Abstract
It has been well documented that deep brain stimulation (DBS) of the subthalamic nucleus (STN) to address some of the disabling motor symptoms of Parkinson’s disease (PD) can evoke unintended effects, especially on non-motor behavior. This observation has catalyzed more than a decade of research concentrated on establishing trends and identifying potential mechanisms for these non-motor effects. While many issues remain unresolved, the collective result of many research studies and clinical observations has been a general recognition of the role of the STN in mediating limbic function. In particular, the STN has been implicated in impulse control and the related construct of valence processing. A better understanding of STN involvement in these phenomena could have important implications for treating impulse control disorders (ICDs). ICDs affect up to 40% of PD patients on dopamine agonist therapy and approximately 15% of PD patients overall. ICDs have been reported to be associated with STN DBS. In this paper we will focus on impulse control and review pre-clinical, clinical, behavioral, imaging, and electrophysiological studies pertaining to the limbic function of the STN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The subthalamic nucleus (STN) is a small, biconvex structure located deep within the brain (Tan et al. 2006). Over the past decade, the STN has been the subject of significant study because of its dual role in movement and in non-motor behaviors. This small structure is a widely used target for high frequency electrical stimulation therapy, also called deep brain stimulation (DBS). STN DBS has been applied to movement disorders and is best known as a treatment for Parkinson’s disease (PD). While much of the STN is preserved in PD, modulation of its function has been shown to significantly reduce the motor symptoms associated with the disease (Shapiro et al. 2007; Vesper et al. 2002; Williams et al. 2014).
STN Involvement in Limbic Function
DBS of the STN Affects Non-motor Behavior
STN DBS has also been associated with adverse effects in non-motor domains and a wide range of neuropsychiatric changes, including depression (Funkiewiez et al. 2004), anxiety (Kim et al. 2015), suicidal ideation (Funkiewiez et al. 2004), apathy (Drapier et al. 2006), explosive-aggressive behavior (Funkiewiez et al. 2004; Sensi et al. 2004), mania, hypomania (Krack et al. 2001), and impulse control disorders (ICDs) (Volkmann et al. 2010; Moum et al. 2012). Studies that have sought to identify clinical predictors or neurobiological correlates of these changes in human DBS patients suggest that their etiology is multi-factorial, including reduction in dopaminergic medication, certain preoperative risk factors, and the possibility of stimulation infiltration into limbic regions in the vicinity of STN (i.e., serotenergic ascending pathways of the zona incerta (ZI), the medial forebrain bundle (Coenen et al. 2009), or the lateral hypothalamus (Mallet et al. 2002)).
It is worth noting, however, that reports of the neuropsychiatric effects of STN DBS have been inconsistent and, in large part, observations have been collected in an unstandardized fashion (Couto et al. 2014). Contrary findings, particularly those demonstrating improvement rather than exacerbation of behavioral disorders, can be found in the literature. For a comprehensive recent review see the work of Kim and colleagues (Kim et al. 2015).
STN Involvement in Limbic Function: Theories and Clinical Evidence
There is growing evidence to support the hypothesis that the STN itself is involved in processing limbic information and that these adverse changes derive from modulation of limbic-processing neurons within the STN rather than simply nearby structures (Rodriguez-Oroz et al. 2012; Mallet et al. 2007; Krack et al. 2010; Fukaya and Yamamoto 2015). In this view, the STN serves as a way station capable of processing associative and limbic information before being sent to cortical and subcortical regions, thus serving to influence changes in behavior (Demetriades et al. 2011; Temel et al. 2005, 2006). Detailed hypotheses of STN limbic involvement have been advanced (Baláž et al. 2011; Haegelen et al. 2009). For excellent reviews of evidence regarding STN limbic involvement, see the work by Baunez and Lardeaux (Baunez and Lardeux 2011) and Marceglia and colleagues (Marceglia et al. 2011).
The question of how DBS actually alters STN limbic function has received considerable attention, although solutions remain speculative. In addition to the possibility of unintentional electrical current diffusion, the relatively compact size of the STN suggests that a putative limbic region of the STN could be subject to microtraumatic injury during DBS electrode placement (Borden et al. 2014). Alternatively, the small size of the target may contribute to variable electrode placement within the STN, potentially exposing limbic regions to variable amounts of current (York et al. 2009; Accolla et al. 2014). In both cases, these potential outcomes could conceivably vary on a case-by-case basis and explain the relatively unpredictable nature of adverse cognitive outcomes of STN DBS that has been observed (Demetriades et al. 2011; Tsai et al. 2007). Further clinical evidence that modulation of STN activity, rather than that of nearby structures, is occurring in these patients includes the findings that a STN region infarct can result in clinically relevant behavioral changes (Park et al. 2011) and that subthalamotomies have been associated with cognitive and neuropsychiatric changes, including improvement in apathy and depression and increases in disinhibition (Bickel et al. 2010). Finally, it should be noted that the effects of stimulation of structures in the vicinity of the STN have not been comprehensively studied. However, one small study comparing stimulation via contacts in or near the ZI resulted in fewer cognitive-related side effects than contacts within the STN (Burrows et al. 2012). Case reports of hypomania following STN DBS have shown that the hypomanic state can be induced by stimulation via an electrode contact located in the anteromedial STN while contacts at the boundaries of the STN had no effects on such behavior (Alkemade et al. 2015; Mallet et al. 2007).
Electrophysiological and Animal Evidence
Tracer Studies Show Connectivity between STN and Limbic Regions
Haynes and Haber’s well known tracer studies in macaque monkeys demonstrated the presence of cortical projections from limbic-related areas in prefrontal cortex to the STN (Haynes and Haber 2013). A study utilizing both retrograde and anterograde tract tracing in non-human primates recently demonstrated connections between the thalamic parafascicular nucleus (PF) and the STN, although these connections were weak in comparison to the striatum (Tandé et al. 2006). The findings of this study could be interpreted to support the idea of a limbic circuitry connecting known limbic portions of the globus pallidus externus (GPe), globus pallidus internus (GPi), and the substantia nigra pars reticulata (SNr) to the STN and striatum via a PF nucleus way station. Finally, another tracer study identified direct projections from the limbic territory of the globus pallidus externus (GPe) to the anterior and medioventral portion of the STN (Karachi et al. 2005). While neither conclusive nor highly descriptive, the available hodological evidence suggests some role for the STN in the brain’s limbic-related circuits.
STN Lesions, Stimulation in Rodents Elicit Behavioral Changes
Lesions of the STN have been shown to increase depression-like behavior in rats (Klein et al. 2010). Interestingly, bilateral high frequency stimulation of the STN was shown to exert a positive cognitive effect on rats, eliciting decreases in premature responses in a reaction time task (Desbonnet et al. 2004). One provocative electrophysiological study in rats demonstrated that a very high percentage of neurons in the ventral pallidum (VP), a major limbic output region, changed firing patterns in response to stimulation of the STN (Turner et al. 2001). Another study in rats suggested that STN lesions associated with changes in cognitive-related behaviors induced neuronal dendritic rearrangement in the prefrontal cortex and hippocampal neurons (Camacho-Abrego et al. 2014). STN DBS in rats was shown to modulate neurotransmission in limbic brain regions, and specifically spur an increase in dopamine (DA) in the nucleus accumbens (NAc) and a decrease in γ-aminobutyric acid (GABA) in the ventral tegmental area (VTA) (Winter et al. 2008).
Electrophysiological Studies Show STN Modulation During Non-Motor Behaviors
Electrophysiological recordings of event related electrical activities, in both human subjects and in animals, have facilitated an opportunity to study the function of subcortical brain regions including the STN (Rektor et al. 2014). Electrophysiological studies in both PD patients (Kühn et al. 2005) and in animals (Lardeux et al. 2009) have shown modulation of STN neuronal activity during emotional and motivational processes (Krack et al. 2010). For example, differences in STN neuronal firing have been observed when patients with obsessive compulsive disorder (OCD) were compared to PD counterparts without OCD (Welter et al. 2011). In particular, OCD was associated with lower discharge frequency in subthalamic neurons, and OCD severity was positively correlated with intraburst frequency and more oscillations in the low-frequency bands. In another electrophysiology study in OCD patients, the authors identified a population of STN neurons that modulated during decision making and when the subject was “checking.” In this study, subjects had to determine if two images presented sequentially “matched,” but they were given the option to review the images before submitting their answer for feedback. Checking was defined as a reviewing the images before submitting their answer for feedback, a behavior that is known to be exacerbated in OCD. Interestingly, this study also found that 60% of neurons responded to combinations of movement and feedback, suggesting that the STN plays a role in integrating different types of information (Burbaud et al. 2013).
Mounting evidence also suggests that the STN is involved in reward processing and impulse control on some level, and we will discuss these findings later in this review.
Neuroimaging Shows Connectivity between STN and Limbic Regions
Neuroimaging studies have provided compelling evidence supporting the limbic pathway involvement of STN. Studies have shown connectivity between limbic areas of cortex and STN (Brunenberg et al. 2012). Intraoperative fMRI imaging of PD patients during STN stimulation demonstrated simultaneous activation of limbic regions (cingulate and insular cortices) (Knight et al. 2015). Similar studies using PET showed increased activation during STN stimulation in the dorsolateral prefrontal cortex bilaterally (Haegelen et al. 2005) and the anterior cingulate (Sestini 2002).
Neuroimaging Shows Metabolic Changes in STN During Non-Motor Behaviors
A diffusion weighted imaging study demonstrated that the integrity of white matter tracts projecting from cortex to STN predicts the ability to “brake” during a response initiation (Coxon et al. 2012). This phenomenon has been hypothesized to occur to create time needed for action reprogramming (e.g., stopping the interference effects) (Coxon et al. 2012). Another imaging study reported an association between premature responding in healthy humans during a stop signal task and decreased intrinsic connectivity of bilateral STN with bilateral subgenual cingulate and right ventral striatum (Morris et al. 2015). Another intriguing study analyzed changes in autonomic response (via heart rate variability) in “expected” and “unexpected” stimulations of the STN in dorsal, middle, and ventral subthalamic regions. It was shown that the autonomic response threshold was higher in the “unexpected” than “expected” condition for both heart rate and sympathetic responses but only in the ventral part of STN (Lanotte et al. 2005). This finding corroborated results of a previous study which showed that ventral STN DBS evoked variable autonomic responses while the dorsal STN did not tie closely to such responses (Benedetti et al. 2004). Additionally, STN DBS has been shown to induce metabolic modifications in brain regions known to be involved in limbic circuitry (Le Jeune et al. 2010; Hilker et al. 2004). Finally, PET imaging studies have demonstrated that STN DBS can increase apathy and reduce recognition of emotional prosody (Péron et al. 2010) and facial emotion (Le Jeune et al. 2008).
The Tripartite Hypothesis
In 1997, Joel and Weiner comprehensively reviewed primate data pertaining to the topographical organization of STN afferents from the globus pallidus externus (GPe) and STN efferents to the pallidum, striatum and SNr (Joel and Weiner 1997). Based on assumptions drawn from this data, they postulated that the STN was divided into motor, associative, and limbic territories, with each projecting to corresponding areas in the pallidum and striatum (Joel and Weiner 1997).
Since then, imaging studies have been used to support the hypothesis of functional sub-regions within the STN (Sauleau et al. 2005), with limbic, associative and motor regions situated within the anterior, mid and posterior portions of the nucleus respectively (Lambert et al. 2012). Findings of morphological and chemical heterogeneity in the nucleus support this functional organization. For example, GABA-ergic interneurons were found to be significantly more numerous in the ventral STN, a region associated with limbic function (Lévesque and Parent 2005). Furthermore, microinjection of the GABA antagonist bicuculline into the anteromedial STN induced behavioral changes, whereas bicuculline injection into the posterolateral STN induced motor but not behavioral changes (Karachi et al. 2009). Electrophysiological findings bolstered supporters of this hypothesis as a recent study of STN local field potentials (LFPs) found that oscillatory behavior differed by region within the STN (Trottenberg et al. 2007). LFPs are essentially measures of the aggregate electrical current produced by neuronal populations in the vicinity of the electrode contact, and thus they provide a window into neural activity at the population level (Łęski, S et al. 2013). LFP signals are typically analyzed in terms of their power spectra, by which the intensity (or power) of certain frequencies or frequency bands in the signal can be observed. It was shown that a significantly larger beta band (13–30 Hz) power was found in the dorsolateral part of STN compared to the ventral (associative/limbic) part of STN (Trottenberg et al. 2007). Finally, many clinical studies have provided evidence for a functional STN topography. For example, a prospective study comparing STN DBS patients who had experienced neuropsychological sequelae to those who had not reported these effects showed that the occurrence of a post-surgical neuropsychological event (i.e., onset of mania, hypomania, impulse control disorder, or depression) was positively correlated to the presence of an anteriorly located active contact within the ventral portion of the STN (Tsai et al. 2007).
The Tripartite Hypothesis Reconsidered
The advent of ultra high resolution MRI studies has enabled visualization of small structures such as the STN (150mm3) (Forstmann et al. 2012; Keuken et al. 2013), and the tripartite subdivision hypothesis has thus become the subject of renewed debate (Alkemade and Forstmann 2014). A recent study employing three-dimensional analysis of motor and non-motor DBS outcomes demonstrated complex relationships between neuroanatomical region within the STN and different aspects of mood and cognition. Ultimately, the authors’ findings did not endorse the idea of a complete functional segregation within the STN, but rather suggested that the STN was likely a convergence site for functionally distinct afferent and efferent information projecting to motor, cognitive, and limbic regions (Eisenstein et al. 2014). In sum, the evidence that the STN mediates limbic processing appears strong, although precisely when, where, and how the STN mediates non-motor behavior is not well understood.
Impulse Control, Parkinson’s Disease, and the STN: Major Concepts
STN DBS can alter impulse control and may induce or exacerbate impulse control disorders (ICDs) in PD patients. Because the STN’s involvement in impulse control is a focus of the present review, this construct and its dysfunction—impulsivity—will now be discussed. And because a considerable majority of the research performed on impulse control and the STN involves PD patients with ICDs, a focus will be on this phenomenon and this disease population.
A concise and consensual definition of impulsivity remains elusive, in part due to the fact that behaviors considered “impulsive” can be heterogenous and because definitions linked to specific cognitive mechanisms and measured with performance tasks do not always correlate to actual observable impulsive behaviors (Kocka and Gagnon 2014). As Kocka and Gagnon noted in their excellent review of impulsivity definitions, there does seem to be a consensus that impulsivity is a multidimensional construct and that it generally “encompasses a multitude of behaviors or responses that are poorly conceived, premature, inappropriate, and that frequently result in unwanted or deleterious outcomes” (Kocka and Gagnon 2014). They further noted that in many cases, the term “disinhibition” is synonymous with impulsivity. In contrast to impulsivity, compulsivity, well articulated by Mosley and Marsh, reflects “a maladaptive perseveration of behavior that is inappropriate to the situation and results in undesirable consequences” (Mosley and Marsh 2015). For our purposes, we will make all attempts to specify a given study’s dimension of interest within the multi-dimensional construct of impulsivity.
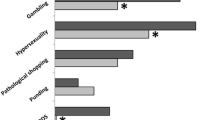
ICDs can manifest in a spectrum of behaviors, including hypersexuality, pathological gambling, compulsive buying, binge eating, hobbyism, and punding (Balarajah and Cavanna 2013). ICDs represent a particular manifestation of dysfunction in impulse/action control; phenomenologically they involve both impulsive (with respect to response initiation) and compulsive (with respect to response termination) dimensions (Mosley and Marsh 2015). ICDs are a fairly common comorbidity of PD, with a prevalence of 6–14% in the PD population (Kim et al. 2015; Zhang et al. 2014). The development of ICDs has been largely associated with the chronic use of dopaminergic agonist (DA) medications; approximately 40% of PD patients on DA therapy may experience an ICD (Garcia-Ruiz et al. 2014). Decreases in these medications often ameliorate ICDs. It would be expected then that reductions in dopaminergic drugs following STN DBS would be associated with a decrease in ICDs. However, change in ICD status post-STN DBS has been at best inconsistent across reported studies (Santangelo et al. 2013). In two large studies, ICDs have been shown to worsen after DBS (Gee et al. 2015). In others, STN DBS has been associated with improvement in, (Gee et al. 2015) and resolution of, pre-existing ICDs, even in studies that control for dopaminergic medications (Demetriades et al. 2011) (Ardouin et al. 2006).
Many of the changes in impulsivity observed clinically have been reproduced in animal models. For example, Baunez and colleagues showed that lesioning the STN (a procedure with comparable motor outcomes to DBS) improved akinesia but induced impulsive behavior in the 6-hydroxy-dopamine rat model of PD (Baunez et al. 1995; Krack et al. 2010) and, later, that bilateral stimulation of the STN induced perseverative behavior in dopamine-depleted rats and in controls (Baunez et al. 2007).
Here, we review the clinical and basic science literature pertaining to the STN’s role in impulse control and related constructs, such as valence processing. Improved understanding of the dynamic role of the STN in impulse control may lead to improved therapies for ICDs in PD and improved methods for delivery of DBS and medication optimization that may possibly decrease ICD-related risk.
Sub-facets of Impulsivity
As Bari and Robbins have noted, impulsivity requires the co-occurrence of dysfunctional inhibitory processes and strong desires or urges to act (Bari and Robbins 2013). Despite the term’s widespread use, “impulsivity” is a heterogeneous construct. Indeed, it has been argued that impulsivity may actually consist of multiple sub-facets that can be studied independently (Dick et al. 2010). Given this heterogeneity, any discussion of the role of the STN in impulse control first requires a framework for what constitutes impulsivity. Recent models have linked five dispositions or traits to so-called rash action: positive urgency (the tendency to act rashly when experiencing a positive mood), negative urgency (the tendency to act rashly when experiencing a negative mood), lack of planning (tendency to act without forethought), lack of perseverance (a failure to remain focused despite distraction), and sensation seeking (a tendency to seek out novel or thrilling stimulation) (Dick et al. 2010).
Given the inherent subjectivity of assessing the aforementioned traits, laboratory tasks have been designed to objectively measure variability in different cognitive processes believed to contribute to impulsive behavior (Dick et al. 2010). In particular, tasks measuring five aspects of impulsive behavior have been advanced: 1) prepotent response inhibition, which refers to “the ability suppress dominant, automatic or prepotent responses”, (examples include the Go-No Go task, the Stop Signal Task, the Continuous Performance Task); 2) resistance to distractor interference, which refers to avoiding interference from task-irrelevant information; 3) resistance to proactive interference, which refers to “resisting memory intrusions of information previously, but no longer relevant to the task”; 4) ability to delay response to obtain a larger reward; and 5) distorted judgments of elapsed time (Dick et al. 2010). Performance on response inhibition and delay discounting tasks are the most widely utilized in human studies, while animal studies utilize these task categories almost exclusively (Dick et al. 2010). Indeed, the latter the two task categories that delineate the most commonly accepted subdivision of impulsivity are response inhibition and delay discounting (Bari and Robbins 2013).
Impulsivity and the Role of Valence
The selection of actions is intimately tied to valence, that is, the degree to which the predicted outcome of a particular action is perceived to be rewarding (positive) or aversive (negative) (Guitart-Masip et al. 2014). It follows that aberrations in the processing of valence may sub-serve aberrations in action control, such as impulsivity. Indeed, ICDs are characterized by repetitive, reward-based behaviors (Okai et al. 2011). A relationship between motor impulsivity scores and neural oscillations during reward and punishment feedback in healthy controls has been recently observed (Leicht et al. 2013). The question then arises: do individuals behave impulsively as a result of a hypersensitivity to prospective reward, a hyposensitivity to prospective loss, some combination of the two, or something else entirely?
Hypersensitivity to reward (Oberg et al. 2011) rather than hyposensitivity to loss (Hewig et al. 2010) has been associated with problem gamblers especially in imaging studies. PD patients with pathological gambling have likewise shown reward hypersensitivity compared to disease-matched controls, with antiglutamatergic therapy (amantadine) associated with decreases in reward hypersensitivity at least in a few patients (Cera et al. 2014). Furthermore, network modeling studies of PD patients with ICDs on dopaminergic medication suggest that these patients are hypersensitive to reward compared to loss, while PD patients with ICDs not on dopaminergic medication were hypersensitive to loss but not to reward (Balasubramani et al. 2015). Meanwhile, PD patients without ICDs showed no difference between reward and punishment learning (Balasubramani et al. 2015). In another study, PD patients with ICDs were shown to exhibit hyposensitivity to loss (Piray et al. 2014). Multiple studies have demonstrated that dopaminergic medications in PD patients—highly associated with ICDs—prevents learning from negative decision outcomes (Frank 2006) (Frank 2005) (Cools et al. 2006) (Frank and O’Reilly 2006). PD was shown to be associated with enhanced devaluation of future rewards, which can be viewed as an indicator of an increased impulsive choice (Evens et al. 2015). Furthermore, rodent studies have shown that impaired response inhibition (a surrogate for impulse behavior) is associated with reward hypersensitivity (Diergaarde et al. 2009).
Finally, the recent work by Guitart-Masip and colleagues deserves mention due its instrumental role in demonstrating not only the interaction between action control and valence, but also the role of the STN in mediating this interaction. In one study, the potential role of STN DBS in modulating a PD patient’s ability to act in different valence contexts was explored. PD patients (both ON and OFF stimulation) and healthy controls (HC) performed the go/no-go task where valence and action were decoupled. Task performance was better for HC when acting for a reward versus acting to avoid punishment. PD patients showed comparable behavior only when STN stimulation was ON, and, in fact performance ultimately exceeded that of HC (Wagenbreth et al. 2015).
In another study, this same task was administered in healthy younger and healthy older adults during structural MRI. Assuming rational behavior, all four action-valence conditions in this task should have been learned equally well. However, it was shown that the study participants, regardless of age, were better at learning to act for reward and not acting to avoid punishment (Chowdhury et al. 2013). These behavioral biases are believed to be hard-wired tendencies that “corrupt” attempts at instrumental learning, that is, attempts to efficiently and appropriately tailor responses to obtain desired outcomes. Interestingly, older participants that were able to overcome these biases exhibited greater structure integrity of the STN (Chowdhury et al. 2013).
A Subthalamic Locus for Valence Processing
It has been hypothesized that stopping an ongoing response involves cortical areas sending a “stop command” to basal ganglia structures, which intercept the go response and in turn decrease excitability of M1 (Bari and Robbins 2013; Greenhouse et al. 2011). The STN serves as a main recipient of cortical stop commands via direct connections to the pre-supplementary motor area (pre-SMA) and inferior frontal gyrus (IFG) (Aron et al. 2007; Bari and Robbins 2013). This so-called hyperdirect pathway permits rapid inhibition of ongoing actions by increasing inhibitory signals from the globus pallidus, thus inhibiting output from the basal ganglia (Bari and Robbins 2013). The engagement of the STN during response inhibition in this manner has been well documented, mainly through electrophysiological studies of PD patients as well as through studies in rats and monkeys (Bari and Robbins 2013). Interestingly, this widely accepted model for STN involvement in response inhibition conceptualizes the STN as a way station for a cortical stop commands but makes no claim about the role of the STN in the valence processingthat is integral to action control. Yet a growing body of evidence suggests STN involvement in value processing. Studies in rats (Kantak et al. 2013; Lardeux and Baunez 2007; Teagarden and Rebec 2007) (Breysse et al. 2015), monkeys (Espinosa-Parrilla et al. 2013) (Espinosa-Parrilla et al. 2015), and humans (Brücke et al. 2007) implicate the STN in mediating valence processing (Mulder et al. 2014).
Valence Processing in the STN: Rodent Studies
STN Neurons Modulate in Response to Reward Expectation, Delivery, and Magnitude
Teagarden and colleagues demonstrated in a rat model that STN neurons increased firing rate prior to reward-based reinforcement and decreased after reward delivery. They also observed increased STN firing rates when reward reinforcement was unexpectedly withheld. Importantly, STN and striatal (STR) neurons were examined concurrently in this study. It was found that both the STN and STR neurons responded to valence-related cues but that response profiles differed, suggesting that STN and STR encode different information (Teagarden and Rebec 2007). A later study by Lardeux and colleagues corroborated these findings, and, in addition, provided evidence that the STN encodes the magnitude of the reward received (Lardeux et al. 2009). In their experiment, rats performed a lever cue test with variable sucrose rewards while single unit activity of STN neurons was recorded. Behavioral data showed significantly faster movement times towards the lever for a 32% sucrose solution compared to a 4% sucrose solution. However, when the 32% sucrose was replaced by pure water, the time to reach the 4% did not change, suggesting that higher reward was preferred to a known and available lesser reward. STN neurons modulated firing rate differentially in response to the receipt of different outcomes, suggesting that STN neurons encode information based on preferred rewards for events, not merely reward in general.
STN Neurons Encode Reward Preference and Reward Prediction Error
A later study by Lardeux and colleagues showed that STN neurons modulated firing rates differentially in response to cocaine and sucrose rewards. Interestingly, this same study showed that when reward values changed (i.e., an expected reward was delayed, changed, not delivered), STN activity also modulated in a way consistent with a role in processing errors in reward prediction (Lardeux et al. 2013).
STN Stimulation and STN Lesions Modulate Perceptions of Reward
A recent study showed that STN stimulation in rats diminished c-Fos gene activation in the striatum that is typically observed during acute cocaine administration, suggesting that STN DBS might decrease the reinforcing properties of the drug (Hachem-Delaunay et al. 2015). In another study, it was shown that STN-lesioned rats showed less impulsive behavior compared to non-lesioned controls in a delay-discounting task where impulsivity was defined as selection of a small immediate reward over a large delayed reward. This finding, however, does not preclude the possibility that STN-lesioned rats are merely more sensitive to reward and may therefore modulate their behavior simply to attain greater reinforcement (Winstanley et al. 2005).
STN lesions (Baunez et al. 2005) and STN DBS (Rouaud et al. 2010) have also been shown to alter rats’ preference for rewards in a context dependent way: both lesioning and stimulation have shown to reduce a rats’ motivation to work for cocaine and to increase motivation to work for food (sucrose), suggesting that STN DBS may exert an effect on the positive affective properties of these rewards (Rouaud et al. 2010). Interestingly, another study by Lardeux and Baunez demonstrated that STN lesions could affect reward motivation in an even more complex way; they showed that STN lesions enhanced the motivation for alcohol in rats with a preexisting high alcohol preference but decreased it in rats that showed a preexisting low preference for alcohol (Lardeux and Baunez 2007). Bilateral STN lesions have also been shown to enhance motivation for food in rats (Baunez et al. 2002).
Valence Processing in the STN: Primate Studies
In a study of single neuron activity in two behaving monkeys, Espinosa-Parrilla and colleagues demonstrated that STN neurons modulated in response to reward during task performance as well as to the expectation of reward delivery when the reward was delayed in time (Espinosa-Parrilla et al. 2013). Interestingly, it was shown that movement-related modulations were combined with reward delivery modulations, suggesting a “convergence of signals related to the animal’s movement and its outcome in the same neurons” (Espinosa-Parrilla et al. 2013). Furthermore, the receipt of a reward was sufficient to induce this modulation; reward-related modulations were not contingent on instrumental response or reward-predictive cues (Espinosa-Parrilla et al. 2013). DBS of the anterior STN in monkeys dramatically reduced stereotypies following bicuculline-induced dysfunction of the GPe (Karachi et al. 2009).
STN Neurons Modulate in Response to Reward Expectation and Delivery in Monkeys
In 2005, Darbaky and colleagues investigated STN neuronal activity in a monkey performing arm-reaching movements to obtain a liquid reward. They showed that neurons were modulated during movement, just before reward delivery, and during reward delivery (Darbaky et al. 2005).
Valence Processing in the STN: Human Studies
It is important to note that currently electrophysiological recordings of the STN can only be performed in patients with a medical need for DBS (Alkemade et al. 2015).
Neuroimaging: STN Activity Increases During Reward Processing
Functional neuroimaging studies of opioid dependent men showed significant increases in the STN in response to heroin-related stimuli, suggesting involvement of the STN in reward processing (Zijlstra et al. 2009). Probabilistic tractography has demonstrated a structural connection between ventromedial prefrontal cortex (vmPFC) and STN and that the strength of this connection predicts the magnitude of value-driven choice bias (Mulder et al. 2014).
Computational Modeling of STN Function Predicts Valence-Dependent Behavior
An important study by Frank and colleagues found that PD patients in the ON STN DBS state (off dopaminergic medications) responded more rapidly to high conflict trials (when valences were more similar) in a probabilistic learning task, indicating a tendency toward impulsive action. Non-DBS patients on dopaminergic medication, however, did not exhibit this trend. However, the patients in this study had an impaired ability to learn from negative outcomes, a finding consistent with other studies mentioned above. Most interestingly, an a priori computational model of the basal ganglia in decision-making predicted the observed dynamics, while suggesting that the STN receives value-based information from cortical regions and processes this information in such a way as to provide a dynamic modulatory signal during facilitation and suppression responses (Frank 2006).
Treatment of this topic would be incomplete without mentioning that the work by Frank et al. motivated a subsequent study by Ballanger and colleagues to investigate whether the observed “braking” function of the STN occurred not only in high-conflict scenarios but in low-conflict scenarios as well (Ballanger et al. 2009). The authors postulated that the STN plays a central role in the inhibition of any response. To test this hypothesis, the authors had PD patients post-STN DBS perform both a Go-No Go task and a control Go task, under stimulation ON and OFF conditions. They found that STN DBS induced a global decrease in reaction time but impaired response inhibition (Ballanger et al. 2009).
Electrophysiology: Theta Band Coherence Associated with Impulsivity
Rodriguez-Oroz and colleagues recorded LFPs from electrodes implanted in the STN of 28 PD patients (Rodriguez-Oroz et al. 2011). They compared oscillatory activity in a subgroup of the population diagnosed with an ICD (n = 10) to a non-ICD subgroup. LFPs were recorded in both ON and OFF stimulation state. In the ON state, the presence of an ICD was associated with oscillations in the 4–10 Hz frequency band (theta-alpha), originating from the ventral subthalamic area. In addition, they observed cortico-subthalamic coherence, which in the ICD patients was more frequent in the 4–7.5 Hz range (Rodriguez-Oroz et al. 2011) compared to non-ICD controls. Coherence is a measure of the correlation in neuronal activity between brain regions, with activity being measured in frequency components.
Electrophysiology: Dopamine Modulates STN Response to Positive Valence
In another study, LFPs were recorded from the STN while PD patients undergoing DBS electrode placement performed an emotional categorization task (Buot et al. 2013). In this task, responses varied according to the perceived valence of stimuli (pleasant, unpleasant, or neutral). Pleasant, unpleasant, and neutral stimuli evoked an event related potential (ERP). The authors found that ERP amplitudes were significantly larger for the unpleasant pictures compared to the neutral ones, with the magnitude of this effect greatest in the ventral part of the STN.
Electrophysiology: Valence Encoding by the STN is Asymmetric
In a study examining spontaneous spiking activity of STN neurons in 17 PD patients undergoing DBS electrode placement, emotive auditory stimuli evoked changes in STN neuronal activity in the right ventral STN, but these changes were not observed in the left ventral STN or the right and left dorsal STN (Eitan et al. 2013).
Impulsivity and the STN
We have already discussed at length both the cross-sectional and anecdotal evidence demonstrating that STN DBS can induce changes in impulse control. In this section, we provide an overview of the laboratory-based studies that have been explicitly designed to elucidate mechanisms underlying the putative involvement of the STN in these changes. We focus here on recent and seminal work in this field, while also seeking to highlight unanswered questions. For comprehensive reviews, see Zavala et al. (Zavala et al. 2015) and Jahanshahi (Jahanshahi 2013).
Behavioral Data Shows Contrasting Effects of STN DBS on Impulse Control
In a test of risky decision-making, PD patients post-STN DBS took more risks than healthy controls; however, they behaved more conservatively than their PD counterparts who had not received DBS. Moreover, this effect was more pronounced in the DBS ON condition versus OFF (Brandt et al. 2015). A study by Boller and colleagues corroborated these findings, demonstrating higher risk behavior in the DBS off condition versus DBS on. Interestingly, they were also able to show that DBS made no difference in the patient’s ability to make use of negative feedback (Boller et al. 2014). However, a study by Plessow and colleagues had somewhat contrasting results (Plessow et al. 2014). Performance of PD patients post STN-DBS on a behavioral task were compared in the DBS on and DBS off states. The behavioral task tested the relationship between automatic response impulses and goal-directed action selection. Results showed enhanced automatic response activation during stimulation (i.e., increased susceptibility to impulsive responses). These findings in humans align with a rodent study which reported that STN DBS increased premature responding in a rat gambling task (Aleksandrova et al. 2013).
Finally, one recent study in humans demonstrated both positive and negative effects of STN DBS on impulsivity, highlighting the dynamic and complex nature of impulse control in the context of goal-directed behavior. Wylie and colleagues compared PD patients post STN DBS (both ON and OFF stim) and HC in regard to performance on a reaction time task. They showed that stimulation increased fast response errors, suggesting that stimulation increased impulsive, premature responding in high conflict situations. Interestingly, stimulation actually improved the capacity to suppress interference effects of the task as time passed (Wylie et al. 2010).
The contrasting results of these studies suggest that STN DBS does exert an effect on impulse control but that this effect may not be uniform. This interpretation conforms to the findings in clinical studies where both improvement and worsening of ICD symptoms have been observed. As we’ve discussed, impulsivity is a complex construct. It seems plausible the pre-existing factors may predispose patients toward a given outcome of stimulation, but certainly further research is needed to elucidate these factors.
Conclusions and Future Directions
There is significant evidence from a variety of sources that the STN plays an important role in limbic function. However, the precise mechanisms of STN involvement in cognition, emotion, and behavior remain elusive. Meanwhile, the functional topography of the STN—once widely accepted to be clearly divided into motor, associative, and limbic regions—has been called into question. Consequently, the STN’s role in non-motor behavior may be regarded as, in the least, dynamic and complex. Among the limbic functions involving the STN, impulse control has arguably been the most intensely studied in recent years. As reviewed above, STN dysfunction does not lead to impulsivity in all behavioral situations (Frank 2006). These findings suggest not only that STN function is complex but also that the constructs of action control and impulsivity, which the STN sub-serves, are as well. We have discussed the importance of valence to these constructs, and we have demonstrated that there is empirical support indicating that the STN processes valence and that it does so in the context of action selection. These data further suggest that changes in valence processing in an STN-dependent circuit may influence action selection and potentially contribute to impulsive behavior. What remains unclear from the behavioral studies mentioned above is whether (and how) STN neuronal activity modulates during action selection in valence-dependent ways. Future studies are required to elucidate the neural correlates of the observed behavioral differences between PD patients with ICD versus those without ICD, between PD patients versus healthy controls, and between other combinations of populations mentioned in the studies described above. Most importantly, such future studies should seek to investigate neural correlates emerging from STN activity, as the evidence suggests that this structure plays an integral role in determining these behavioral differences. Newly available electrophysiological techniques enabling researchers to chronically record subcortical LFP data from awake, behaving humans—as well as simultaneously record these LFPs from multiple brain regions (Gunduz et al. 2015)—may result in provocative insights when applied to these open questions about limbic STN.
References
Accolla, E. A., Dukart, J., Helms, G., Weiskopf, N., Kherif, F., Lutti, A., et al. (2014). Brain tissue properties differentiate between motor and limbic basal ganglia circuits. Human Brain Mapping, 35, 5083–5092.
Aleksandrova, L. R., Creed, M. C., Fletcher, P. J., Lobo, D. S. S., Hamani, C., & Nobrega, J. N. (2013). Deep brain stimulation of the subthalamic nucleus increases premature responding in a rat gambling task. Behavioural Brain Research, 245, 76–82.
Alkemade, A., & Forstmann, B. U. (2014). Do we need to revise the tripartite subdivision hypothesis of the human subthalamic nucleus (STN)? NeuroImage, 95, 326–329.
Alkemade A., Schnitzler A., Forstmann B. U. (2015). Topographic organization of the human and non-human primate subthalamic nucleus. Brain Struct. Funct.
Ardouin, C., Voon, V., Worbe, Y., Abouazar, N., Czernecki, V., Hosseini, H., et al. (2006). Pathological gambling in Parkinson’s disease improves on chronic subthalamic nucleus stimulation. Movement Disorders, 21, 1941–1946.
Aron, A. R., Behrens, T. E., Smith, S., Frank, M. J., & Poldrack, R. A. (2007). Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27, 3743–3752.
Balarajah, S., & Cavanna, A. E. (2013). The pathophysiology of impulse control disorders in Parkinson disease. Behavioural Neurology, 26, 237–244.
Balasubramani, P. P., Chakravarthy, V. S., Ali, M., Ravindran, B., Moustafa, A. A. (2015). Identifying the Basal Ganglia Network Model Markers for Medication-Induced Impulsivity in Parkinson’s Disease Patients [Internet]. PLoS ONE 10[cited 2015 Aug 4] Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4456385/
Baláž, M., Bočková, M., Rektorová, I., & Rektor, I. (2011). Involvement of the subthalamic nucleus in cognitive functions -- a concept. Journal of Neurological Sciences, 310, 96–99.
Ballanger, B., van Eimeren, T., Moro, E., Lozano, A. M., Hamani, C., Boulinguez, P., et al. (2009). Stimulation of the subthalamic nucleus and impulsivity: release your horses. Annals of Neurology, 66, 817–824.
Bari, A., & Robbins, T. W. (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Progress in Neurobiology, 108, 44–79.
Baunez, C., & Lardeux, S. (2011). Frontal cortex-like functions of the subthalamic nucleus. Frontiers in Systems Neuroscience, 5, 83.
Baunez, C., Nieoullon, A., & Amalric, M. (1995). In a rat model of parkinsonism, lesions of the subthalamic nucleus reverse increases of reaction time but induce a dramatic premature responding deficit. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 15, 6531–6541.
Baunez, C., Amalric, M., & Robbins, T. W. (2002). Enhanced food-related motivation after bilateral lesions of the subthalamic nucleus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22, 562–568.
Baunez, C., Dias, C., Cador, M., & Amalric, M. (2005). The subthalamic nucleus exerts opposite control on cocaine and ‘natural’ rewards. Nature Neuroscience, 8, 484–489.
Baunez, C., Christakou, A., Chudasama, Y., Forni, C., & Robbins, T. W. (2007). Bilateral high-frequency stimulation of the subthalamic nucleus on attentional performance: transient deleterious effects and enhanced motivation in both intact and parkinsonian rats. European Journal of Neuroscience, 25, 1187–1194.
Benedetti, F., Colloca, L., Lanotte, M., Bergamasco, B., Torre, E., & Lopiano, L. (2004). Autonomic and emotional responses to open and hidden stimulations of the human subthalamic region. Brain Research Bulletin, 63, 203–211.
Bickel, S., Alvarez, L., Macias, R., Pavon, N., Leon, M., Fernandez, C., et al. (2010). Cognitive and neuropsychiatric effects of subthalamotomy for Parkinson’s disease. Parkinsonism & Related Disorders, 16, 535–539.
Boller, J. K., Barbe, M. T., Pauls, K. A. M., Reck, C., Brand, M., Maier, F., et al. (2014). Decision-making under risk is improved by both dopaminergic medication and subthalamic stimulation in Parkinson’s disease. Experimental Neurology, 254, 70–77.
Borden, A., Wallon, D., Lefaucheur, R., Derrey, S., Fetter, D., Verin, M., et al. (2014). Does early verbal fluency decline after STN implantation predict long-term cognitive outcome after STN-DBS in Parkinson’s disease? Journal of Neurological Sciences, 346, 299–302.
Brandt, J., Rogerson, M., Al-Joudi, H., Reckess, G., Shpritz, B., Umeh, C. C., et al. (2015). Betting on DBS: Effects of subthalamic nucleus deep brain stimulation on risk taking and decision making in patients with Parkinson’s disease. Neuropsychology, 29, 622–631.
Breysse, E., Pelloux, Y., Baunez, C. (2015). The Good and Bad Differentially Encoded within the Subthalamic Nucleus in Rats,, [Internet]. eNeuro; 2[cited 2015 Oct 26] Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4607759/
Brücke, C., Kupsch, A., Schneider, G.-H., Hariz, M. I., Nuttin, B., Kopp, U., et al. (2007). The subthalamic region is activated during valence-related emotional processing in patients with Parkinson’s disease. European Journal of Neuroscience, 26, 767–774.
Brunenberg, E. J. L., Moeskops, P., Backes, W. H., Pollo, C., Cammoun, L., Vilanova, A., et al. (2012). Structural and resting state functional connectivity of the subthalamic nucleus: identification of motor STN parts and the hyperdirect pathway. PloS One, 7, e39061.
Buot, A., Welter, M.-L., Karachi, C., Pochon, J.-B., Bardinet, E., Yelnik, J., et al. (2013). Processing of emotional information in the human subthalamic nucleus. Journal of Neurology, Neurosurgery, and Psychiatry, 84, 1331–1338.
Burbaud, P., Clair, A.-H., Langbour, N., Fernandez-Vidal, S., Goillandeau, M., Michelet, T., et al. (2013). Neuronal activity correlated with checking behaviour in the subthalamic nucleus of patients with obsessive-compulsive disorder. Brain: A Journal of Neurology, 136, 304–317.
Burrows, A. M., Ravin, P. D., Novak, P., Peters, M. L. B., Dessureau, B., Swearer, J., et al. (2012). Limbic and motor function comparison of deep brain stimulation of the zona incerta and subthalamic nucleus. Neurosurgery, 70, 125–130.
Camacho-Abrego, I., Tellez-Merlo, G., Melo, A. I., Rodríguez-Moreno, A., Garcés, L., De La Cruz, F., et al. (2014). Rearrangement of the dendritic morphology of the neurons from prefrontal cortex and hippocampus after subthalamic lesion in Sprague–Dawley rats. Synapse (New York), 68, 114–126.
Cera, N., Bifolchetti, S., Martinotti, G., Gambi, F., Sepede, G., Onofrj, M., et al. (2014). Amantadine and cognitive flexibility: decision making in Parkinson’s patients with severe pathological gambling and other impulse control disorders. Neuropsychiatric Disease and Treatment, 10, 1093–1101.
Chowdhury, R., Guitart-Masip, M., Lambert, C., Dolan, R. J., & Düzel, E. (2013). Structural integrity of the substantia nigra and subthalamic nucleus predicts flexibility of instrumental learning in older-age individuals. Neurobiology of Aging, 34, 2261–2270.
Coenen, V. A., Honey, C. R., Hurwitz, T., Rahman, A. A., McMaster, J., Bürgel, U., et al. (2009). Medial forebrain bundle stimulation as a pathophysiological mechanism for hypomania in subthalamic nucleus deep brain stimulation for Parkinson’s disease. Neurosurgery, 64, 1106–1114.
Cools, R., Altamirano, L., & D’Esposito, M. (2006). Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsychologia, 44, 1663–1673.
Couto, M. I., Monteiro, A., Oliveira, A., Lunet, N., & Massano, J. (2014). Depression and anxiety following deep brain stimulation in Parkinson’s disease: systematic review and meta-analysis. Acta Médica Portuguesa, 27, 372–382.
Coxon, J. P., Impe, A. V., Wenderoth, N., & Swinnen, S. P. (2012). Aging and inhibitory control of action: cortico-subthalamic connection strength predicts stopping performance. Journal of Neuroscience, 32, 8401–8412.
Darbaky, Y., Baunez, C., Arecchi, P., Legallet, E., & Apicella, P. (2005). Reward-related neuronal activity in the subthalamic nucleus of the monkey. Neuroreport, 16, 1241–1244.
Demetriades, P., Rickards, H., Cavanna, A. E. (2011). Impulse Control Disorders Following Deep Brain Stimulation of the Subthalamic Nucleus in Parkinson’s Disease: Clinical Aspects [Internet]. Park. Dis. 2011; [cited 2015 Aug 4] Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3043299/
Desbonnet, L., Temel, Y., Visser-Vandewalle, V., Blokland, A., Hornikx, V., & Steinbusch, H. W. M. (2004). Premature responding following bilateral stimulation of the rat subthalamic nucleus is amplitude and frequency dependent. Brain Research, 1008, 198–204.
Dick, D. M., Smith, G., Olausson, P., Mitchell, S. H., Leeman, R. F., O’Malley, S. S., et al. (2010). Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology, 15, 217–226.
Diergaarde, L., Pattij, T., Nawijn, L., Schoffelmeer, A. N. M., & De Vries, T. J. (2009). Trait impulsivity predicts escalation of sucrose seeking and hypersensitivity to sucrose-associated stimuli. Behavioral Neuroscience, 123, 794–803.
Drapier, D., Drapier, S., Sauleau, P., Haegelen, C., Raoul, S., Biseul, I., et al. (2006). Does subthalamic nucleus stimulation induce apathy in Parkinson’s disease? Journal of Neurology, 253, 1083–1091.
Eisenstein, S. A., Koller, J. M., Black, K. D., Campbell, M. C., Lugar, H. M., Ushe, M., et al. (2014). Functional anatomy of subthalamic nucleus stimulation in Parkinson disease. Annals of Neurology, 76, 279–295.
Eitan, R., Shamir, R. R., Linetsky, E., Rosenbluh, O., Moshel, S., Ben-Hur, T., et al. (2013). Asymmetric right/left encoding of emotions in the human subthalamic nucleus. Frontiers in Systems Neuroscience, 7, 69.
Espinosa-Parrilla, J.-F., Baunez, C., & Apicella, P. (2013). Linking reward processing to behavioral output: motor and motivational integration in the primate subthalamic nucleus. Frontiers in Computational Neuroscience, 7, 175.
Espinosa-Parrilla, J. F., Baunez, C., & Apicella, P. (2015). Modulation of neuronal activity by reward identity in the monkey subthalamic nucleus. European Journal of Neuroscience, 42, 1705–1717.
Evens, R., Stankevich, Y., Dshemuchadse, M., Storch, A., Wolz, M., Reichmann, H., et al. (2015). The impact of Parkinson’s disease and subthalamic deep brain stimulation on reward processing. Neuropsychologia, 75, 11–19.
Forstmann, B. U., Keuken, M. C., Jahfari, S., Bazin, P.-L., Neumann, J., Schäfer, A., et al. (2012). Cortico-subthalamic white matter tract strength predicts interindividual efficacy in stopping a motor response. NeuroImage, 60, 370–375.
Frank, M. J. (2005). Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. Journal Cognitive Neuroscience, 17, 51–72.
Frank, M. J. (2006). Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Network Official Journal International Neural Networks Society, 19, 1120–1136.
Frank, M. J., & O’Reilly, R. C. (2006). A mechanistic account of striatal dopamine function in human cognition: psychopharmacological studies with cabergoline and haloperidol. Behavioral Neuroscience, 120, 497–517.
Fukaya, C., & Yamamoto, T. (2015). Deep brain stimulation for Parkinson’s disease: recent trends and future direction. Neurologia Medico-Chirurgica (Tokyo), 55, 422–431.
Funkiewiez, A., Ardouin, C., Caputo, E., Krack, P., Fraix, V., Klinger, H., et al. (2004). Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 75, 834–839.
Garcia-Ruiz, P. J., Martinez Castrillo, J. C., Alonso-Canovas, A., Herranz Barcenas, A., Vela, L., Sanchez Alonso, P., et al. (2014). Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: a multicentre study. Journal of Neurology, Neurosurgery, and Psychiatry, 85, 840–844.
Gee, L., Smith, H., De La Cruz, P., Campbell, J., Fama, C., Haller, J., et al. (2015). The influence of bilateral subthalamic nucleus deep brain stimulation on impulsivity and prepulse inhibition in Parkinson’s disease patients. Stereotactic and Functional Neurosurgery, 93, 265–270.
Greenhouse, I., Swann, N. C., Aron, A. R. (2011). Fronto-basal-ganglia circuits for stopping action [Internet]. Neural Basis Motiv. Cogn. Control; 189[cited 2015 Aug 5] Available from: http://books.google.com/books?hl=en&lr=&id=A_eoYgtLmFMC&oi=fnd&pg=PA189&dq=info:tSbAIu32TzcJ:scholar.google.com&ots=YdmRHP-rLW&sig=3BZtsfOcSmRKyoFxZ5PrkzpxD1c
Guitart-Masip, M., Duzel, E., Dolan, R., & Dayan, P. (2014). Action versus valence in decision making. Trends in Cognitive Sciences, 18, 194–202.
Gunduz, A., Morita, H., Rossi, P. J., Allen, W. L., Alterman, R. L., Bronte-Stewart, H., et al. Proceedings of the Second Annual Deep Brain Stimulation Think Tank: What’s in the Pipeline. Int. J. Neurosci. 2015: 1–11.
Hachem-Delaunay, S., Fournier, M.-L., Cohen, C., Bonneau, N., Cador, M., Baunez, C., et al. (2015). Subthalamic nucleus high-frequency stimulation modulates neuronal reactivity to cocaine within the reward circuit. Neurobiology of Disease, 80, 54–62.
Haegelen, C., Verin, M., Broche, B. A., Prigent, F., Jannin, P., Gibaud, B., et al. (2005). Does subthalamic nucleus stimulation affect the frontal limbic areas? a single-photon emission computed tomography study using a manual anatomical segmentation method. Surgical and Radiologic Anatomy, 27, 389–394.
Haegelen, C., Rouaud, T., Darnault, P., & Morandi, X. (2009). The subthalamic nucleus is a key-structure of limbic basal ganglia functions. Medical Hypotheses, 72, 421–426.
Haynes, W. I. A., & Haber, S. N. (2013). The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: implications for basal ganglia models and deep brain stimulation. Journal of Neuroscience, 33, 4804–4814.
Hewig, J., Kretschmer, N., Trippe, R. H., Hecht, H., Coles, M. G. H., Holroyd, C. B., et al. (2010). Hypersensitivity to reward in problem gamblers. Biological Psychiatry, 67, 781–783.
Hilker, R., Voges, J., Weisenbach, S., Kalbe, E., Burghaus, L., Ghaemi, M., et al. (2004). Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson’s disease. Journal of Cerebral Blood Flow and Metabolism Official journal of the International Society for Cerebral Blood Flow and Metabolism, 24, 7–16.
Jahanshahi, M. (2013). Effects of deep brain stimulation of the subthalamic nucleus on inhibitory and executive control over prepotent responses in Parkinson’s disease. Frontiers in Systems Neuroscience, 7, 118.
Joel, D., & Weiner, I. (1997). The connections of the primate subthalamic nucleus: indirect pathways and the open-interconnected scheme of basal ganglia-thalamocortical circuitry. Brain Research. Brain Research Reviews, 23, 62–78.
Kantak, K. M., Yager, L. M., & Brisotti, M. F. (2013). Impact of medial orbital cortex and medial subthalamic nucleus inactivation, individually and together, on the maintenance of cocaine self-administration behavior in rats. Behavioural Brain Research, 238, 1–9.
Karachi, C., Yelnik, J., Tandé, D., Tremblay, L., Hirsch, E. C., & François, C. (2005). The pallidosubthalamic projection: an anatomical substrate for nonmotor functions of the subthalamic nucleus in primates. Movement Disorders Official Journal Movement Disorder Society, 20, 172–180.
Karachi, C., Grabli, D., Baup, N., Mounayar, S., Tandé, D., François, C., et al. (2009). Dysfunction of the subthalamic nucleus induces behavioral and movement disorders in monkeys. Movement Disorders Official Journal Movement Disorder Society, 24, 1183–1192.
Keuken, M. C., Bazin, P.-L., Schäfer, A., Neumann, J., Turner, R., & Forstmann, B. U. (2013). Ultra-high 7T MRI of structural age-related changes of the subthalamic nucleus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 33, 4896–4900.
Kim, H.-J., Jeon, B. S., & Paek, S. H. (2015). Nonmotor symptoms and subthalamic deep brain stimulation in Parkinson’s disease. Journal Movement Disorders, 8, 83–91.
Klein, J., Winter, C., Coquery, N., Heinz, A., Morgenstern, R., Kupsch, A., et al. (2010). Lesion of the medial prefrontal cortex and the subthalamic nucleus selectively affect depression-like behavior in rats. Behavioural Brain Research, 213, 73–81.
Knight, E. J., Testini, P., Min, H.-K., Gibson, W. S., Gorny, K. R., Favazza, C. P., et al. (2015). Motor and nonmotor circuitry activation induced by subthalamic nucleus deep brain stimulation in patients with Parkinson disease: intraoperative functional magnetic resonance imaging for deep brain stimulation. Mayo Clinic Proceedings, 90, 773–785.
Kocka, A., & Gagnon, J. (2014). Definition of impulsivity and related terms following traumatic brain injury: a review of the different concepts and measures used to assess impulsivity, disinhibition and other related concepts. Behavioral Sciences (Basel Switzerland), 4, 352–370.
Krack, P., Kumar, R., Ardouin, C., Dowsey, P. L., McVicker, J. M., Benabid, A. L., et al. (2001). Mirthful laughter induced by subthalamic nucleus stimulation. Movement Disorders Official Journal Movement Disorder Society, 16, 867–875.
Krack, P., Hariz, M. I., Baunez, C., Guridi, J., & Obeso, J. A. (2010). Deep brain stimulation: from neurology to psychiatry? Trends in Neurosciences, 33, 474–484.
Kühn, A. A., Hariz, M. I., Silberstein, P., Tisch, S., Kupsch, A., Schneider, G.-H., et al. (2005). Activation of the subthalamic region during emotional processing in Parkinson disease. Neurology, 65, 707–713.
Lambert, C., Zrinzo, L., Nagy, Z., Lutti, A., Hariz, M., Foltynie, T., et al. (2012). Confirmation of functional zones within the human subthalamic nucleus: patterns of connectivity and sub-parcellation using diffusion weighted imaging. NeuroImage, 60, 83–94.
Lanotte, M., Lopiano, L., Torre, E., Bergamasco, B., Colloca, L., & Benedetti, F. (2005). Expectation enhances autonomic responses to stimulation of the human subthalamic limbic region. Brain, Behavior, and Immunity, 19, 500–509.
Lardeux, S., & Baunez, C. (2007). Alcohol preference influences the subthalamic nucleus control on motivation for alcohol in rats. Neuropsychopharmacology, 33, 634–642.
Lardeux, S., Pernaud, R., Paleressompoulle, D., & Baunez, C. (2009). Beyond the reward pathway: coding reward magnitude and error in the rat subthalamic nucleus. Journal of Neurophysiology, 102, 2526–2537.
Lardeux, S., Paleressompoulle, D., Pernaud, R., Cador, M., & Baunez, C. (2013). Different populations of subthalamic neurons encode cocaine vs. sucrose reward and predict future error. Journal of Neurophysiology, 110, 1497–1510.
Le Jeune, F., Péron, J., Biseul, I., Fournier, S., Sauleau, P., Drapier, S., et al. (2008). Subthalamic nucleus stimulation affects orbitofrontal cortex in facial emotion recognition: a PET study. Brain: A Journal of Neurology, 131, 1599–1608.
Le Jeune, F., Péron, J., Grandjean, D., Drapier, S., Haegelen, C., Garin, E., et al. (2010). Subthalamic nucleus stimulation affects limbic and associative circuits: a PET study. European Journal of Nuclear Medicine and Molecular Imaging, 37, 1512–1520.
Leicht, G., Troschütz, S., Andreou, C., Karamatskos, E., Ertl, M., Naber, D., et al. (2013). Relationship between oscillatory neuronal activity during reward processing and trait impulsivity and sensation seeking. PloS One, 8, e83414.
Łęski, S., Lindén, H., Tetzlaff, T., Pettersen, K. H., Einevoll, G. T. (2013). Frequency Dependence of Signal Power and Spatial Reach of the Local Field Potential [Internet]. PLoS Comput. Biol.; 9[cited 2015 Nov 5] Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3715549/
Lévesque, J.-C., & Parent, A. (2005). GABAergic interneurons in human subthalamic nucleus. Movement Disorders Official Journal Movement Disorder Society, 20, 574–584.
Mallet, L., Mesnage, V., Houeto, J.-L., Pelissolo, A., Yelnik, J., Behar, C., et al. (2002). Compulsions, Parkinson’s disease, and stimulation. Lancet (London, England), 360, 1302–1304.
Mallet, L., Schüpbach, M., N’Diaye, K., Remy, P., Bardinet, E., Czernecki, V., et al. (2007). Stimulation of subterritories of the subthalamic nucleus reveals its role in the integration of the emotional and motor aspects of behavior. Proceedings of the National Academy of Sciences of the United States of America, 104, 10661–10666.
Marceglia, S., Fumagalli, M., & Priori, A. (2011). What neurophysiological recordings tell us about cognitive and behavioral functions of the human subthalamic nucleus. Expert Review of Neurotherapeutics, 11, 139–149.
Morris, L. S., Kundu, P., Baek, K., Irvine, M. A., Mechelmans, D. J., Wood, J., et al. (2015) Jumping the Gun: Mapping Neural Correlates of Waiting Impulsivity and Relevance Across Alcohol Misuse. Biol. Psychiatry.
Mosley, P. E., & Marsh, R. (2015). The psychiatric and neuropsychiatric symptoms after subthalamic stimulation for Parkinson’s disease. Journal of Neuropsychiatry and Clinical Neurosciences, 27, 19–26.
Moum, S. J., Price, C. C., Limotai, N., Oyama, G., Ward, H., Jacobson, C., et al. (2012). Effects of STN and GPi deep brain stimulation on impulse control disorders and dopamine dysregulation syndrome. PloS One, 7, e29768.
Mulder, M. J., Boekel, W., Ratcliff, R., & Forstmann, B. U. (2014). Cortico-subthalamic connection predicts individual differences in value-driven choice bias. Brain Structure and Function, 219, 1239–1249.
Oberg, S. A. K., Christie, G. J., & Tata, M. S. (2011). Problem gamblers exhibit reward hypersensitivity in medial frontal cortex during gambling. Neuropsychologia, 49, 3768–3775.
Okai, D., Samuel, M., Askey-Jones, S., David, A. S., & Brown, R. G. (2011). Impulse control disorders and dopamine dysregulation in Parkinson’s disease: a broader conceptual framework. European Journal of Neurology Official Journal European Federation Neurology Society, 18, 1379–1383.
Park, H. K., Kim, H.-J., Kim, S. J., Kim, J. S., Shin, H.-W., & Kim, J. S. (2011). From Jekyll to Hyde after limbic subthalamic nucleus infarction. Neurology, 77, 82–84.
Péron, J., Grandjean, D., Le Jeune, F., Sauleau, P., Haegelen, C., Drapier, D., et al. (2010). Recognition of emotional prosody is altered after subthalamic nucleus deep brain stimulation in Parkinson’s disease. Neuropsychologia, 48, 1053–1062.
Piray, P., Zeighami, Y., Bahrami, F., Eissa, A. M., Hewedi, D. H., & Moustafa, A. A. (2014). Impulse control disorders in Parkinson’s disease are associated with dysfunction in stimulus valuation but not action valuation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 34, 7814–7824.
Plessow, F., Fischer, R., Volkmann, J., & Schubert, T. (2014). Subthalamic deep brain stimulation restores automatic response activation and increases susceptibility to impulsive behavior in patients with Parkinson’s disease. Brain and Cognition, 87, 16–21.
Rektor, I., Bočková, M., Chrastina, J., Rektorová, I., Baláž, M. (2014). The modulatory role of subthalamic nucleus in cognitive functions - A viewpoint. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol.
Rodriguez-Oroz, M. C., López-Azcárate, J., Garcia-Garcia, D., Alegre, M., Toledo, J., Valencia, M., et al. (2011). Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson’s disease. Brain: A Journal of Neurology, 134, 36–49.
Rodriguez-Oroz, M. C., Moro, E., & Krack, P. (2012). Long-term outcomes of surgical therapies for Parkinson’s disease. Movement Disorders, 27, 1718–1728.
Rouaud, T., Lardeux, S., Panayotis, N., Paleressompoulle, D., Cador, M., & Baunez, C. (2010). Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proceedings of the National Academy of Sciences of the United States of America, 107, 1196–1200.
Santangelo, G., Barone, P., Trojano, L., & Vitale, C. (2013). Pathological gambling in Parkinson’s disease. A comprehensive review. Parkinsonism & Related Disorders, 19, 645–653.
Sauleau, P., Raoul, S., Lallement, F., Rivier, I., Drapier, S., Lajat, Y., et al. (2005). Motor and non motor effects during intraoperative subthalamic stimulation for Parkinson’s disease. Journal of Neurology, 252, 457–464.
Sensi, M., Eleopra, R., Cavallo, M. A., Sette, E., Milani, P., Quatrale, R., et al. (2004). Explosive-aggressive behavior related to bilateral subthalamic stimulation. Parkinsonism & Related Disorders, 10, 247–251.
Sestini, S., di Scotto Luzio, A., Ammannati, F., De Cristofaro, M. T. R., Passeri, A., Martini, S., et al. (2002). Changes in regional cerebral blood flow caused by deep-brain stimulation of the subthalamic nucleus in Parkinson’s disease. Journal of Nuclear Medicine Official Publisher Society of Nuclear Medicine, 43, 725–732.
Shapiro, M. B., Vaillancourt, D. E., Sturman, M. M., Metman, L. V., Bakay, R. A. E., & Corcos, D. M. (2007). Effects of STN DBS on rigidity in Parkinson’s disease. IEEE Transactions on Neural Systems and Rehabilitation Engineering Publisher: IEEE Engineering in Medicine and Biology Society, 15, 173–181.
Tan, S. K. H., Temel, Y., Blokland, A., Steinbusch, H. W. M., & Visser-Vandewalle, V. (2006). The subthalamic nucleus: from response selection to execution. Journal of Chemical Neuroanatomy, 31, 155–161.
Tandé, D., Féger, J., Hirsch, E. C., & François, C. (2006). Parafascicular nucleus projection to the extrastriatal basal ganglia in monkeys. Neuroreport, 17, 277–280.
Teagarden, M. A., & Rebec, G. V. (2007). Subthalamic and striatal neurons concurrently process motor, limbic, and associative information in rats performing an operant task. Journal of Neurophysiology, 97, 2042–2058.
Temel, Y., Blokland, A., Steinbusch, H. W. M., & Visser-Vandewalle, V. (2005). The functional role of the subthalamic nucleus in cognitive and limbic circuits. Progress in Neurobiology, 76, 393–413.
Temel, Y., Kessels, A., Tan, S., Topdag, A., Boon, P., & Visser-Vandewalle, V. (2006). Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review. Parkinsonism & Related Disorders, 12, 265–272.
Trottenberg, T., Kupsch, A., Schneider, G.-H., Brown, P., & Kühn, A. A. (2007). Frequency-dependent distribution of local field potential activity within the subthalamic nucleus in Parkinson’s disease. Experimental Neurology, 205, 287–291.
Tsai, S. T., Lin, S. H., Lin. S. Z., Chen, J. Y., Lee, C. W., Chen. S. Y. (2007) Neuropsychological effects after chronic subthalamic stimulation and the topography of the nucleus in Parkinson’s disease. Neurosurgery 61, E1024–1029; discussion E1029–1030.
Turner, M. S., Lavin, A., Grace, A. A., & Napier, T. C. (2001). Regulation of limbic information outflow by the subthalamic nucleus: excitatory amino acid projections to the ventral pallidum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 21, 2820–2832.
Vesper, J., Klostermann, F., Stockhammer, F., Funk, T., & Brock, M. (2002). Results of chronic subthalamic nucleus stimulation for Parkinson’s disease: a 1-year follow-up study. Surgical Neurology, 57, 306–311. discussion 311–313.
Volkmann, J., Daniels, C., & Witt, K. (2010). Neuropsychiatric effects of subthalamic neurostimulation in Parkinson disease. Nature Reviews Neurology, 6, 487–498.
Wagenbreth, C., Zaehle, T., Galazky, I., Voges, J., Guitart-Masip, M., Heinze, H.-J., et al. (2015). Deep brain stimulation of the subthalamic nucleus modulates reward processing and action selection in Parkinson patients. Journal of Neurology, 262, 1541–1547.
Welter, M.-L., Burbaud, P., Fernandez-Vidal, S., Bardinet, E., Coste, J., Piallat, B., et al. (2011). Basal ganglia dysfunction in OCD: subthalamic neuronal activity correlates with symptoms severity and predicts high-frequency stimulation efficacy. Transcultural Psychiatry, 1, e5.
Williams, N. R., Foote, K. D., & Okun, M. S. (2014). STN vs. GPi deep brain stimulation: translating the rematch into clinical practice. Movement Disorders-Clinical Practice, 1, 24–35.
Winstanley, C. A., Baunez, C., Theobald, D. E. H., & Robbins, T. W. (2005). Lesions to the subthalamic nucleus decrease impulsive choice but impair autoshaping in rats: the importance of the basal ganglia in Pavlovian conditioning and impulse control. European Journal of Neuroscience, 21, 3107–3116.
Winter, C., Lemke, C., Sohr, R., Meissner, W., Harnack, D., Juckel, G., et al. (2008). High frequency stimulation of the subthalamic nucleus modulates neurotransmission in limbic brain regions of the rat. Experimental Brain Research, 185, 497–507.
Wylie, S. A., Ridderinkhof, K. R., Elias, W. J., Frysinger, R. C., Bashore, T. R., Downs, K. E., et al. (2010). Subthalamic nucleus stimulation influences expression and suppression of impulsive behaviour in Parkinson’s disease. Brain, 133, 3611–3624.
York, M. K., Wilde, E. A., Simpson, R., & Jankovic, J. (2009). Relationship between neuropsychological outcome and DBS surgical trajectory and electrode location. Journal of Neurological Sciences, 287, 159–171.
Zavala, B., Zaghloul, K., & Brown, P. (2015). The subthalamic nucleus, oscillations, and conflict. Movement Disorders-Clinical Practice, 30, 328–338.
Zhang, G., Zhang, Z., Liu, L., Yang, J., Huang, J., Xiong, N., et al. (2014). Impulsive and compulsive behaviors in Parkinson’s disease. Frontiers in Aging Neuroscience, 6, 318.
Zijlstra, F., Veltman, D. J., Booij, J., van den Brink, W., & Franken, I. H. A. (2009). Neurobiological substrates of cue-elicited craving and anhedonia in recently abstinent opioid-dependent males. Drug and Alcohol Dependence, 99, 183–192.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossi, P.J., Gunduz, A. & Okun, M.S. The Subthalamic Nucleus, Limbic Function, and Impulse Control. Neuropsychol Rev 25, 398–410 (2015). https://doi.org/10.1007/s11065-015-9306-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-015-9306-9