Abstract
To determine the clinical and demographic correlates of persistent, remitting, and new-onset impulse control behaviors (ICBs) before and after subthalamic deep brain stimulation (STN-DBS) in Parkinson’s disease (PD). We compared the pre- and post-surgical prevalence of ICBs, classified as impulse control disorders (ICD), dopamine dysregulation syndrome (DDS), and punding in 150 consecutive PD STN-DBS-treated patients and determined the association with motor, cognitive, neuropsychological, and neuropsychiatric endpoints. At baseline (before STN-DBS), ICBs were associated with younger age (p = 0.045) and male gender (85 %; p = 0.001). Over an average follow-up of 4.3 ± 2.1 years of chronic STN-DBS there was an overall trend for reduction in ICBs (from 17.3 to 12.7 %; p = 0.095) with significant improvement in hypersexuality (12–8.0 %; p = 0.047), gambling (10.7–5.3 %; p = 0.033), and DDS (4.7–0 %; p < 0.001). ICB remitted in 18/26 patients (69 %) and persisted in 8/26 (31 %); the latter group was characterized by higher levodopa equivalent daily dose. Patients who developed a new-onset ICB during follow-up (n = 11/150) were characterized by younger age (p = 0.042), lower dyskinesia improvement (p ≤ 0.035), and a gender distribution with higher prevalence of women (p = 0.018). In addition, new-onset ICB was more common among patients with borderline, schizoid, and/or schizotypal traits of personality disorders; persistent ICB in those with obsessive–compulsive traits. PD-related ICBs exhibit a complex outcome after STN-DBS, with a tendency for overall reduction but with age, gender, dopaminergic therapy, and neuropsychiatric features exerting independent effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impulse control behaviors (ICBs) is a class of psychiatric disorders characterized by failure to resist an impulse even if harmful to oneself or others, including three disorders: dopamine dysregulation syndrome (DDS) [1]; impulse control disorder (ICD) [2, 3]; and punding [4]. This complex syndrome has a prevalence of 13.6 % in Parkinson’s disease (PD) compared to 1 % in the general population [5], and may be thought of as the neuropsychiatric equivalent of levodopa-induced dyskinesia [6–8]. The risk of ICBs in PD is higher in males with younger age at disease onset, and in the context of dopamine agonists use [5, 9], pathological personality traits [10, 11], depression [12], and history of addictive behaviors [13, 14], as well as those with selected single nucleotide polymorphisms related to dopamine metabolism [15, 16] or parkin mutation [17]. Therapeutic strategies against ICB may be based on discontinuing dopamine agonists, with or without down titration of other dopaminergic therapies [18]. The role of subthalamic nucleus deep brain stimulation (STN-DBS) has been conflicting. Some studies reported improvement or resolution of ICB after STN-DBS [19–23] whereas others yielded mixed results, with some patients improving, worsening, or even developing de-novo ICB after surgery [24–28]. The data currently available are insufficient to ascertain whether ICB changes after STN-DBS are related to beneficial effects related to post-surgical decrease in dopaminergic treatment, to modulation of basal ganglia oscillatory patterns, or a combination thereof [29].
In this follow-up study, we sought to compare the rate of remission, persistence, and new appearance of ICBs after STN-DBS, as related to clinical/demographic features, dopaminergic therapies, stimulation parameters, mood, anxiety, apathy, and neuropsychological data.
Methods
Patients and clinical evaluations
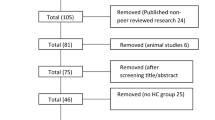
Data from 172 consecutive PD patients treated with STN-DBS at the Department of Neuroscience, University of Turin, from June 2004 and June 2015 were retrospectively analyzed, comparing the pre-surgical and post-surgical prevalence of ICBs, specified as ICD (hypersexuality, gambling, pathological shopping, or a combination thereof [multiple ICD]), DDS (defined as an addictive pattern of dopaminergic drug use, with doses in excess of those required to control motor symptoms), and punding (defined as compulsive fascination with and prolonged performance of repetitive mechanical tasks). The presence/absence of ICBs was assessed by means of a clinical diagnostic interview based on specific diagnostic criteria [30], comparing their prevalence at baseline vs. post-surgical follow-up (regular evaluations were performed every 3–6 months collecting information from patients and caregivers), and classifying patients as follows: no ICB (before and after STN-DBS), remitting ICB; persistent ICB, and new-onset ICB after STN-DBS. Pre- and post-surgical ICBs prevalence was compared with demographic features (age and gender) and clinical, cognitive and pharmacological endpoints. Motor severity was assessed by means of the Unified Parkinson’s Disease Rating Scale (UPDRS), evaluating the ON (maximal dopaminergic efficacy) and OFF conditions (reemergence of parkinsonian features, after at least 12 h since the last levodopa dose) in the preoperative assessment, and the MED-ON/STIM-ON (maximal dopaminergic efficacy/Stimulator ON) and MED-OFF/STIM-OFF conditions (at least 12 h since the last levodopa dose/Stimulator OFF) in the post-operative assessment.
Before surgery all patients received a comprehensive neuropsychiatric assessment aiming at assessing reasoning (Raven’s Colored Progressive Matrices), memory (Digit Span, Bi-syllabic Words Repetition Test (Verbal Span), Corsi’s Block Tapping Test (Spatial Span), and Paired Associative Learning), frontal executive function (Digit Cancellation Test, Trail Making Test part A and B, Nelson Modified Card Sorting Test, Frontal Assessment Battery and Phonemic Verbal Fluency) and language (Category Verbal Fluency). In addition, at the pre-surgical assessment patients were screened for personality disorders by means of the “Structured Clinical Interview and Questionnaire for DSM-IV Personality Disorders (SCID-II)” [31]. Subjects with personality disorders were excluded from DBS, while those with “personality disorder traits” were carefully selected for DBS according to a case-by-case discussion with the psychiatrist and neuropsychologist. Mood and anxiety were evaluated by means of the Beck Depression Inventory (BDI) [32], State-Trait Anxiety Inventory (STAI)-X1 (reaction to episodic stress conditions) and STAI-X2 (predisposition to experiencing persistent anxiety) [33]. Apathy was evaluated using the Marin Apathy Scale (MAS).
Finally, medications were logged as levodopa equivalent daily doses (LEDD) for all dopaminergic medications and for dopamine agonists; stimulation parameters as total electrical energy delivered (TEED = voltage2 × pulse width × frequency/impedance [34]).
Statistical analyses
Continuous variables were reported as average ± standard deviation (range). Cramer’s V, Mann–Whitney and Kruskal–Wallis tests were used for inter-group comparisons, Wilcoxon test and repeated-measures ANOVA for longitudinal comparisons between groups. All tests were performed using SPSS 21.0, considering two-tailed p values with 0.05 as the statistical threshold. Bonferroni correction was used for multiple comparisons in post hoc analyses. The ethical committee approved the study (CS/855; Prot. no. 475/2016) and patients provided written informed consent.
Results
Complete clinical and neuropsychological data were available for 150/172 patients (15 patients were followed-up in other Centers and 7 had incomplete data). The cohort consisted of 86 men and 64 women with PD treated with STN-DBS at the age of 59.1 ± 7.2 years (range 37–70 years), after 12.9 ± 1.5 years from symptom onset (range 7–25 years). At baseline, all patients (n = 150) were receiving L-dopa; 81.3 % (n = 122) were treated with dopamine agonists; 37.3 % (n = 56) with COMT inhibitors; and 16.0 % (n = 24) with MAO-B inhibitors.
Pre- vs. post-surgical ICBs
The pre-surgical prevalence of ICBs was 17.3 % (n = 26) (Table 1). Multiple ICDs were identified in 6.0 % of patients (n = 9), single ICD in 5.3 % (n = 8), multiple ICD + DDS in 3.3 % (n = 5), single ICD + punding in 1.3 % (n = 2), single ICD + DDS (n = 1) and single ICD + DDS + punding (n = 1) in 0.7 % each. Post-surgical data showed a trend for ICBs prevalence reduction in STN-DBS treated patients (mean follow-up of 4.3 ± 2.1 years), from 17.3 to 12.7 % (p = 0.095): 69.2 % (n = 18/26) remitted after 22.1 ± 15.3 months (range 8–60) and 30.8 % (n = 8/26) had persistent ICB after 41.4 ± 22.1 months of follow-up (range 11–82) (Table 2). During the entire follow-up period, 7.3 % of patients (n = 11/150) developed a new-onset ICB, after a mean follow-up of 40.6 ± 25.9 months (range 6–84). Among new-onset ICB, 45.4 % (n = 5/11) developed multiple ICDs (gambling + compulsive shopping in 3 patients and hypersexuality + compulsive shopping in 2 patients); 27.3 % (n = 3/11) single ICD (hypersexuality in 2 and compulsive shopping in 1); 18.2 % (n = 2/11) single ICD + punding (hypersexuality and compulsive shopping in 1 patient each); and 9.1 % (n = 1/11) punding alone.
Overall, there was a reduction in the prevalence of hypersexuality (12–8.0 %; p = 0.047), gambling (10.7–5.3 %; p = 0.033), and DDS (4.7–0 %; p < 0.001). There were no changes in the prevalence of multiple ICD (9.3–6.7 %; p = 0.197), compulsive shopping (6.7–5.3 %; p = 0.617), and punding (2.0–3.3 %; p = 0.414) (Fig. 1).
Impulsive control behaviors (baseline and follow-up). Number of patients with ICB before and after surgery. There was a significant reduction in the prevalence of gambling, hypersexuality, and DDS. No significant changes were observed in the prevalence of compulsive shopping and punding. *Significant difference between pre- and post-surgical prevalence (p < 0.05). DDS dopamine dysregulation syndrome
Dyskinesia and UPDRS-III motor score
Baseline data showed no significant differences in motor symptoms, dyskinesia severity (item 33 of UPDRS), and dyskinesia duration (item 32 of UPDRS) between patients with and without ICB (Table 1). Repeated-measure ANOVA showed a different improvement (Table 2) of dyskinesia duration and severity in the four groups (p = 0.033 and p = 0.035), with new-onset ICB patients reporting lower amelioration compared to other groups (post hoc comparisons: p ≤ 0.035 and p ≤ 0.029). There were no differences in UPDRS-III motor score between new-onset, remitting, persistent, and no ICB (Table 2).
Dopaminergic therapies and Stimulation Parameters
There were no differences in dopaminergic therapy (total LEDD, dopamine agonist LEDD, or use of dopamine agonists) between patients with and without ICB at baseline (Table 1). The LEDD post-surgical reduction was evident in all groups (−29.6 % in new-onset ICB; −28.3 % in remitting ICB; −21.0 % in persistent ICB; −37.3 % in no ICB), although the cohort with persistent ICB maintained higher total LEDD compared to other groups (p = 0.021; Table 2). Stimulation parameters and TEED did not differ across all ICB groups (Table 2).
Demographic features
There was a higher prevalence of ICBs in men at baseline (88.5 % men; 11.5 % women; p = 0.001) (Table 1), while the gender distribution significantly changed after STN-DBS (the prevalence of women increased from 11.5 to 42.1 %; p = 0.018) (Table 2). Age was younger both in patients with ICBs at baseline (56.9 ± 5.5 vs. 59.6 ± 6.9 years; p = 0.045) and in new-onset ICBs patients (59.1 ± 7.3 years) compared to no ICBs (64.2 ± 7.5 years), persistent ICB (61.8 ± 8.5 years) and remitting ICBs (61.1 ± 7.8 years) (p = 0.042).
Cognitive and neuropsychological data
Cognitive and neuropsychological data were similar between patients with and without ICBs at baseline. Post-surgical data revealed higher prevalence of obsessive–compulsive (p = 0.042) traits of personality disorder in persistent ICBs, and higher prevalence of borderline (p = 0.007), schizoid (p = 0.046) and schizotypal (p = 0.010) traits of personality disorder in new-onset ICBs (Table 3). There were no changes in cognitive performance between baseline and follow-up among new-onset, remitting, persistent, and no ICB groups (Table 4). No baseline differences were observed in BDI (p = 0.368), anxiety (STAI-X1, p = 0.415; STAI-X2, p = 0.149), and apathy (p = 0.400) scores, while post-surgical data showed a trend for BDI worsening in new-onset ICBs and persistent ICBs (Table 4).
Discussion
We confirmed a trend towards reduction of ICBs in PD patients treated with STN-DBS. However, selected features emerged as risk factors for persistent ICB (higher LEDD and obsessive compulsive personality trait) and new-onset ICB (younger age, borderline, schizoid and schizotypal traits of personality disorder), indicating that the beneficial effects of STN-DBS on ICBs may be partially reduced in patients with less pronounced decrease in dopaminergic medications, younger age, and/or specific traits of personality disorders. Interestingly, we observed a different ICB gender distribution before and after STN-DBS surgery, suggesting a differential sensitivity between men and women to dopaminergic therapies reduction and/or STN-DBS modulation. Hypersexuality, gambling, and DDS were significantly improved, whereas punding, compulsive shopping and multiple ICD were not (trend toward improvement in the latter two). These data confirm and extend previous reports on the varying effects of STN-DBS on ICD and DDS [19–28], highlighting the presence of “risk factors” for ICB persistence.
The reduction of dopaminergic medications partly explains the beneficial effects of STN-DBS on selected ICBs; it has been suggested that the imbalanced impairment of the dorsal striatum motor area and the ventral striatum limbic area typical of PD might have a role in the development of ICB [35, 36]. Antiparkinsonian medications, while compensating for dopamine depletion of the motor dorsal striatum, could ‘over-dose’ the otherwise non-deficient limbic ventral striatum, thus facilitating the development of impulsive behaviors [37]. This theory is in agreement with the hypothesis that STN-DBS effects on ICBs should be primarily attributed to the post-surgical reduction of dopaminergic medications. It should be noted, however, that several STN-DBS studies reported mixed results [24–28], suggesting that other factors might be involved. Neuropsychiatric comorbidities and personality disorders have been frequently associated with ICBs in the general population, but their clinical relevance in PD has been poorly understood and insufficiently recognized [11]. Greater obsessive–compulsive symptoms and novelty seeking behaviors interact in the pathophysiology of PD-associated ICBs [6]. A substantial range (23–92 %) of non-PD pathological gamblers have at least one personality disorder, namely obsessive–compulsive, borderline, antisocial, narcissistic, or dependent personality [38, 39], and recent evidences support the hypothesis that PD patients with Cluster A personality disturbances might be at higher risk of developing ICBs [40]. We observed that patients with traits of obsessive compulsive personality disorder had higher risk of falling in the category of persistent ICB, and that patients with traits of borderline, schizoid and schizotypal personality disorder were at higher risk of developing new-onset ICB after DBS, in spite of a significant reduction in dopaminergic therapies. There was a trend towards BDI worsening in the groups of new-onset ICB and persistent ICB, while no differences were observed in apathy score and other neuropsychological or cognitive outcomes. Our results also support the lack of correlation between cognitive impairment and impulsive behaviors [12, 41], arguing against a causative link between specific cognitive dysfunction and ICD.
Finally, we found a correlation between new-onset ICB and reduced amelioration of post-surgical dyskinesia. The contention that ICBs may represent the neuropsychiatric equivalent of hyperdopaminergic motor complications [5–7] was only supported in the persistent ICB cohort, which showed higher total post-STN-DBS LEDD, and a trend towards higher LEDD values before surgery. No associations were found with the magnitude of dopamine agonists reduction or with stimulation parameters as themselves influencing the ICBs outcome. However, more accurate evaluation of electrode placement will be necessary in future studies to analyze whether differences between stimulation of the ventral vs. dorsal portion of the STN play a differential role in whether ICBs remit or persistence.
This study has several limitations. The retrospective design and lack of randomization may have resulted in possible ascertainment biases, which limit the generalization of results. Our findings may only be applicable to the highly selected group of patients that fulfill the strict CAPSIT-PD clinical and neuropsychological criteria for STN-DBS eligibility. We did not use a specific scale for the assessment of ICB, such as the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease-Rating scale (QUIP-RS), which has been validated only in recent years [42]. Moreover, the list of ICBs that have been considered in this study is incomplete, since we did not include compulsive eating. Finally, we analyzed personality disorder traits in patients without overt abnormalities in personality structure, because all subjects with severe personality disorder or any other relevant neuropsychiatric comorbidity were excluded at the time of STN-DBS surgical selection.
In conclusion, our results suggest that: (a) hypersexuality, gambling, and DDS are significantly improved by STN-DBS and may be considered potential targets for surgical treatment; (b) Different mechanisms may be involved in presurgical versus new-onset postsurgical ICB; (c) ICBs appear unrelated to cognitive function; and (d) Higher LEDD and obsessive compulsive personality traits may increase the risk for persistent ICB after STN-DBS; (e) younger age, female gender, and specific personality traits (borderline, schizoid and schizotypal) may represent potential risk factors for ICB in the cohort of post-surgical STN-DBS patients. A prospective randomized controlled clinical trial will be required to confirm and extend these preliminary observations and further clarify the complex association between STN-DBS and impulsive behaviors in relationship with the clinical, demographic, pharmacological factors, and patient’s pre-surgical neuropsychiatric assessment.
References
Voon V, Fox SH (2007) Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch Neurol 64:1089–1096
Adler CH (2005) Nonmotor complications in Parkinson’s disease. Mov Disord 20:S23–S29
O’Sullivan SS, Evans AH, Lees AJ (2009) Dopamine dysregulation syndrome: an overview of its epidemiology, mechanisms and management. CNS Drugs 23:157–170
Evans AH, Katzenschlager R, Paviour D, O’Sullivan JD, Appel S, Lawrence AD, Lees AJ (2004) Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord 19:397–405
Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE (2010) Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 67:589–595
Voon V, Mehta AR, Hallett M (2011) Impulse control disorders in Parkinson’s disease: recent advances. Curr Opin Neurol 24:324–330
Voon V, Fernagut PO, Wickens J, Baunez C, Rodriguez M, Pavon N, Juncos JL, Obeso JA, Bezard E (2009) Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol 8:1140–1149
Silveira-Moriyama L, Evans AH, Katzenschlager R, Lees AJ (2006) Punding and dyskinesias. Mov Disord 21:2214–2217
Voon V, Hassan K, Zurowski M, de Souza M, Thomsen T, Fox S, Lang AE, Miyasaki J (2006) Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology 67:1254–1257
Callesen MB, Weintraub D, Damholdt MF, Møller A (2014) Impulsive and compulsive behaviors among Danish patients with Parkinson’s disease: prevalence, depression, and personality. Parkinsonism Relat Disord 20:22–26
Poletti M, Bonuccelli U (2012) Impulse control disorders in Parkinson’s disease: the role of personality and cognitive status. J Neurol 259:2269–2277
Pontieri FE, Assogna F, Pellicano C, Cacciari C, Pannunzi S, Morrone A, Danese E, Caltagirone C, Spalletta G (2015) Sociodemographic, neuropsychiatric and cognitive characteristics of pathological gambling and impulse control disorders NOS in Parkinson’s disease. Eur Neuropsychopharmacol 25:69–76
Weintraub D (2009) Impulse control disorders in Parkinson’s disease: prevalence and possible risk factors. Parkinsonism Relat Disord 15:S110–S113
Pontone G, Williams JR, Bassett SS, Marsh L (2006) Clinical features associated with impulse control disorders in Parkinson disease. Neurology 67:1258–1261
Cormier F, Muellner J, Corvol JC (2013) Genetics of impulse control disorders in Parkinson’s disease. J Neural Transm (Vienna) 120:665–671
Eisenegger C, Knoch D, Ebstein RP, Gianotti LR, Sándor PS, Fehr E (2010) Dopamine receptor D4 polymorphism predicts the effect of L-DOPA on gambling behavior. Biol Psychiatry 67:702–706
Morgante F, Fasano A, Ginevrino M, Petrucci S, Ricciardi L, Bove F, Criscuolo C, Moccia M, De Rosa A, Sorbera C, Bentivoglio AR, Barone P, De Michele G, Pellecchia MT, Valente EM (2016) Impulsive-compulsive behaviors in parkin-associated Parkinson disease. Neurology. doi:10.1212/WNL.0000000000003177 (Epub ahead of print)
Samuel M, Rodriguez-Oroz M, Antonini A, Brotchie JM, Ray Chaudhuri K, Brown RG, Galpern WR, Nirenberg MJ, Okun MS, Lang AE (2015) Management of impulse control disorders in Parkinson’s disease: controversies and future approaches. Mov Disord 30:150–159
Amami P, Dekker I, Piacentini S, Ferré F, Romito LM, Franzini A, Foncke EM, Albanese A (2015) Impulse control behaviours in patients with Parkinson’s disease after subthalamic deep brain stimulation: de novo cases and 3-year follow-up. J Neurol Neurosurg Psychiatry 86:562–564
Hack N, Akbar U, Thompson-Avila A, Fayad SM, Hastings EM, Moro E, Nestor K, Ward H, York M, Okun MS (2014) Impulsive and compulsive behaviors in Parkinson Study Group (PSG) centers performing deep brain stimulation surgery. J Parkinsons Dis 4:591–598
Moum SJ, Price CC, Limotai N, Oyama G, Ward H, Jacobson C, Foote KD, Okun MS (2012) Effects of STN and GPi deep brain stimulation on impulse control disorders and dopamine dysregulation syndrome. PLoS One 7:e29768
Ardouin C, Voon V, Worbe Y, Abouazar N, Czernecki V, Hosseini H, Pelissolo A, Moro E, Lhommée E, Lang AE, Agid Y, Benabid AL, Pollak P, Mallet L, Krack P (2006) Pathological gambling in Parkinson’s disease improves on chronic subthalamic nucleus stimulation. Mov Disord 21:1941–1946
Witjas T, Baunez C, Henry JM, Delfini M, Regis J, Cherif AA, Peragut JC, Azulay JP (2005) Addiction in Parkinson’s disease: impact of subthalamic nucleus deep brain stimulation. Mov Disord 20:1052–1055
Eusebio A, Witjas T, Cohen J, Fluchère F, Jouve E, Régis J, Azulay JP (2013) Subthalamic nucleus stimulation and compulsive use of dopaminergic medication in Parkinson’s disease. J Neurol Neurosurg Psychiatry 84:868–874
Kim YE, Kim HJ, Kim HJ, Lee JY, Yun JY, Kim JY, Paek SH, Jeon BS (2013) Impulse control and related behaviors after bilateral subthalamic stimulation in patients with Parkinson’s disease. J Clin Neurosci 20:964–969
Lim SY, O’Sullivan SS, Kotschet K, Gallagher DA, Lacey C, Lawrence AD, Lees AJ, O’Sullivan DJ, Peppard RF, Rodrigues JP, Schrag A, Silberstein P, Tisch S, Evans AH (2009) Dopamine dysregulation syndrome, impulse control disorders and punding after deep brain stimulation surgery for Parkinson’s disease. J Clin Neurosci 16:1148–1152
Smeding HM, Goudriaan AE, Foncke EMJ, Schuurman PR, Speelman JD, Schmand B (2007) Pathological gambling after bilateral subthalamic nucleus stimulation in Parkinson disease. J Neurol Neurosurg Psychiatr 78:517–519
Hälbig TD, Tse W, Frisina PG, Baker BR, Hollander E, Shapiro H, Tagliati M, Koller WC, Olanow CW (2009) Subthalamic deep brain stimulation and impulse control in Parkinson’s disease. Eur J Neurol 16:493–497
Rodriguez-Oroz MC, Lopez-Azcarate J, Garcia-Garcia D, Alegre M, Toledo J, Valencia M, Guridi J, Artieda J, Obeso JA (2011) Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson’s disease. Brain 134:36–49
Voon V, Fox SH (2007) Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch Neurol 64:1089–1096
First MB, Gibbon M, Spitzer RL et al (1997) Structured Clinical Interview for DSM-IV Axis II Personality Disorders, (SCID-II). American Psychiatric Press, Washington (DC)
Beck AT (1987) Beck depression inventory. Psychological Corporation, San Antonio (TX)
Spielberger CD, Gorsuch RL, Lushene RE (1980) S.T.A.I. Questionnaire for state and trait anxiety self-assessment. Organizzazioni Speciali, Firenze (IT)
Koss AM, Alterman RL, Tagliati M, Shils JL (2005) Calculating total electrical energy delivered by deep brain stimulation systems. Ann Neurol 58:168–169
Castrioto A, Funkiewiez A, Debû B, Cools R, Lhommée E, Ardouin C, Fraix V, Chabardès S, Robbins TW, Pollak P, Krack P (2015) Iowa gambling task impairment in Parkinson’s disease can be normalised by reduction of dopaminergic medication after subthalamic stimulation. J Neurol Neurosurg Psychiatry 86:186–190
Kish SJ, Shannak K, Hornykiewicz O (1988) Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med 318:876–880
Cools R, Altamirano L, D’Esposito M (2006) Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsychologia 44:1663–1673
Odlaug BL, Schreiber LR, Grant JE (2012) Personality disorders and dimensions in pathological gambling. J Pers Disord 26:381–392
Bagby RM, Vachon DD, Bulmans E, Quilty LC (2008) Personality disorders and pathological gambling: a review and re-examination of prevalence rates. J Pers Disord 22:191–207
Brusa L, Pavino V, Massimetti MC, Ceravolo R, Stefani S, Stanzione P (2016) Pathological Gambling in Parkinson’s disease patients: dopaminergic medication or personality traits fault? J Neurol Sci 366:167–170
Siri C, Cilia R, Reali E, Pozzi B, Cereda E, Colombo A, Meucci N, Canesi M, Zecchinelli AL, Tesei S, Mariani CB, Sacilotto G, Zini M, Pezzoli G (2015) Long-term cognitive follow-up of Parkinson’s disease patients with impulse control disorders. Mov Disord 30:696–704
Weintraub D, Mamikonyan E, Papay K, Shea JA, Xie SX, Siderowf A (2012) Questionnaire for impulsive-compulsive disorders in Parkinson’s disease-rating scale. Mov Disord 27:242–247
Acknowledgments
Authors acknowledge the contributions of the Neurology Unit staff at the San Giovanni Battista Hospital, Turin and the Gardner Family Center for Parkinson’s Disease and Movement Disorders at the University of Cincinnati.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Merola has received grant support from UCB Pharma and speaker honoraria from CSL Behring, UCB Pharma and Teva Pharmaceuticals. He has received personal compensation from Edge Consulting S.r.l., MediK S.r.l. and Sthetos S.r.l. Dr. Romagnolo has received grant support from AbbVie and travel grants from Novartis and Merck Serono. He has received personal compensation from Edge Consulting S.r.l. and Sthetos S.r.l. Dr. Rizzi declares no conflicts of interests. Dr. Rizzone received speaker and/or consulting honoraria from Medtronic, Lundbeck, UCB Pharma and AbbVie. Dr. Zibetti received speaker and/or consulting honoraria from Medtronic, Lundbeck, UCB Pharma and AbbVie. Prof. Lanotte received honoraria for lecturing and travel grants from Medtronic. Dr. Duker has served as a consultant for Merz Pharmaceuticals, US World Meds, and Auspex Pharmaceuticals and has received honoraria from UCB. Dr. Mandybur received honoraria for lecturing and travel grants from Medtronic. Dr. Espay is supported by the NIH (K23MH092735) and has received grant support from CleveMed/Great Lakes Neurotechnologies, Davis Phinney Foundation, and Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Solvay, Abbott, Chelsea Therapeutics, TEVA, Impax, Merz, Lundbeck, and Eli Lilly; honoraria from TEVA, UCB, the American Academy of Neurology, and the Movement Disorders Society; and publishing royalties from Lippincott Williams & Wilkins and Cambridge University Press. Prof. Lopiano received honoraria for lecturing and travel grants from Medtronic, UCB Pharma and AbbVie.
Funding statement
Nothing to declare.
Ethical standards
The authors declare that they acted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The ethical committees approval was obtained (Comitato Etico Interaziendale Città della Salute e della Scienza di Torino; CS/855; Prot. no. 475/2016) and all patients gave their written informed consent to participate at the study.
Rights and permissions
About this article
Cite this article
Merola, A., Romagnolo, A., Rizzi, L. et al. Impulse control behaviors and subthalamic deep brain stimulation in Parkinson disease. J Neurol 264, 40–48 (2017). https://doi.org/10.1007/s00415-016-8314-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8314-x