Abstract
Epileptic seizures are characterized by a rich dynamic spectrum consisting of excessive, abnormal and synchronized firing of neuron ensembles. Such abnormal firing has been quantitatively characterized via power laws in neural avalanches. The term “neural avalanche” has been used to illustrate the excessively amplified neural firing patterns that lead to epileptic seizures. The pattern of amplified firing in neural avalanches betrays a modular signature in the spread of activation across cortical minicolumns. According to this modular approach of epilepsy, the excessive amplification of neural firing in a cortical minicolumn results from a defect within the “inhibitory curtain” surrounding the pyramidal cells. The functional basis of this approach provides insights into potential clinical interventions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Anatomical connectivity patterns of brain complexity have revealed a wiring organization that can be captured by neural modules that are neither regular nor random (Mountcastle 1997; Opris and Casanova 2014). In many instances the emergence of behaviors and pathological states are accompanied by various changes in brain connectivity patterns (Varela et al. 2001). Recently, it has been discovered that functional connectivity patterns obtained from electrophysiological recordings, functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) signals during different pathological and cognitive brain states (including epilepsy) display structural and functional signatures of modular properties (Vaessen et al. 2014). Experimental studies have also shown that brain functions are coordinated via scattered specialized modules, forming a “brainweb” of neural ensembles (Varela et al. 2001). Modularity is a basic concept in the complex networks of cells endowed with columnar laminar structure (Buxhoeveden and Casanova 2002; Mountcastle 1997). A module can be defined as a group of cells within a network, in which connections between cells in the group are denser than connections of the cells with the rest of the network (Mountcastle 1997). Modularity appears to play a key role in the stability, flexibility and robustness of the network (Sole and Valverde 2008). Modular architecture of the brain might play a key role in the integration of microcircuit and of macro-scale brain activities (Opris et al. 2011, 2012a, b, 2013). Occurrence of rhythmic spike wave discharges of high amplitude that synchronizes over multiple cortical areas, suddenly emerging from a normal activity background, is a main characteristic of epilepsy.
Dysfunction of microcircuit and of macro-scale brain activity facilitates the emergence of epileptic discharges. While brain connectivity studies mainly focus on the onset, evolution and onset of epileptic discharges, in this chapter we study the modular signature of brain microcircuits and networks extracted from signals of epileptic patients compared to healthy subjects. Epileptic seizures are pathological activity states in the human brain having a rich dynamic spectrum consisting of excessive, abnormal and synchronized firing of neuronal ensembles. Such abnormal firing has been quantitatively characterized via power laws that are characteristic to avalanche processes (Wu et al. 2014).
The term “neural avalanche” illustrates excessively amplified neural firing patterns. The pattern of amplified firing in neural avalanches betrays a modular signature in the spread of activation across cortical minicolumns and large-scale networks. According to this modular view of epilepsy, one could posit that excessive amplification of neural firing in a cortical minicolum may be the outcome of pyramidal cells crosstalk that pierce the inhibitory wall surrounding the minicolumns.
15.2 Neural Avalanches
15.2.1 Definition of Neural Avalanches
Neuronal populations exhibit a type of activity named neuronal avalanches. A neuronal avalanche is a cascade of bursts of activity in neuronal networks whose size distribution can be approximated by a power law, as illustrated in critical sand pile models (Bak et al. 1987). The size distribution of neuronal avalanches in cortical networks of the brain has been reported to follow a power law distribution with an exponent close to −3/2, which reflect the long range spatial correlations in spontaneous activity (Klaus et al. 2011).
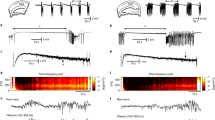
Neuronal avalanche has been demonstrated in cortical seizure-like activity in the anterior cingulate cortex (ACC) induced by 4-aminopyridine (4AP) and bicuculline (Bic) antagonists of selected voltage-gated potassium channels and γ-aminobutyric acid-A (GABAA) receptors, respectively (Wu et al. 2014). To illustrate the concept of neuronal avalanche induced by the application of 4AP and Bic, a layered brain slice together with the recording position of the multi-electrode array (MEA) are depicted in Fig. 15.1a. To detect the active neuronal responses, a threshold was set on the high-pass filtered sweep of negative local field potentials (nLFPs). These nLFPs have been correlated with neuronal spikes (Wu et al. 2014).
Definition of the neuronal avalanche. (a) Brain slice with the recording site. (b) Comparison of seizure-like activity of ictal events, followed by tonic and clonic bursts with spontaneous activity. (c) Example of the collective time step, which is framed in 4 ms time bins from eight channels. The definition of an avalanche is separated by blank activity at the beginning and end of the events. The activated electrodes are counted as the avalanche size, and each event’s lifetime is the summation of the total time frames. (d) Distribution of different avalanche sizes plotted on a log-log scale. The neuronal avalanche could follow the power-law distribution, and its slope could be calculated as the α value. The original data were shuffled in order to disturb their spatiotemporal arrangement. Scale bar = 1 mm in A (Adapted with permission from Wu et al. 2014 BMC Neuroscience)
Seizure-like activity consists of ictal and tonic bursts (Fig. 15.1b, red line, upper panel), followed by clonic bursts (Fig. 15.1b, gray line, upper panel). The time-points at which each nLFPs exceeded the specific threshold is marked as a unit raster in the lower panel of Fig. 15.1b. The time units were summed together in a timescale-binned plot to calculate the avalanche size and lifetime. Figure 15.1c shows the cumulated time units framed by a time window (Δt, gray regions) from eight-channel recording. According to Wu et al. 2014, the avalanche is defined as “a series of activity separated by a blank at the beginning and end of the events”. The avalanche size was calculated as the total number of electrodes with active units, while the lifetime was calculated as the summation of the total time frame in each avalanche event. The distribution of the avalanche size with its probability P(s) were plotted on a log-log scale. A neuronal avalanche that has a fitted straight-line slope of α value indicates a power-law relationship and the event’s dependence. The 4AP-Bic group (red solid line) showed a power-law distribution with the α value around −1.5 (Fig. 15.1d). An important test was performed to assess whether event dependence is essential for the power-law relationship. Thus, the event dependence was disturbed, by randomly shuffling the data with regard to the order of temporal sequence and spatial arrangement of the events. Both the shuffling data (dashed red line) and spontaneous activity data (black line) showed an exponential distribution, which is a type of Poisson distribution in which the events occur independently (Fig. 15.1d).
15.2.2 Power Law Distributions
Power law distributions of event sizes are often seen in complex phenomena including semiconductor devices (Levinshtein et al. 2005), forest fires, earthquakes, phase transitions, financial market fluctuations, snow avalanches and many other instances (Bak 1996). For example, earthquake models integrate local rules in which forces at one site are distributed to nearest neighbors without dissipation. This conservation of forces is similar to the conservation of synaptic strengths (Royer and Pare 2003) and it could be a mechanism responsible for maintaining a network near the critical point. Computer simulations indicate that networks can be kept nearly critical levels when the total sum of synaptic strengths soars near a constant value (Hsu and Beggs 2006). This could be accomplished through a mechanism like synaptic scaling (Turrigiano and Nelson 2000), which has been observed experimentally. Finally, recently “burned” areas in forest fire models are refractory, while unburned areas are more likely to ignite. This balance of refractoriness and excitability combines to maintain the system near the critical point. Recent models of neuronal avalanches (Levina et al. 2005) have suggested that short-term synaptic depression and facilitation may also serve to drive neuronal networks toward the critical point where avalanches occur. Thus, an understanding of power laws in diverse complex systems can suggest mechanisms that might underlie criticality in neuronal networks. Computational models have also explored the potential relationship between neuronal avalanches and epilepsy (Beggs 2008; Hsu and Beggs 2006; Hsu et al. 2007, 2008).
Although the normal brain firing activity can be moderately-synchronous, in epileptic seizures, a group of neurons begin firing in an abnormal, excessive, and synchronized manner (McPhee and Hammer 2010; National Institute for Health and Clinical Excellence 2012; Plenz 2012; Yang et al. 2012). This excessive firing may occur due to structural or functional anomalies within the epileptic brain. Such abnormal activity could be produced either by hyper excited neurons acting independently or it could involve abnormal interactions among many neurons. Many forms of collective activity including waves, spirals, oscillations, synchrony, and neuronal avalanches have been identified in abnormal epileptic firing. All these emergent activity patterns have been hypothesized to show pathologic signatures associated with epilepsy (Hobbs et al. 2010). From this perspective, epileptic activity would occur when regulatory mechanisms failed and the network entered a super critical regimen. Operating at the critical point depends on the appropriate balance between excitation and inhibition implies a structured activity that is far from random. In addition, the activity in one neuron would, on average, lead to activity in other neurons, amplifying activity excessively and possibly leading to seizures (Hobbs et al. 2010). Therefore, when network activity is randomly shuffled, it no longer follows a power law distribution characteristic of avalanches (Beggs 2008).
Hobbs et al. (2010) examined neural activity from human and rat cortical tissue in local cortical networks using 60 channel multielectrode arrays to record local field potentials in brain slices removed from the most active epileptogenic area (as identified by intraoperative electrocorticography). In the human cortical tissue they found periods of pronounced hyperexcitability and lack of a clear power law in avalanche size distributions. Analysis showed that during these periods, there was a significant positive correlation between the branching parameter and the firing rate, suggesting a positive feedback loop. This aspect was not present in the activity examined in rat tissue. These results indicate that cortical tissue removed from pediatric epilepsy patients produces aberrant neuronal avalanches (Hobbs et al. 2010).
15.2.3 Self-Organized Criticality
Self-organized criticality (SOC) represents a property of complex dynamic systems that evolve to a critical state, capable of producing scale-free energy fluctuations. A characteristic feature of dynamical systems exhibiting SOC is the power-law probability distributions that describe the dynamics of energy release. Worrell and colleagues in their study investigated the probability distribution of in vivo pathological energy fluctuations in human epileptic hippocampus by analyzing data from seven consecutive patients with temporal lobe epilepsy who required depth electrode iEEG monitoring during evaluation for epilepsy surgery (Worrell et al. 2002). Contacts that recorded the earliest clear seizure onset on iEEG delineated the seizure onset zone that was determined by visual inspection as located within the mesial (middle) temporal lobe in each patient. Typical waveforms of interest were epileptiform spikes, sharp waves, and sharp and slow wave complexes. They concluded that the probability densities of interictal epileptic energy fluctuations and the quiescent time between successive fluctuations exhibit power-law scaling, which provides evidence for SOC in human epileptic hippocampus. They hypothesized that interictal epileptiform discharges are a mechanism for energy release within epileptic brain, and that these events may provide a method for identifying the network involved in seizure generation and they even may assure a physiological mechanism for preventing seizures (Worrell et al. 2002).
15.3 Epileptic Seizures
15.3.1 Definitions, Cause, Symptoms and Features of Epileptic Seizures
Epilepsy has been defined as a chronic, complex neurological disorder associated with abnormal electrical activity in the brain, marked by sudden recurrent episodes of sensory/motor disturbance, changes in behavior, with or without loss of consciousness, and/or convulsions (Nunes et al. 2012). This excessive activity can be produced by hyperexcited neurons acting independently or involve abnormal interactions among many neurons (Hobbs et al. 2010). Epilepsy is one of the most common chronic neurological affections, with more than 50 million patients worldwide and approximate 2 million new cases each year. Epilepsy responds to treatment in about 70 % of cases. Not all cases of epilepsy are lifelong, a substantial number of people improve to the point that medication is no longer needed. One seizure does not signal epilepsy (up to 10 % of people worldwide have one seizure during their lifetimes) (Chang and Lowenstein 2003). Epilepsy has been defined since 2005 by two or more unprovoked seizures, >24 h apart (Fisher et al. 2005). The definition of epilepsy has been recently revised in order to consider the diagnosis even after occurrence of a single unprovoked seizure, providing that we can demonstrate an enduring predisposition for recurrence, similar to the general recurrence risk after two seizures (more than 60 %) over the next 10 years. For instance a patient can have a single unprovoked seizure after trauma, a stroke, or central nervous system infection. A patient with such brain disorders has a high risk of developing epilepsy after a single unprovoked seizure.
The tendency to respond to particular stimuli with seizures also meets the conceptual definition of epilepsy. Reflex epilepsies are also associated with an enduring abnormal predisposition to have recurrent seizures.
The document also mentions as a general agreement that epilepsy should no longer be considered a disorder but a disease, the term implying a more substantial, long lasting derangement of neuronal functionality (Fisher et al. 2014).
15.3.1.1 Classification of Seizures and Epilepsies
Generalized epileptic seizures are defined as originating within bilaterally spread networks that, at some point, are rapidly involved. These networks do not necessary include the entire cortex and could involve both cortical and subcortical structures. Generalized seizures can be asymmetric in clinical appearance. Focal epileptic seizures are defined as originating within networks restricted to one hemisphere. They may be localized in a small area or have a wider spread. Depending on the seizure type, ictal onset (“area of cortex that initiates clinical seizures” (Rosenow and Luders 2001) is consistent from one seizure to another), with distinctive propagation patterns that can sometimes imply the contralateral hemisphere. Focal seizures might provoke alteration of consciousness or awareness and could evolve into secondary tonic-clonic generalization. Auras are purely subjective clinical manifestation usually occurring at the very onset of a focal seizure as a warning event. The epileptic nature is sometimes difficult to prove if the aura symptoms do not evolve into a more objective clinical pattern (Luders et al. 1998). Focal seizures might develop abruptly without any warning symptoms.
15.3.1.2 Cause Types (Etiology)
The International League Against Epilepsy (ILAE) Commission on Classification and Terminology has simplified the classification of seizures and they proposed the following three concepts:
-
1.
Genetic epilepsy represents the result of a known or supposed genetic error in which seizures are the main symptom of the disorder. In recent years, some types of epilepsy have been correlated to mutations in genes, mostly involving ion channels, assumptions derived from specific molecular genetic studies or from suitable adapted family studies. For example a mutation in the GABA1 gene has been detected in some members of a family with juvenile myoclonic epilepsy (Cossette et al. 2002). No definitive conclusions can be made because there is no knowledge regarding specific surrounding influences as causes of or factors that contribute to these forms of epilepsy.
-
2.
Structural or metabolic: This type of epilepsy refers to a condition or disease with a metabolic or structural background associated with a high risk of developing epilepsy. Structural lesions are frequently associated with focal seizures. They include cerebral changes resulting from, trauma, stroke, perinatal brain damage, malformations, brain tumors, and infections. These epilepsies are characterized by the presence in epileptogenic foci of residual neurons with no afferents and less dendritic spines, destroyed probably by infections, trauma, stroke, or other lesion. Structural lesions of the cerebral cortex can disrupt, inhibitory GABAergic interneurons, thereby minimizing the inhibition that controls large pyramidal cells (Menzler et al. 2011).
-
3.
“Unknown cause”: There are several cases wherein the underlying pathophysiology can’t be identified. These are usually encountered in children and young adults but can occur at any age in persons who have a family history of epilepsy or seizures. This epilepsy may have a genetic defect at its origin or it may be the result of a distinct and yet unknown disorder (Berg et al. 2010).
15.3.2 Power Law Distributions in Epilepsy
Diseases of central nervous system are often associated with altered brain dynamics (Expert et al. 2010). It has been hypothesized that the dynamical properties characterizing a critical state may be considered as an important marker of brain well-being in both health and disease (Plenz 2012). During epileptic seizures the distribution of phase-locking intervals (PLI) is providing additional evidence for the criticality hypothesis. Furthermore, deriving the distribution of PLI from electrocorticogram (ECoG) data as an indicator of critical brain dynamics has shown that the system deviates from scale-free behavior during seizures. All scales closely follow a power-law probability distribution during pre-ictal time intervals with the exponent between 22 and 23.5 (see Fig. 15.2). The apparent robustness of the power-law against exact conditions (different anatomical regions with varying number of channels) strengthens the hypothesis of the relevance of a critical state in human brain dynamics. While the PLI distribution followed a power-law in time intervals preceding the seizure onset, a deviation from power-law behavior was observed in intervals containing the seizure attack.
The distribution of phase-locking intervals deviates from a power-law during epileptic seizures. Top: The electrocorticogram (ECoG) recording shows the onset of a focal epileptic seizure attack around 300 s time. Bottom: Cumulative distributions of phase-locking intervals (PLI) are obtained during three time intervals of 150 s: preictal (left), ictal (middle) and postictal (right). Dashed lines indicate a power-law with exponent 23.1. While the distribution appears to follow a power-law during the pre-ictal period, intervals of increased phase-locking disturb this characteristic distribution with the onset of seizure activity. Data shown are from a patient scale 3, corresponding to the frequency band 25–12.5 Hz (Adapted with permission from Meisel et al. 2012, PLOS Computational Biology)
Figure 15.2 shows the distribution of PLI derived from a pre-ictal, an ictal and a postictal time interval. The probability to find longer PLI increased during attacks thereby destroying the scale-free property of the original distribution. After the seizure this distribution slowly relaxed back to a power-law. In Fig. 15.2 this relaxation is not yet complete in the postictal time interval as there is still some residual seizure dynamics in the ECoG recording.
15.3.3 Self-Organized Criticality in the Brain
Critical systems have been defined as systems that are close to a critical point, near the boundary of an order-disorder phase transition. At criticality, these systems can avoid being trapped in one of two extreme cases: a disordered state (when interactions are too weak and the system is dominated by noise) or a globally ordered state in which all elements are locked (when interactions are too strong and the system is completely static). A dualism is essential for a complex system, like the brain, to function: it must maintain some order to ensure coherent functioning (i.e., generate a reproducible behavior in response to a certain stimulus) while allowing for a certain degree of disorder to enable flexibility (i.e., adapt to varying external conditions). Such dualism is possible at criticality.
While many degrees of order/disorder are possible, the subtle balance between order and disorder at criticality manifests itself in certain general statistical properties: critical systems exhibit spatial and temporal correlations that are long range (i.e., on scales that are larger than those on which mutual interactions take effect) and follow power-law distributions. Recent research has shown that brain networks, by being in the critical state, optimize their response to inputs and maximize their information processing ability (Shew and Plentz 2013).
Haimovici and colleagues have presented a simple brain model that, if tuned to criticality, explains the broad range of experimental observations of human brain activity, in particular, reproducing key findings obtained with functional magnetic resonance imaging (fMRI) (Haimovici et al. 2013). fMRI research has been able to deliver an important observation: the human brain at rest exhibits a large-scale spatiotemporal organization into distinct functional networks—so-called resting state networks (RSN) (Fox and Raichle 2007). RSNs are areas of the resting brain—measured in subjects performing any cognitive, or motor tasks—in which fluctuations of neural activity are correlated, as revealed by the fact that BOLD signal fluctuations within the same network are synchronous. Each RSN can be related to a specific set of cortical areas associated with certain functions: cognitive, sensory (visual, auditory), and motor RSNs, for instance, have been identified (see Fig. 15.3).
Functional magnetic resonance imaging (fMRI) experiments have revealed that the brain at rest is organized into several areas in which fluctuations of brain activities are correlated, so-called resting state networks (RSN). From top to bottom: medial visual (VisM), lateral visual (VisL), auditory (Aud), and sensory-motor (SM) RSNs. (Right columns) Results from the work of Haimovici et al. 2013 show that a simple model can reproduce the statistical properties of RSNs only if the model is tuned to criticality (at TC) (Adapted with permission from Haimovici et al. 2013, Phys Rev Letters)
What Haimovici and colleagues found is that such a model can lead to activity clusters similar to those found for RSN activity (Haimovici et al. 2013). But for the model to match the experimental data, the activation threshold had to be set exactly at the level at which their model becomes critical, as illustrated in Fig. 15.3. At criticality, their model predicts a number of statistical properties that are consistent with experiments: the brain forms activity clusters whose size follows a power law with slope of −3/2, the hallmark of neuronal avalanches (Beggs and Plenz 2003), corresponding to a peak in the size of the second-largest cluster, as found in percolation models (Margolina et al. 1982); the correlation length (the distance at which two points in the system behave independently) and its fluctuations diverge and match those seen in human brain data.
15.4 Modular Signatures in Epileptic Seizures
15.4.1 Functional vs. Structural Modularity of the Epileptic Brain
The human neocortex consists of a large number of minicolumns in parallel vertical arrays (Mountcastle 1957, 1997; Buxhoeveden and Casanova 2002; Casanova et al. 2007; Shepherd and Grillner 2010). Minicolumns are the first step in a nested series of nodes or echelons of increasing complexity (Mountcastle 1997; Szentagothai 1975). Within minicolumns, cortical neurons are aggregated into five horizontal layers (or laminae), namely two supra-granular, one granular and two infra-granular layers. Other levels of modular organization include multiple minicolumns, macrocolumns, and large-scale networks of macrocolumns that are interconnected with the entire brain (Buxhoeveden and Casanova 2002; Opris and Casanova 2014).
In contrast to the sparse but organized connectivity between the modules of control subjects, brain connectivity of epileptic patients shows a configuration where nodes in a functional module are more connected to different functional modules. Recently, Vaessen et al. (2014) examined connectivity of whole brains of children with frontal lobe epilepsy (FLE) and compared their structural and functional connectivity with the same in healthy controls. Their measurements of the functional connectivity was derived from the dynamic fluctuations of the fMRI, while the structural connectivity was determined from fiber tractograms of diffusion weighted MRI. The whole brain network patterns of connectivity were characterized with graph theoretical metrics and further decomposed into modules. Then, the graph metrics with the extracted connectivity within and between modules were related to cognitive performance.
As shown in Fig. 15.4a, the modularity algorithm extracted four modules from the averaged functional connectivity matrix over all subjects. Spatial organization of module #1 was considered the “default mode network” with network nodes distributed in the frontal, temporal and parietal lobes (Vaessen et al. 2014). Module #2 was distributed over the frontal and subcortical regions. Module #3 was localized in the occipital lobe. Module #4 nodes were distributed over frontal, temporal and occipital regions. All four modules seemed to be symmetric to the inter-hemispheric fissure. As shown in Figs. 15.4a, b when the structural connections were organized according to the modularity index of the frontal cortex, the structural modularity revealed bilateral structural sub networks. For the structural connectivity matrix the modularity algorithm determined only two modules, which were highly symmetric over the two hemispheres. After functional connections were organized according to these structural connectivity modules, the sub organization vanished (Fig. 15.4c, d). An interactive view of functional connectivity and structural connectivity was displayed as between-module, within-module averaged over all modules and individual within-module connections (Fig. 15.4e, f). It was further shown that functional “disturbances” of epileptic children were related to increased clustering and stronger modularity compared to healthy controls, which was accompanied by stronger within- and weaker between-module functional connectivity. While structural modularity increased with stronger cognitive impairment, it was concluded that decreased coupling between large-scale functional network modules may represent a hallmark for impaired cognition in childhood FLE.
Functional vs. structural brain modularity. (a) Functional connectivity showing the average connection matrices sorted by module. Colored rectangles indicate the modules. High within-module connectivity is illustrated by the higher values (more hot colors), while between-module connectivity is shown by more sparse (more cold colors). (b) Structural connectivity was sorted by functional modules. The functional modules are organized bilaterally, while the structural connectivity has strong inter-hemispheric connectivity and low intra-hemispheric connectivity clearly visible in the block patterns. (c) Functional connectivity matrix was sorted by the modular organization derived from the structural connectivity. The two structural connectivity modules represent the left and right hemisphere. Functional connectivity shows that strong inter-hemispheric connections are present within the two modules. (d) The structural connectivity was sorted by structural connectivity modularity. Strong intra-hemispheric connections are obvious, while inter-hemispheric connections (and thus between-module connections) are weaker. (e) Illustration of modular organization of functional connectivity. Within-module connections are colored as in panel A. (f) An alternative presentation of the modular organization. The nodes of each separate module are depicted spatially segregated. The gray lines indicate the between-module connections (Adapted with permission from Vaessen et al. 2014, PLOS one)
15.4.2 Spatial Scale of Epileptogenicity Biomarkers Matches Minicolumns Size
High-frequency oscillations (HFO) in the 80(100)–500 Hz range are considered an important biomarker of cortical epileptogenicity. Although HFO may be present in non-epilepogenic brain structures, it has been shown that the ripple (<250 Hz) and particularly fast ripples (>250 Hz) subbands have are highly specific to the seizure onset zone (Zijlmans et al. 2012). Using dense 2D microelectrode arrays (MEA) having a spacing of 0.4 mm between electrodes, Schevon and colleagues have shown that about ~90 % of the recorded HFOs were limited to a single channel (Schevon et al. 2009). This is consistent with the idea that most of the spontaneous HFO events are confined within a minicolumn, and spreading the activity requires a recruitment process of several minicolumns, possibly through an avalanche process following a power-law, as suggested by several other studies using intracranial recordings (Worrell et al. 2002; Wu et al. 2014).
Sub-clinical micro-seizures confined to a spatial domain matching the size of a minicolumn (~0.5 mm) were observed using a combination of micro- and macro-contact cortical grids and depth electrodes on domains in a study by Stead and colleagues (Stead et al. 2010). They hypothesize that ‘relatively sparse pathological cortical columns are the anatomical substrate of focal neocortical epilepsy’, or ‘the sick column hypothesis’. Such minicolumns with pathological neurons or pathological connectivity (intra- or inter-columnar) may be the initiators of a seizure, as epileptiform discharges have been detected early during the ictal onset in microcontacts, before being detected on the macrocontacts (Stead et al. 2010; Zijlmans et al. 2012).
15.4.3 Does Cortical Columnar Firing in Layer 2/3 Get Amplified Across Neighboring Minicolumns?
A great deal of structural and functional implications emerged with the decreased coupling between large-scale functional network modules resulting in impaired cognition in epilepsy. It is intriguing to know what happens at the minicolumn and microcircuit level during epileptic seizures. Does cortical columnar firing in layer 2/3 (Wu et al. 2014; Giresh and Plenz 2008) get amplified across neighboring minicolumns?
During “neural avalanche” the firing pattern of pyramidal cells gets excessively amplified. Such synchronized amplification is also reflected by the 8 Hz multiple harmonics (Fig. 15.5a–c). According to such modular view of epilepsy, one hypothesis posits that such excessive amplification of neural firing in a cortical minicolumn may be the outcome of pyramidal cells crosstalk that pierce the minicolumnar inhibitory wall surrounding the pyramidal cells. Indeed if that would be the case layer 2 and 3 cells that have pyramidal cells extend cortico-cortical projections intra- and inter-hemisphere and the excitation is thus spread across many areas (Fig. 15.6). This excitation is then sent through the minicolumns top-down to the subcortical structures that deal with the behavior, resulting in massive seizures with uncoordinated movements. The minicolumn hypothesis fits also with local generation of epileptic seizures in cortical microcircuits.
Illustration of synchronized burst in epilepsy. (a) EEG data from intracranial depth electrodes showing seizure initiation on contacts B03-B04 and propagation on contacts B01-B02, B06-B07, C01-C02 and T01-T06; (b) and (c) Time-frequency maps for contacts B04(b) and C01(c) showing sustained 8 Hz activity and multiple harmonics
Instantaneous spatial extent of the epileptic seizure. (a) Raw (unfiltered) EEG data from intracranial depth electrodes showing seizure initiation on electrode B and propagation on electrodes C and T. The red arrow represent the timestamp at which the EEG voltage maps are created. Maximum intensity projection voltage maps in axial (b), sagittal (c) and coronal (d) views, where green dots are contacts included in the montage and red dots are contacts not included in the montage (The EEG data is represented in color code: red for higher EEG amplitude (voltage), blue for lower EEG amplitude (voltage))
15.5 Future Directions
15.5.1 Future Directions in Treating Epilepsy
In contrast to the rapid advances in other therapies, epilepsy medical therapy was usually limited to anti-epileptic drugs. Yet, approximately 34 % of the patients suffering from epilepsy are described as medically intractable epilepsy patients which still suffer from sustained frequent seizures in defiance of receiving adequate treatment with anti-epileptic drugs of sufficient duration (Kwan and Brodie 2000). Recent studies show that the notable advance in the field of inflammation and immunology are estimated to be important elements of the pathobiology of epilepsy that may offer future directions in treatment of this disease. Inflammatory processes have been noticed in the brain tissue of both experimental animal models and subjects with epilepsy. Anti-inflammatory and immunotherapies demonstrated considerable anticonvulsant properties both in experimental and in clinical findings. These arguments denote the fact that modulation of inflammatory processes and immunity could represent a new particular objective to accomplish eventual anticonvulsant effects for the subjects with epilepsy, especially for those suffering of medically intractable epilepsy (Yua et al. 2013). For modern neurosurgery, the aim is to find a procedure which is minimally invasive and does not involve a large craniotomy thus avoiding cognitive morbidity and decreasing hospital stay.
15.5.1.1 Responsive Neurostimulation (RNS)
An additional treatment option for those suffering from medically intractable epilepsy, consists in direct brain stimulation with a system that provides responsive focal cortical stimulation (RNS System). The aim is to interrupt epileptiform abnormalities before the seizure starts. The RNS System detects epileptiform activity with electrodes placed in the brain. A programmable brain implanted neurostimulator provides control on seizure focus. Subdural cortical strip leads and/or one or two brain depth electrodes are surgically implanted, according to the seizure onset zone. These electrodes are connected to the stimulator. This device permanently detects and analyze brain electrical activity and is programmed to select specific electrical patterns and then to generate pulses to control seizures in a closed loop feed-back. Heck and coworkers studied the safety and effectiveness of this method in 191 patients with medically intractable partial onset seizures emerging from one or two foci. After a 2 years of post-implant follow-up they demonstrated that responsive stimulation of the seizure onset zone, decreased the frequency of seizures and revealed seizure reduction over time, being acceptably safe and well tolerated (Heck et al. 2014).
15.5.1.2 Stereotactic Laser Ablation
Stereotactic laser ablation of the epileptogenic structures may be an alternative to standard epilepsy surgery. Laser ablation leaves areas situated in the pathway to the target largely intact. This technique could be correlated with fewer deficits by avoiding important network regions and critical white matter pathways. Particularly when guided by MRI it allows real time thermal monitoring of the resection and also provides feedback for the laser energy delivery (Curry et al. 2012; Tovar-Spinoza et al. 2013).
15.5.1.3 Vagus Nerve Stimulation
Vagus nerve stimulation can be used to prevent epileptic seizures by sending regular, mild electrical pulses to the brain via the vagus nerve. These pulses are supplied by a pacemaker-like device. The pulse generator is surgically placed in the upper part of the chest, under the skin. A connecting wire passes from the stimulator to an electrode that is adjacent to the vagus nerve, which is accessible through a small incision in the cervical area. After it is implanted, depending on the patient’s tolerance, the device may be programmed to stimulate the nerve at regular intervals. The settings on the stimulator are adjustable, and the electrical pulses are gradually raised as the subject’s tolerance to the pulses increases. A certain number of patients suffering from medically intractable epilepsy have undergone implantation of the left vagus nerve. Less than half of the subjects reported more than 50 % diminution in seizure frequency and few patients became seizure free (Ramsey et al. 1991).
AspireSR generator is a new technology which provides automatic stimulation in response to seizure detection. This technology is based on the fact that seizures are most of the time being accompanied by ictal tachycardia. This generator can analyze relative heart rate changes in order to adapt to seizures control. The stimulation can be activated manually when the patient anticipate the onset of a seizure, by using a hand-held magnet. This kind of stimulation has been proven to reduce seizure severity, shorten or stop a seizure, and ameliorate or reduce the postictal recovery period (Cyberonics 2014).
15.5.1.4 Transcranial Magnetic Stimulation
Repetitive transcranial magnetic stimulation (rTMS) may be considered a new therapeutic tool in treating epilepsy. It can be used to control seizures even though its safety and tolerability among the patients with epilepsy are still discussed in the literature. Hsu et al. (2011) did a meta-analysis on 164 participants in previous studies during twenty years period (1990–2010) to estimate the antiepileptic efficacy of rTMS (low frequency repetitive transcranial magnetic stimulation) in medically intractable epilepsy. They concluded the fact that low frequency rTMS has a favorable effect on seizure control, particularly evident in subjects with cortical dysplasia or neocortical epilepsy. These findings require confirmation in extensive studies.
15.5.1.5 Biomarkers for Seizure Detection
15.5.1.5.1 Electrophysiological Biomarkers
It is necessary to identify and validate electrophysiological biomarkers of epilepsy in order to diagnose, prevent, treat and cure epilepsy. The aim is to identify the epileptogenic zone and obtain functional mapping that could be used to determine if epilepsy surgery can be performed and to define risk /benefit ratio. Tailored resections are recommended. Electrophysiological biomarkers of epilepsy may be high-frequency oscillations which consist of ripples 80(100)–250 Hz and fast ripples >250 Hz. High-frequency oscillations (HFO) could be present in normal brain, for instance in area CA1 of the hippocampus (Buzsaki et al. 1992). Sensory-evoked high-frequency oscillations could also be present in CA3, subiculum and the entorhinal cortex (Chrobak and Buzsaki 1996, Csicsvari et al. 1999). Interictal fast ripples (250–600 Hz) are strongly associated with brain areas capable of generating spontaneous seizures (Zijlmans et al. 2012). Ripples in the dentate gyrus and the neocortex should be considered pathological HFOs. The association between pathological HFOs and epileptogenicity suggests pathological HFOs could support the localization of the seizure onset zone and might identify the epileptogenic zone more accurately. Interictal spikes represent a good spatial biomarker for the seizure onset zone and the irritative zone. However, there is little evidence that interictal spikes predict seizure frequency or the disease's severity. The functional role of interictal spikes in epilepsy is not known, but some interictal spikes might reduce ictal discharges. In a different view, the presence and clustering of interictal spikes after induced status epilepticus in rats could predict the subsequent appearance of spontaneous seizure (Staba et al. 2014).
15.5.1.5.2 Cortical Excitability (CE)
Transcranial magnetic stimulation (TMS) is a noninvasive and safe method to explore cortical excitability (CE). Bauer et al. 2014 reviewed fifty studies that determined CE in patients with epilepsy and most of them revealed cortical hyperexcitability, which can be improved with anti-epileptic drug treatment. Other studies state that reduction of CE after resection (epilepsy surgery) is an important predictor of good seizure outcome. Cortical direct electrical stimulation (DES) studies have indeed confirmed an increased excitability in the seizure onset zone (Iwasaki et al. 2010; Enatsu et al 2012). Cortical excitability may therefore be a feasible biomarker for epilepsy.
15.5.1.5.3 Molecular Biomarkers
Notable advance has been made recently concerning biomarkers of epilepsy. Analysis on the genomic, proteomic and mRNA levels were made from samples of brain tissue or cerebrospinal fluid, blood, plasma, or serum. The purpose is to discover specific molecular biomarkers. Similar to the role of molecular biomarkers for brain tumors, a temporary dysfunction of the blood–brain barrier may contribute to epileptogenesis. Combining molecular analysis with other procedures, such as imaging and electrophysiological biomarkers could be beneficial for understanding and treatment of epileptic patients (Lukasiuk and Becker 2014).
15.5.2 Control of Epilepsy
Device controlled epilepsy is an option that has been developed recently to help patients with drug resistant epilepsy. Among the emerging applications are anti-epilepsy neural implants and the nanostructures such as carbon nanowire/nanotubes that allow the drug to reach the critical location in the brain (Opris 2013).
15.5.2.1 Anti-epilepsy Neural Implants
An anti-seizure neural implant should operate on the brain signals that cause the epileptic seizures in order to maintain its proper functioning. Anderson et al. (2013) has shown high-frequency spectral changes in the prefrontal cortex of primates with potential use in neuoroprosthetics. In another study (Johnson et al. 2012), 5 human subjects were implanted with electrocorticography (ECoG) arrays for long-term epilepsy monitoring. Subjects were trained on a brain-computer interface to control a cursor on a computer screen by modulating the spindle activity involved in learning. The fact that the pattern of increased spindle activity was locally modulated by training on a brain-computer interface, supports the idea of future neural implants to control epilepsy.
15.5.2.2 Nanotechnological Applications to Control Epilepsy
The nanomaterials play a critical role in controlling the multitude of events that determine the composition and structure of the elements for brain machine interface. A number of approaches that allow the control at the nano-level (metals, carbon nanowires/nanotubes and organic conducting polymers) have revealed a critical relationship between nanostructure features and cellular behavior (Wallace et al. 2012). In this regard, epilepsy related drugs, delivery systems and devices, seem to operate successfully based on nanotechnology (Bennewitz and Saltzman 2009; Pathan et al. 2010). Thus, the nanotechnology for delivery of drugs to the brain for epilepsy treatment use drug loaded nanoparticles with improved efficiency.
15.5.2.3 Nano-devices for Epilepsy Control
The possibility to use small scale, monolithic integrated circuits, allows the easy and efficient implantation of a whole energy efficient system. Neuroprosthetics for Parkinson’s patients send electrical pulses to the deep brain structures, with the aim of reducing or eliminating symptoms. Still, to have a more complete system, one could take into consideration the feedback-loop design. In such a case, the device would pick up the signals related to the intention of the user and format them back to the brain in such a way that the effects of neurodegenerative diseases are reduced.
The implementation of such as device should be as minimally invasive and precise as possible. Battery supply of such devices can be either long-term, depending on energy consumption or rechargeable using induction coils. One major obstacle for Brain-Machine Interfaces (BMI) today represents the lack of upgrade-ability of the hardware of a device, once implanted. For each physical change, the patient must be operated again. A proposed solution to this question is the “Neural Dust” (Seo et al. 2013), based on micrometre size, ultra-low power CMOS “node” sensors used for detection and reporting of extracellular data and a central control unit. The device aims to replace the electrode implantation that is in use today. The free-floating sensors are powered ultrasonically by the transmitters and use backscatter communication. In detail, the main control unit is powered by a standalone radio frequency (RF) transmitter on the skin of the skull, completely wireless. This type of power and communication transmission reduces significantly the risk of infection associated with standard wire implants.
Ultrasound EM (electromagnetic waves) frequency was chosen for power and communication transfer because the tissue attenuation is far less when compared to standard micro-wave frequencies used for wireless today. The sensors are using piezo-electric transducers, since they seem to be best suited for the application. The software of the system is based on the function of the device: feedback-loops will allow the control of Parkinson’s Disease and Epilepsy. Currently solutions are employed with varying success on breaking down the neural electrical code. Sending the right electrical signals to the epileptic tissue should stop its propagation. But being able to recognise the beginning of an ictal phase is possible only using learning algorithms such as support vector machines, since the patients signals are different. The algorithm itself is used as an early-warning system, based on the analysis of electroencephalogram recordings (EEG) that enables the controlling part of the device to enable suppressing signals to the affected tissue. Current best-performing algorithms allow around 70 % accuracy. Improvement in this field will produce an automated stimulator for the patients (Wang 2013).
In conclusion, as recent results point out, the pattern of amplified firing in neural avalanches betrays a modular signature in the spread of activation across cortical minicolumns. According to such modular approach of epilepsy, it is likely that the excessive amplification of neural firing in a cortical minicolum results from a defect within the “inhibitory curtain” surrounding the pyramidal cells (Opris and Casanova 2014). The functional basis of this approach provides valuable insights into potential clinical interventions.
References
Anderson NR, Blakely T, Brunner P et al (2013) High-frequency spectral changes in Dorsolateral Prefrontal Cortex for potential neuoroprosthetics. In: Conference proceedings of the IEEE engineering in medicine and biology society 2013, pp 2247–2250
Bak P (1996) How nature works: the science of self-organized criticality. Copernicus, New York
Bak P, Tang C, Wiesenfeld K (1987) Self-organized criticality: an explanation of the 1/f noise. Phys Rev Lett 59:381–384
Bauer PR, Kalitzin S, Zijlmans M et al (2014) Cortical excitability as a potential clinical marker of epilepsy: a review of the clinical application of transcranial magnetic stimulation. Int J Neural Syst 24(2):1430001. doi:10.1142/S0129065714300010
Beggs JM (2008) The criticality hypothesis: how local cortical networks might optimize information processing. Phil Trans R Soc A 366(1864):329–343
Beggs JM, Plenz D (2003) Neuronal avalanches in neocortical circuits. J Neurosci 23:11167–11177
Bennewitz MF, Saltzman WM (2009) Nanotechnology for delivery of drugs to the brain for epilepsy. Neurotherapeutics 6(2):323–336
Berg AT, Brkovic SF, Brodie MJ et al (2010) Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005–2009. Epilepsia 51(4):676–685
Blackspear M, Roberts JA, Terry JR et al (2006) A unifying explanation of primary generalized seizures through nonlinear brain modeling and bifurcation analysis. Cereb Cortex 16(9):1296–1313
Buxhoeveden D, Casanova M (2002) The minicolumn Hypothesis in neuroscience. Brain 125:935–951
Buzsaki G, Horvath Z, Urioste R et al (1992) High-frequency network oscillation in the hippocampus. Science 256:1025–1027
Casanova MF, Trippe J, Switala A (2007) A temporal continuity to the vertical organization of the human neocortex. Cereb Cortex 17(1):130–137
Chang BS, Lowenstein DH (2003) Epilepsy. N Engl J Med 349(13):1257–1266. doi:10.1056/NEJMra022308, PMID 14507951
Chrobak JJ, Buzsaki G (1996) High-frequency oscillations in the output of the hippocampal–entorhinal axis of the freely behaving rat. J Neurosci 16:3056–3066
Cossette P, Liu L, Brisebois K et al (2002) Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat Genet 31(2):184–189
Csicsvari J, Hirase H, Czurko A et al (1999) Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci 19(1):274–287
Curry DJ, Gowda A, McNichols RJ et al (2012) MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav 24(4):408–414
Cyberonics (2014) Cyberonics announces CE mark approval of the aspireSR™ generator for VNS therapy®. http://ir.cyberonics.com/releasedetail.cfm?ReleaseID=826981 Accessed 22 July 2014
Enatsu R, Piao Z, O'Connor T et al (2012) Cortical excitability varies upon ictal onset patterns in neocortical epilepsy: a cortico-cortical evoked potential study. Clin Neurophysiol 123:252–260
Expert P, Lambiotte R, Chialvo DR et al (2010) Self-similar correlation function in brain resting-state functional magnetic resonance imaging. J R Soc Interface 8(57):472–479
Fisher RS, van Emde BW, Blume W et al (2005) Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46:470–472
Fisher RS, Acevedo C, Arzimanoglou A et al (2014) A practical clinical definition of epilepsy. Epilepsia 55(4):475–482
Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8(9):700–711
Giresh E, Plenz D (2008) Neuronal avalanches organize as nested theta and beta/gamma-oscillations during development of cortical layer 2/3. Proc Natl Acad Sci U S A 105(21):7576–7581
Haimovici A, Tagliazucchi E, Balenzuela P et al (2013) Brain organization into resting state networks emerges at criticality on a model of the human connectome. Phys Rev Lett 110:178101
Heck CN, King-Stephens D, Massey AD et al (2014) Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia 55(3):432–441
Hobbs JP, Smith JL, Beggs JM (2010) Aberrant neuronal avalanches in cortical tissue removed from juvenile epilepsy patients. J Clin Neurophysiol 27:380–386
Hsu D, Beggs JM (2006) Neuronal avalanches and criticality: a dynamical model for homeostasis. Neurocomputing 69:1134–1136
Hsu D, Tang A, Hsu M et al (2007) Simple spontaneously active Hebbian learning model: homeostasis of activity and connectivity, and consequences for learning and epileptogenesis. Phys Rev E Stat Nonlin Soft Matter Phys 76(4 Pt 1):041909
Hsu D, Chen W, Hsu M et al (2008) An open hypothesis: is epilepsy learned, and can it be unlearned? Epilepsy Behav 13(3):511–522
Hsu WY, Cheng CH, Lin MW et al (2011) Antiepileptic effects of low frequency repetitive transcranial magnetic stimulation: a meta-analysis. Epilepsy Res 96(3):231–240
Iwasaki M, Enatsu R, Matsumoto R et al (2010) Accentuated cortico-cortical evoked potentials in neocortical epilepsy in areas of ictal onset. Epileptic Disord 12(4):292–302. doi:10.1684/epd.2010.0334
Johnson LA, Blakely T, Hermes D et al (2012) Sleep spindles are locally modulated by training on a brain-computer interface. Proc Natl Acad Sci U S A 109(45):18583–18588
Klaus A, Yu S, Plenz D (2011) Statistical analyses support power law distributions found in neuronal avalanches. PLoS ONE 6(5):e19779. doi:10.1371/journal.pone.0019779
Kwan P, Brodie MJ (2000) Early identification of refractory epilepsy. N Engl J Med 342:314–319
Levina A, Herrmann JM, Geisel T (2005) Dynamical synapses give rise to a power law distribution of neuronal avalanches. Neural Information Processing Systems (NIPS). Adv Neural Inf Process Syst 18:771–778
Levinshtein M, Kostamovaara J, Vainshtein S (2005) Breakdown phenomena in semiconductors and semiconductor devices. World Scientific, Singapore
Lüders H, Acharya J, Baumgartner C et al (1998) Semiological seizure classification. Epilepsia 39(9):1006–1013
Lukasiuk K, Becker AJ (2014) Molecular biomarkers of epileptogenesis. Neurotherapeutics 11(2):319–323
Margolina A, Herrmann HJ, Stauffer D (1982) Size of largest and second-largest cluster in random percolation. Phys Lett 49A:73–75
McPhee SJ, Hammer GD (2010) Pathophysiology of disease: an introduction to clinical medicine, 6th edn. McGraw-Hill Medical, New York
Meisel C, Storch A, Hellmeyer-Elgner S et al (2012) Failure of adaptive self-organized criticality during epileptic seizure attacks. PLoS Comput Biol 8(1):1–8
Menzler K, Thiel P, Hermsen A et al (2011) The role of underlying structural cause for epilepsy classification: clinical features and prognosis in mesial temporal lobe epilepsy caused by hippocampal sclerosis versus cavernoma. Epilepsia 52(4):707–711
Mountcastle VB (1957) Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol 20(4):408–434
Mountcastle VB (1997) The columnar organization of the neocortex. Brain 120:701–722
National Institute for Health and Clinical Excellence (2012) The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. National Clinical Guideline Centre, London, pp 21–28
Nunes VD, Sawyer L, Neilson J et al (2012) Diagnosis and management of the epilepsies in adults and children: summary of BMJ. BMJ 344:e281. doi: 10.1136/bmj.e281
Opris I (2013) Inter-laminar microcircuits across the neocortex: repair and augmentation. frontiers. In: Lebedev MA, Opris I, Casanova MF (ed) Systems neuroscience research topic: “Augmentation of brain function: facts, fiction and controversy”. doi:10.3389/fnsys.2013.00080
Opris I, Casanova MF (2014) Prefrontal cortical minicolumn: from executive control to disrupted cognitive processing. Brain. doi:10.1093/brain/awt359
Opris I, Hampson RE, Stanford TR et al (2011) Neural activity in frontal cortical cell layers: evidence for columnar sensorimotor processing. J Cogn Neurosci 23(6):1507–1521. doi:10.1162/jocn.2010.21534
Opris I, Fuqua JL, Huettl PF et al (2012a) Closing the loop in primate prefrontal cortex: inter-laminar processing. Front Neural Circuits 6:88. doi:10.3389/fncir.2012.00088
Opris I, Hampson RE, Gerhardt GA et al (2012b) Columnar processing in primate pFC: evidence for executive control microcircuits. J Cogn Neurosci 24(12):2334–2347
Opris I, Santos L, Gerhardt GA et al (2013) Prefrontal cortical microcircuits bind perception to executive control. Sci Rep 3:2285. doi:10.1038/srep02285
Pathan SA, Jain GK, Akhter S et al (2010) Insights into the novel three ‘D’s of epilepsy treatment: drugs, delivery systems and devices. Drug Discov Today 15(17–18):717–732
Plenz D (2012) Neuronal avalanches and coherence potentials. Eur Phys J Spec Top 205(1):259–301
Ramsey RE, Uthman B, Ben-Menachem E et al (1991) Efficacy of vagal nerve stimulation in partial seizueres: double blind comparison of two stimulus paradigms. Epilepsia 32(suppl):90–91
Rosenow F, Lüders HO (2001) Presurgical evaluation of epilepsy. Brain 124:1683–1700
Royer S, Pare D (2003) Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature 422(6931):518–522
Schevon CA, Trevelyan AJ, Schroeder CE et al (2009) Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain 132(Pt 11):3047–3059
Seo D, Carmena JM, Rabaey JM et al (2013) Neural dust: an ultrasonic, low power solution for chronic brain-machine interfaces, Cornell University Library, arXiv:1307.2196 [q-bio.NC]
Shepherd G, Grillner S (2010) Handbook of brain microcircuits. Oxford University Press, Oxford
Shew W, Plentz D (2013) The functional benefits of criticality in the cortex. Neuroscientist 19:88–100
Sole RV, Valverde S (2008) Spontaneous emergence of modularity in cellular networks. J R Soc Interface 5:129–133
Staba RJ, Stead M, Worrell GA (2014) Electrophysiological biomarkers of epilepsy. Neurotherapeutics 11(2):334–346. doi:10.1007/s13311-014-0259-0
Stead M, Bower M, Brinkmann BH et al (2010) Microseizures and the spatiotemporal scales of human partial epilepsy. Brain 133(9):2789–2797. doi:10.1093/brain/awq190
Szentagothai J (1975) The module-concept in cerebral cortex architecture. Brain Res 95:475–496
Tovar-Spinoza Z, Carter D, Ferrone D et al (2013) The use of MRI-guided laser-induced thermal ablation for epilepsy. Childs Nerv Syst 29(11):2089–2094
Turrigiano GG, Nelson SB (2000) Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol 10:358–364
Vaessen MJ, Jansen JFA, Braakman HMH et al (2014) Functional and structural network impairment in childhood frontal lobe epilepsy. PLoS ONE 9(3):e90068
Varela F, Lachaux JP, Rodriguez E et al (2001) The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci 2:229–239
Wallace GG, Higgins MJ, Moultonand SE et al (2012) Nanobionics: the impact of nanotechnology on implantable medical bionic devices. Nanoscale 4:4327–4347
Wang S (2013) Online seizure prediction using an adaptive learning approach. IEEE Trans Knowl Data Eng 25(12):2854–2866
Worrell GA, Cranstoun SD, Echauz J et al (2002) Evidence for self-organized criticality in human epileptic hippocampus. Neuroreport 13:2017–2021
Wu JJS, Chang WP, Shih HC et al (2014) Cingulate seizure-like activity reveals neuronal avalanche regulated by network excitability and thalamic inputs. BMC Neurosci 15(3):1–17
Yang H, Shew WL, Roy R et al (2012) Maximal variability of phase synchrony in cortical networks with neuronal avalanches. J Neurosci 32(3):1061–1072. doi:10.1523/JNEUROSCI. 2771-11.2012
Yua N, Liub H, Dia Q (2013) Modulation of immunity and the inflammatory response: a new target for treating drug-resistant epilepsy. Curr Neuropharmacol 11:114–127
Zijlmans M, Jiruska P, Zelmann R et al (2012) High-frequency oscillations as a new biomarker in epilepsy. Ann Neurol 71(2):169–178
Acknowledgments
Ana Ciurea was supported by the strategic grant POSDRU/159/1.5/S/133652, “Integrated system to improve the quality of doctoral and postdoctoral research in Romania and promotion of the role of science in society” cofinanced by the European Social Found within the Sectorial Operational Program Human Resources Development 2007–2013.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Ciurea, A. et al. (2015). Modular Signatures and Neural Avalanches in Epileptic Brain Networks. In: Casanova, M., Opris, I. (eds) Recent Advances on the Modular Organization of the Cortex. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9900-3_15
Download citation
DOI: https://doi.org/10.1007/978-94-017-9900-3_15
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9899-0
Online ISBN: 978-94-017-9900-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)