Sinha, N., Dauwels, J., Kaiser, M., Cash, S.S., Brandon Westover, M., Wang, Y., Taylor, P.N.: Predicting neurosurgical outcomes in focal epilepsy patients using computational modelling. Brain 140, 319–332 (2017)
Goodfellow, M., Rummel, C., Abela, E., Richardson, M.P., Schindler, K., Terry, J.R.: Estimation of brain network ictogenicity predicts outcome from epilepsy surgery. Sci. Rep. 6, 29215 (2016)
Benjamin, O., Fitzgerald, T.H., Ashwin, P., Tsaneva-Atanasova, K., Chowdhury, F., Richardson, M.P., Terry, J.R.: A phenomenological model of seizure initiation suggests network structure may explain seizure frequency in idiopathic generalised epilepsy. J. Math. Neurosci. 2, 1 (2012)
Terry, J.R., Benjamin, O., Richardson, M.P.: Seizure generation: the role of nodes and networks. Epilepsia 53, e166–e169 (2012)
Bayati, M., Valizadeh, A.: Effect of synaptic plasticity in the structure and dynamics of disordered networks of coupled neurons. Physical Review E. 86, 011925 (2012)
Torres, J.J., Kappen, H.J.: Emerging phenomena in neural networks with dynamic synapses and their computational implications. Front. Comput. Neurosci. 7, 30 (2013)
Schindewolf, C., Kim, D., Bel, A., Rotstein, H.G.: Complex patterns in networks of hyperexcitable neurons. Theoret. Comput. Sci. 633, 71–82 (2016)
Volo, M.D., Torcini, A.: Transition from asynchronous to oscillatory dynamics in balanced spiking networks with instantaneous synapses. Phys. Rev. Lett. 121, 128301 (2018)
Majak, K., Pitkänen, A.: Do seizures cause irreversible cognitive damage? Evidence from animal studies. Epilepsy Behav. 5, 35–44 (2004)
Citraro, R., Iannone, M., Leo, A., De Caro, C., Nesci, V., Tallarico, M., Abdalla, K., Palma, E., Arturi, F., De Sarro, G., Constanti, A., Russo, E.: Evaluation of the effects of liraglutide on the development of epilepsy and behavioural alterations in two animal models of epileptogenesis. Brain Res. Bull. 153, 133–142 (2019)
Hempel, C.M., Hartman, K.H., Wang, X.-J., Turrigiano, G.G., Nelson, S.B.: Multiple forms of short-term plasticity at excitatory synapses in rat medial prefrontal cortex. J. Neurophysiol. 83, 3031–3041 (2000)
Bernasconi, N., Natsume, J., Bernasconi, A.: Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology 65, 223–228 (2005)
Bragin, A., Wilson, C.L., Engel, J.: Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia 41, S144–S152 (2000)
Bernasconi, N.: Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain 126, 462–469 (2003)
Yang, C., Liu, Z., Luan, G., Wang, Q.: The extension of epileptogenicity as the driving force of the epileptogenic network evolution and complex symptoms. Brain Res. 1748, 147073 (2020)
Kobylarek, D., Iwanowski, P., Lewandowska, Z., Limphaibool, N., Szafranek, S., Labrzycka, A., Kozubski, W.: Advances in the potential biomarkers of epilepsy. Front. Neurol. 10, 685 (2019)
Liguori, C., Costa, C., Franchini, F., Izzi, F., Spanetta, M., Cesarini, E.N., Di Santo, S., Manfredi, N., Farotti, L., Romoli, M., Lanari, A., Salvadori, N., Parnetti, L., Calabresi, P., Mercuri, N.B., Placidi, F.: Cognitive performances in patients affected by late-onset epilepsy with unknown etiology: a 12-month follow-up study. Epilepsy Behav. 101, 106592 (2019)
Süße, M., Hamann, L., Flöel, A., von Podewils, F.: Nonlesional late-onset epilepsy: semiology, EEG, cerebrospinal fluid, and seizure outcome characteristics. Epilepsy Behav. 91, 75–80 (2019)
Ochoa, J.G., Hentgarden, D., Paulzak, A., Ogden, M., Pryson, R., Lammle, M., Rusyniak, W.G.: Subtle pathological changes in neocortical temporal lobe epilepsy. Epilepsy Behav. 71, 17–22 (2017)
Jacobs, J., Staba, R., Asano, E., Otsubo, H., Wu, J.Y., Zijlmans, M., Mohamed, I., Kahane, P., Dubeau, F., Navarro, V., Gotman, J.: High-frequency oscillations (HFOs) in clinical epilepsy. Prog. Neurobiol. 98, 302–315 (2012)
Jirsch, J.D.: High-frequency oscillations during human focal seizures. Brain 129, 1593–1608 (2006)
Bragin, A., Engel, J., Wilson, C.L., Fried, I., Mathern, G.W.: Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia 40, 127–137 (1999)
Leung, H., Zhu, C.X.L., Chan, D.T.M., Poon, W.S., Shi, L., Mok, V.C.T., Wong, L.K.S.: Ictal high-frequency oscillations and hyperexcitability in refractory epilepsy. Clin. Neurophysiol. 126, 2049–2057 (2015)
Kobayashi, K., Agari, T., Oka, M., Yoshinaga, H., Date, I., Ohtsuka, Y., Gotman, J.: Detection of seizure-associated high-frequency oscillations above 500Hz. Epilepsy Res. 88, 139–144 (2010)
Hao, J., Cui, Y., Liu, B., Yu, L., Lin, Y., Xia, Y., Yao, D., Guo, D.: Roles of very fast ripple (500–1000Hz) in the hippocampal network during status epilepticus. Int. J. Neural Syst. (2020). https://doi.org/10.1142/S0129065721500027
Frauscher, B., Bartolomei, F., Kobayashi, K., Cimbalnik, J., van 't Klooster, M.A., Rampp, S., Otsubo, H., Höller, Y., Wu, J.Y., Asano, E., Engel, J., Kahane, P., Jacobs, J., Gotman, J.: High-frequency oscillations: the state of clinical research. Epilepsia. 58, 1316–1329 (2017)
Koc, G., Gokcil, Z., Bek, S., Kasikci, T., Eroglu, E., Odabasi, Z.: Effects of continuous theta burst transcranial magnetic stimulation on cortical excitability in patients with idiopathic generalized epilepsy. Epilepsy Behav. 77, 26–29 (2017)
Koenig, T., Smailovic, U., Jelic, V.: Past, present and future EEG in the clinical workup of dementias. Psychiatry Res. Neuroimaging 306, 111182 (2020)
Tatum, W.O., Rubboli, G., Kaplan, P.W., Mirsatari, S.M., Radhakrishnan, K., Gloss, D., Caboclo, L.O., Drislane, F.W., Koutroumanidis, M., Schomer, D.L., Kasteleijn-Nolst Trenite, D., Cook, M., Beniczky, S.: Clinical utility of EEG in diagnosing and monitoring epilepsy in adults. Clin. Neurophysiol. 129, 1056–1082 (2018)
Liu, X., Wu, S., Daif, A., Sun, T., Chauhan, V., Issa, N.P., Rose, S., Tao, J.X.: Clinical implications of scalp ictal EEG pattern in patients with temporal lobe epilepsy. Clin. Neurophysiol. 130, 1604–1610 (2019)
Fangsaad, T., Assawabumrungkul, S., Visudtibhan, A.: Clinical course and long-term outcome in children with alteration of consciousness underwent continuous EEG monitoring: a prospective observational study in Thailand. J. Clin. Neurosci. 47, 93–96 (2018)
van Mierlo, P., Carrette, E., Hallez, H., Vonck, K., Van Roost, D., Boon, P., Staelens, S.: Accurate epileptogenic focus localization through time-variant functional connectivity analysis of intracranial electroencephalographic signals. Neuroimage 56, 1122–1133 (2011)
Chionis, D., Dokhane, A., Ferroukhi, H., Pautz, A.: Application of causality analysis on nuclear reactor systems. Chaos 29, 043126 (2019)
Bartolomei, F., Chauvel, P., Wendling, F.: Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain 131, 1818–1830 (2008)
Lehnertz, K., Elger, C.E.: Spatio-temporal dynamics of the primary epileptogenic area in temporal lobe epilepsy characterized by neuronal complexity loss. Electroencephalogr. Clin. Neurophysiol. 95, 108–117 (1995)
Andrzejak, R.G., Widman, G., Lehnertz, K., Rieke, C., David, P., Elger, C.E.: The epileptic process as nonlinear deterministic dynamics in a stochastic environment: an evaluation on mesial temporal lobe epilepsy. Epilepsy Res. 44, 129–140 (2001)
Andrzejak, R.G., Schindler, K., Rummel, C.: Nonrandomness, nonlinear dependence, and nonstationarity of electroencephalographic recordings from epilepsy patients. Phys. Rev. E 86, 046206 (2012)
Yang, C., Luan, G., Liu, Z., Wang, Q.: Dynamical analysis of epileptic characteristics based on recurrence quantification of SEEG recordings. Physica A 523, 507–515 (2019)
Li, X., Ouyang, G., Yao, X., Guan, X.: Dynamical characteristics of pre-epileptic seizures in rats with recurrence quantification analysis. Phys. Lett. A 333, 164–171 (2004)
Suffczynski, P., da Silva, F.H.L., Parra, J., Velis, D.N., Bouwman, B.M., van Rijn, C.M., van Hese, P., Boon, P., Khosravani, H., Derchansky, M., Carlen, P., Kalitzin, S.: Dynamics of epileptic phenomena determined from statistics of Ictal transitions. IEEE Trans. Biomed. Eng. 53, 524–532 (2006)
Panahi, S., Aram, Z., Jafari, S., Ma, J., Sprott, J.C.: Modeling of epilepsy based on chaotic artificial neural network. Chaos, Solitons Fractals 105, 150–156 (2017)
Panahi, S., Shirzadian, T., Jalili, M., Jafari, S.: A new chaotic network model for epilepsy. Appl. Math. Comput. 346, 395–407 (2019)
Yan, J., Wang, Y., Ouyang, G., Yu, T., Li, X.: Using max entropy ratio of recurrence plot to measure electrocorticogram changes in epilepsy patients. Physica A 443, 109–116 (2016)
Acharya, U.R., Sree, S.V., Chattopadhyay, S., Yu, W., Ang, P.C.A.: Application of recurrence quantification analysis for the automated identification of epileptic EEG signals. Int. J. Neural Syst. 21, 199–211 (2011)
Ngamga, E.J., Bialonski, S., Marwan, N., Kurths, J., Geier, C., Lehnertz, K.: Evaluation of selected recurrence measures in discriminating pre-ictal and inter-ictal periods from epileptic EEG data. Phys. Lett. A 380, 1419–1425 (2016)
Lehnertz, K., Elger, C.E.: Can epileptic seizures be predicted? evidence from nonlinear time series analysis of brain electrical activity. Physiol. Rev. Lett. 80, 5019–5022 (1998)
Stacey, W., Le Van Quyen, M., Mormann, F., Schulze-Bonhage, A.: What is the present-day EEG evidence for a preictal state? Epilepsy Res. 97, 243–251 (2011)
Suffczynski, P., Kalitzin, S.: Epileptic transitions: model predictions and experimental validation. J. Clin. Neurophysiol. 22, 13 (2005)
Laxpati, N.G., Kasoff, W.S., Gross, R.E.: Deep brain stimulation for the treatment of epilepsy: circuits, targets, and trials. Neurotherapeutics 11, 508–526 (2014)
Kalitzin, S.N., Velis, D.N., da Silva, F.H.L.: Stimulation-based anticipation and control of state transitions in the epileptic brain. Epilepsy Behav. 17, 310–323 (2010)
Balamurugan, E., Aggarwal, M., Lamba, A., Dang, N., Tripathi, M.: Perceived trigger factors of seizures in persons with epilepsy. Seizure 22, 743–747 (2013)
Gilby, K.L., O’Brien, T.J.: Epilepsy, autism, and neurodevelopment: kindling a shared vulnerability? Epilepsy Behav. 26, 370–374 (2013)
da Silva, F.H.L., Blanes, W., Kalitzin, S.N., Parra, J., Suffczynski, P., Velis, D.N.: Epilepsies as dynamical diseases of brain systems: basic models of the transition between normal and epileptic activity. Epilepsia 44, 72–83 (2003)
Freeman, W.J., Kozma, R., Werbos, P.J.: Biocomplexity: adaptive behavior in complex stochastic dynamical systems. Biosystems 59, 109–123 (2001)
Tankus, A.: Exploring human epileptic activity at the single-neuron level. Epilepsy Behav. 58, 11–17 (2016)
Kalitzin, S., Velis, D., Suffczynski, P., Parra, J., da Silva, F.H.L.: Electrical brain-stimulation paradigm for estimating the seizure onset site and the time to ictal transition in temporal lobe epilepsy. Clin. Neurophysiol. 116, 718–728 (2005)
Barker, B.S., Nigam, A., Ottolini, M., Gaykema, R.P., Hargus, N.J., Patel, M.K.: Pro-excitatory alterations in sodium channel activity facilitate subiculum neuron hyperexcitability in temporal lobe epilepsy. Neurobiol. Dis. 108, 183–194 (2017)
Truccolo, W., Donoghue, J.A., Hochberg, L.R., Eskandar, E.N., Madsen, J.R., Anderson, W.S., Brown, E.N., Halgren, E., Cash, S.S.: Single-neuron dynamics in human focal epilepsy. Nat. Neurosci. 14, 635–641 (2011)
Litt, B., Esteller, R., Echauz, J., D’Alessandro, M., Shor, R., Henry, T., Pennell, P., Epstein, C., Bakay, R., Dichter, M., Vachtsevanos, G.: Epileptic seizures may begin hours in advance of clinical onset: a report of five patients. Neuron 30, 51–64 (2001)
Avoli, M., de Curtis, M., Köhling, R.: Does interictal synchronization influence ictogenesis? Neuropharmacology 69, 37–44 (2013)
Righes Marafiga, J., Vendramin Pasquetti, M., Calcagnotto, M.E.: GABAergic interneurons in epilepsy: more than a simple change in inhibition. Epilepsy Behav. (2020). https://doi.org/10.1016/j.yebeh.2020.106935
Rich, S., Chameh, H.M., Rafiee, M., Ferguson, K., Skinner, F.K., Valiante, T.A.: Inhibitory network bistability explains increased interneuronal activity prior to seizure onset. Front. Neural Circuits 13, 81 (2020)
Proix, T., Jirsa, V.K., Bartolomei, F., Guye, M., Truccolo, W.: Predicting the spatiotemporal diversity of seizure propagation and termination in human focal epilepsy. Nat. Commun. 9, 1088 (2018)
Perucca, P., Dubeau, F., Gotman, J.: Intracranial electroencephalographic seizure-onset patterns: effect of underlying pathology. Brain 137, 183–196 (2014)
Wang, Y., Goodfellow, M., Taylor, P.N., Baier, G.: Phase space approach for modeling of epileptic dynamics. Phys. Rev. E. 85, 061918 (2012)
Jirsa, V.K., Stacey, W.C., Quilichini, P.P., Ivanov, A.I., Bernard, C.: On the nature of seizure dynamics. Brain 137, 2210–2230 (2014)
Guo, D., Xia, C., Wu, S., Zhang, T., Zhang, Y., Xia, Y., Yao, D.: Stochastic fluctuations of permittivity coupling regulate seizure dynamics in partial epilepsy. Sci. China Technol. Sci. 60, 995–1002 (2017)
Proix, T., Bartolomei, F., Chauvel, P., Bernard, C., Jirsa, V.K.: Permittivity coupling across brain regions determines seizure recruitment in partial epilepsy. J. Neurosci. 34, 15009–15021 (2014)
Zhang, L., Wang, Q., Baier, G.: Dynamical features of a focal epileptogenic network model for stimulation-based control. IEEE Trans. Neural Syst. Rehabil. Eng. 28, 1856–1865 (2020)
Zhang, L., Wang, Q., Baier, G.: Spontaneous transitions to focal-onset epileptic seizures: a dynamical study. Chaos 30, 103114 (2020)
Badawy, R.A.B., Harvey, A.S., Macdonell, R.A.L.: Cortical hyperexcitability and epileptogenesis: understanding the mechanisms of epilepsy–Part 1. J. Clin. Neurosci. 16, 355–365 (2009)
Whelan, C.D., Altmann, A., Botía, J.A., et al.: Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain 141, 391–408 (2018)
Focke, N.K., Yogarajah, M., Bonelli, S.B., Bartlett, P.A., Symms, M.R., Duncan, J.S.: Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage 40, 728–737 (2008)
van Mierlo, P., Carrette, E., Hallez, H., Raedt, R., Meurs, A., Vandenberghe, S., Van Roost, D., Boon, P., Staelens, S., Vonck, K.: Ictal-onset localization through connectivity analysis of intracranial EEG signals in patients with refractory epilepsy. Epilepsia 54, 1409–1418 (2013)
Wilke, C., van Drongelen, W., Kohrman, M., He, B.: Identification of epileptogenic foci from causal analysis of ECoG interictal spike activity. Clin. Neurophysiol. 120, 1449–1456 (2009)
Zhang, L., Liang, Y., Li, F., Sun, H., Peng, W., Du, P., Si, Y., Song, L., Yu, L., Xu, P.: Time-varying networks of inter-ictal discharging reveal epileptogenic zone. Front. Comput. Neurosci. 11, 77 (2017)
Ben-Ari, Y., Tremblay, E., Ottersen, O.P., Naquet, R.: Evidence suggesting secondary epileptogenic lesions after kainic acid: pretreatment with diazepam reduces distant but not local brain damage. Brain Res. 165, 362–365 (1979)
Alamir, M., Welsh, J.S., Goodwin, G.C.: Synaptic plasticity based model for epileptic seizures. Automatica 47, 1183–1192 (2011)
Wiemann, M., Altrup, U., Speckmann, E.-J.: Epileptic neurons induce augmenting synaptic depolarizations in non-epileptic neurons (buccal ganglia, Helix pomatia). Neurosci. Lett. 237, 101–104 (1997)
Lehnertz, K., Ansmann, G., Bialonski, S., Dickten, H., Geier, C., Porz, S.: Evolving networks in the human epileptic brain. Physica D 267, 7–15 (2014)
Kurths, J.: Bistable firing pattern in a neural network model. Front. Comput. Neurosci. 13, 8 (2019)
Gilson, M.: STDP in recurrent neuronal networks. Front. Comput. Neurosci. 4, 23 (2010)
Luccioli, S., Angulo-Garcia, D., Cossart, R., Malvache, A., Módol, L., Sousa, V.H., Bonifazi, P., Torcini, A.: Modeling driver cells in developing neuronal networks. PLoS Comput. Biol. 14, e1006551 (2018)
Bayati, M., Valizadeh, A., Abbassian, A., Cheng, S.: Self-organization of synchronous activity propagation in neuronal networks driven by local excitation. Front. Comput. Neurosci. 9, 69 (2015)
Raiesdana, S., Firoozabadi, S.M.P., Gholpayegani, S.M.H.: An evolutionary network model of epileptic phenomena. Neurocomputing 74, 617–628 (2011)
Zucker, R.S., Regehr, W.G.: Short-term synaptic plasticity. Annu. Rev. Physiol. 64, 355–405 (2002)
Fuhrmann, G., Segev, I., Markram, H., Tsodyks, M.: Coding of temporal information by activity-dependent synapses. J. Neurophysiol. 87, 140–148 (2002)
Thomson, A.M.: Activity-dependent properties of synaptic transmission at two classes of connections made by rat neocortical pyramidal axons in vitro. J. Physiol. 502, 131–147 (1997)
Gonzalez-Burgos, G.: Synaptic efficacy during repetitive activation of excitatory inputs in primate dorsolateral prefrontal cortex. Cereb. Cortex 14, 530–542 (2004)
Molaee-Ardekani, B., Benquet, P., Bartolomei, F., Wendling, F.: Computational modeling of high-frequency oscillations at the onset of neocortical partial seizures: from ‘altered structure’ to ‘dysfunction.’ Neuroimage 52, 1109–1122 (2010)
Gutkin, B.S., Laing, C.R., Colby, C.L., Chow, C.C., Ermentrout, G.B.: Turning on and off with excitation: the role of spike-timing asynchrony and synchrony in sustained neural activity. J. Comput. Neurosci. 11, 121–134 (2001)
Yang, C., Liu, Z., Wang, Q., Luan, G., Zhai, F.: Epileptic seizures in a heterogeneous excitatory network with short-term plasticity. Cogn Neurodyn. (2020). https://doi.org/10.1007/s11571-020-09582-w
González, O.C., Krishnan, G.P., Timofeev, I., Bazhenov, M.: Ionic and synaptic mechanisms of seizure generation and epileptogenesis. Neurobiol. Dis. 130, 104485 (2019)
Jiang, H., Cai, Z., Worrell, G.A., He, B.: Multiple oscillatory push–pull antagonisms constrain seizure propagation. Ann. Neurol. 86, 683–694 (2019)
Wasling, P., Hanse, E., Gustafsson, B.: Long-term depression in the developing hippocampus: low induction threshold and synapse nonspecificity. J. Neurosci. 22, 1823–1830 (2002)
Lippman-Bell, J.J., Zhou, C., Sun, H., Feske, J.S., Jensen, F.E.: Early-life seizures alter synaptic calcium-permeable AMPA receptor function and plasticity. Mol. Cell. Neurosci. 76, 11–20 (2016)
Lynch, G., Kramár, E.A., Babayan, A.H., Rumbaugh, G., Gall, C.M.: Differences between synaptic plasticity thresholds result in new timing rules for maximizing long-term potentiation. Neuropharmacology 64, 27–36 (2013)
David, O., Woźniak, A., Minotti, L., Kahane, P.: Preictal short-term plasticity induced by intracerebral 1 Hz stimulation. Neuroimage 39, 1633–1646 (2008)
Bertram, E.: The relevance of kindling for human epilepsy. Epilepsia 48, 65–74 (2007)
Wang, Y., Chen, Z.: An update for epilepsy research and antiepileptic drug development: toward precise circuit therapy. Pharmacol. Ther. 201, 77–93 (2019)
Ma, J., Yang, Z., Yang, L., Tang, J.: A physical view of computational neurodynamics. J. Zhejiang Univ. Sci. A. 20, 639–659 (2019)
Ma, J., Tang, J.: A review for dynamics in neuron and neuronal network. Nonlinear Dyn. 89, 1569–1578 (2017)
Roth, B.L.: DREADDs for neuroscientists. Neuron 89, 683–694 (2016)
Adamantidis, A.R., Zhang, F., Aravanism, A.M., Deisseroth, K., Lecea, L.D.: Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424 (2007)
Ma, J., Tang, J.: A review for dynamics of collective behaviors of network of neurons. Sci. China Technol. Sci. 58, 2038–2045 (2015)
Yang, L.-X., Jin, C.-L., Zhu-Ge, Z.-B., Wang, S., Wei, E.-Q., Bruce, I.C., Chen, Z.: Unilateral low-frequency stimulation of central piriform cortex delays seizure development induced by amygdaloid kindling in rats. Neuroscience 138, 1089–1096 (2006)
Sun, H., Zhang, S., Zhong, K., Xu, Z., Zhu, W., Fang, Q., Wu, D., Hu, W., Xiao, B., Chen, Z.: Mode-dependent effect of low-frequency stimulation targeting the hippocampal CA3 subfield on amygdala-kindled seizures in rats. Epilepsy Res. 90, 83–90 (2010)
Neal, E.G., Maciver, S., Schoenberg, M.R., Vale, F.L.: Surgical disconnection of epilepsy network correlates with improved outcomes. Seizure 76, 56–63 (2020)
Briellmann, R.S., Jackson, G.D., Pell, G.S., Mitchell, L.A., Abbott, D.F.: Structural abnormalities remote from the seizure focus: a study using T2 relaxometry at 3 T. Neurology 63, 2303–2308 (2004)
Bernasconi, N., Bernasconi, A., Caramanos, Z., Andermann, F., Dubeau, F., Arnold, D.L.: Morphometric MRI analysis of the parahippocampal region in temporal lobe epilepsy. Ann. N. Y. Acad. Sci. 911, 495–500 (2006)
Scott, R.C.: Abnormalities in hippocampi remote from the seizure focus: a T2 relaxometry study. Brain 126, 1968–1974 (2003)
Klviinen, R., Salmenper, T.: Do recurrent seizures cause neuronal damage? A series of studies with MRI volumetry in adults with partial epilepsy. Prog. Brain Res. 135, 279–295 (2002)
Meldrum, B.S.: Concept of activity-induced cell death in epilepsy: historical and contemporary perspectives. Prog. Brain Res. 135, 3–11 (2002)
Wu, J., Gao, M., Shen, J., Qiu, S., Kerrigan, J.F.: Mechanisms of intrinsic epileptogenesis in human gelastic seizures with hypothalamic hamartoma. CNS Neurosci. Ther. 21, 104–111 (2015)
Castro, L.H., Ferreira, L.K., Teles, L.R., Jorge, C.L., Arantes, P.R., Ono, C.R., Adda, C.C., Valerio, R.F.: Epilepsy syndromes associated with hypothalamic hamartomas. Seizure 16, 50–58 (2007)
Scholly, J., Staack, A.M., Kahane, P., Scavarda, D., Régis, J., Hirsch, E., Bartolomei, F.: Hypothalamic hamartoma: epileptogenesis beyond the lesion? Epilepsia 58, 32–40 (2017)
Scholly, J., Valenti, M.-P., Staack, A.M., Strobl, K., Bast, T., Kehrli, P., Steinhoff, B.J., Hirsch, E.: Hypothalamic hamartoma: is the epileptogenic zone always hypothalamic? Arguments for independent (third stage) secondary epileptogenesis. Epilepsia 54, 123–128 (2013)
Goddard, G.V.: Development of epileptic seizures through brain stimulation at lowintensity. Nature 214, 1020–1021 (1967)
Goodfellow, M., Taylor, P.N., Wang, Y., Garry, D.J., Baier, G.: Modelling the role of tissue heterogeneity in epileptic rhythms: tissue heterogeneity in epileptic rhythms. Eur. J. Neurosci. 36, 2178–2187 (2012)
Wang, Y., Trevelyan, A.J., Valentin, A., Alarcon, G., Taylor, P.N., Kaiser, M.: Mechanisms underlying different onset patterns of focal seizures. PLoS Comput. Biol. 13, e1005475 (2017)
Wang, Y., Goodfellow, M., Taylor, P.N., Baier, G.: Dynamic mechanisms of neocortical focal seizure onset. PLoS Comput. Biol. 10, e1003787 (2014)
Kalitzin, S., Koppert, M., Petkov, G., Velis, D., da Silva, F.H.L.: Computational model prospective on the observation of proictal states in epileptic neuronal systems. Epilepsy Behav. 22, S102–S109 (2011)
Kalitzin, S., Koppert, M., Petkov, G., da Silva, F.H.L.: Multiple oscillatory states in models of collective neuronal dynamics. Int. J. Neural Syst. 24, 1450020 (2014)
Hodgkin, A.L., Huxley, A.F.: The components of membrane conductance in the giant axon of Loligo. J. Physiol. 116, 473–496 (1952)
Hindmarsh, J.L., Rose, R.M.: A model of neuronal bursting using three coupled first order differential equation. Proc. R. Soc. Lond. Ser. B. 221, 87–102 (1984)
Burkitt, A.N.: A review of the integrate-and-fire neuron model: I Homogeneous synaptic input. Biol. Cybern. 95, 1–19 (2006)
Jansen, B.H., Zouridakis, G., Brandt, M.E.: A neurophysiologically-based mathematical model of flash visual evoked potentials. Biol. Cybern. 68, 275–283 (1993)
Wendling, F., Bartolomei, F., Bellanger, J.J., Chauvel, P.: Epileptic fast activity can be explained by a model of impaired GABAergic dendritic inhibition. Eur. J. Neurosci. 15, 1499–1508 (2002)
Wang, Z., Tian, C., Dhamala, M., Liu, Z.: A small change in neuronal network topology can induce explosive synchronization transition and activity propagation in the entire network. Sci. Rep. 7, 561 (2017)
Gerster, M., Berner, R., Sawicki, J., Zakharova, A., Škoch, A., Hlinka, J., Lehnertz, K., Schöll, E.: FitzHugh–Nagumo oscillators on complex networks mimic epileptic-seizure-related synchronization phenomena. Chaos 30, 123130 (2020)
Liu, Z., Luan, G., Yang, C., Guan, Y., Liu, C., Wang, J., Wang, M., Wang, Q.: Distinguishing dependent-stage secondary epileptogenesis in a complex case of giant hypothalamic hamartoma with assistance of a computational method. Front. Neurol. 11, 478 (2020)
Acknowledgements
This research was supported by the National Science Foundation of China (Grants 11932003, 81790650, 81790654).
Author information
Authors and Affiliations
Contributions
CY contributed to conceptualization, investigation, writing–original draft. ZL contributed to resources, writing—review, and editing. QW contribted to writing—review and editing. QW contributed to project administration and funding acquisition. ZL contributed to investigation, writing—review and editing. GL supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no any interest conflict with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, C., Liu, Z., Wang, Q. et al. Epilepsy as a dynamical disorder orchestrated by epileptogenic zone: a review. Nonlinear Dyn 104, 1901–1916 (2021). https://doi.org/10.1007/s11071-021-06420-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-021-06420-4