Abstract
Brain injury caused by stroke has a high rate of mortality and remains a major medical challenge worldwide. In recent years, there has been significant attention given to the use of human Umbilical cord-derived Mesenchymal Stem Cells (hUC-MSCs) for the treatment of stroke in different adult and neonate animal models of stroke. However, using hUC-MSCs by systemic administration to treat ischemic stroke has not been investigated sufficiently. In this study, we conducted various experiments to explore the neuroprotection of hUC-MSCs in rats. Our findings demonstrate that an intravenous injection of a high dose of hUC-MSCs at 2 × 10^7 cells/kg markedly ameliorated brain injury resulting from ischemic stroke. This improvement was observed one day after inducing transient middle cerebral artery occlusion (MCAO) and subsequent reperfusion in rats. Notably, the efficacy of this single administration of hUC-MSCs surpassed that of edaravone, even when the latter was used continuously over three days. Mechanistically, secretory factors derived from hUC-MSCs, such as HGF, BDNF, and TNFR1, ameliorated the levels of MDA and T-SOD to regulate oxidative stress. In particular, TNFR1 also improved the expression of NQO-1 and HO-1, important proteins associated with oxidative stress. More importantly, TNFR1 played a significant role in reducing inflammation by modulating IL-6 levels in the blood. Furthermore, TNFR1 was observed to influence the permeability of the blood–brain barrier (BBB) as demonstrated in the evan’s blue experiment and protein expression of ZO-1. This study represented a breakthrough in traditional methods and provided a novel strategy for clinical medication and trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke, the second leading cause of death in the world, has an extremely high proportion of disability and mortality, which can be classified into hemorrhagic stroke and ischemic stroke [1]. Notably, the rate of ischemic stroke accounts for 85% of all strokes and affects approximately 15 million people annually [2, 3]. Generally, patients with stroke show hemiplegia, inarticulate, and ataxia, which significantly impact their quality of life and place a substantial burden on their families [4]. Previous research has indicated that cardiovascular diseases, including hypertension and hyperlipidemia, as well as metabolic diseases like diabetes and lipid metabolism disorder, play a crucial role in triggering stroke [5]. Currently, cerebrovascular embolism is widely recognized as the primary cause of ischemic stroke [6]. The major pathological mechanism of ischemic stroke involves the inadequate supply of oxygen and essential substrates, like glucose, to the brain during transient ischemia, resulting in damage to the affected brain region. Reperfusion of cerebral blood flow after ischemia has also garnered significant interest among researchers due to its potential to cause irreversible secondary damage in the ischemic region [7]. Besides, permanent ischemia is associated with higher mortality rates [8, 9]. Thus, considering the complex nature of pathological mechanisms and the variability of diseases, ischemic stroke continues to be a significant global medical challenge that requires resolution.
Previous research has demonstrated that inflammation, oxidative stress, cellular excitatory toxicity, and cell death processes are the primary molecular mechanisms of stroke-induced brain injury [10]. According to the pathogenesis, thrombolysis is the predominant effective therapeutic strategy apart from mechanical thrombectomy. Recombinant tissue plasminogen activator (rt-PA) is an example of such strategies, as it can timely protect patients from ischemic stroke injury. However, the side effects of rt-PA, particularly the potential for cerebral hemorrhage and worsening stroke damage, contribute to a poor prognosis for patients [11]. Therefore, longer therapeutic time windows, enhanced repair capabilities, and prolonged effectiveness of new therapeutic drugs and strategies received the world’s attention.
Mesenchymal Stem Cells (MSCs) are pluripotent stem cells that share common characteristics with other types of stem cells, such as self-renewal and multi-directional differentiation. MSCs can be derived from various tissues, including bone marrow, adipose tissue, and umbilical cord blood, and thus have their unique treatment characteristics [12].With the swift advancement in research, the transplantation of MSCs has emerged as a promising therapeutic method for resolving ischemic diseases, including stroke, coronary artery disease, and peripheral artery disease, with excellent therapeutic effects [13,14,15]. Multiple reports have demonstrated the remarkable neuroprotective effect of MSCs in protecting patients from ischemic stroke injuries [16,17,18]. Additionally, MSCs possess vital characteristics that promote the repair of damaged tissues [19], which is particularly beneficial for patients who are unable to receive timely treatment when an ischemic stroke occurs. So far, mechanism studies have demonstrated that MSCs can repair neural function, reduce the area of cerebral infarction, and promote vascular and nerve regeneration to alleviate brain damage caused by ischemic stroke, whose beneficial effects are attributed to the paracrine secretion, immunoregulation, and regulation of cell apoptosis [20,21,22]. In recent years, researchers have been increasingly interested in using human umbilical cord-derived MSCs (hUC-MSCs) to treat complex diseases such as liver fibrosis, subacute spinal cord injury, and acute kidney injury [23,24,25].
Still, there is inadequate research on the preclinical phase and mechanisms of hUC-MSCs especially by systemic administration in mitigating brain injury from ischemic stroke. Thus, in this study, we aimed to determine the administration route, therapeutic time window, and multiple-dose study of hUC-MSCs in an in vivo model of ischemic stroke. Edaravone, a clinically proven neuroprotective agent in ischemic stroke, effectively clears free radicals during the acute phase [26, 27]. In this study, we hypothesized that the mechanism of hUC-MSCs is similar to that of edaravone. Thus, we selected edaravone as the positive control. Additionally, we further investigated the mechanism of action of hUC-MSCs to provide a research foundation and new therapeutic strategy for future clinical medication and trials of ischemic stroke.
Materials and Methods
Human UC-MSCs Culture
Human UC-MSCs were obtained from Baylx Biotech Co., Ltd (Beijing, China) and cultured in Dulbecco’s modified Eagle’s medium: nutrient mixture F12 (DMEM/F12, Invitrogen, California, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, California, USA) and incubated at 37 °C in a 5% CO2 environment. For the experiments, cells from passages 6 to 7 were used. The characteristics of the hUC-MSCs were determined by using a flow cytometer (Beckman, Indiana, USA) and FITC- or PE-conjugated antibodies against specific membrane markers (CD19, CD34, CD31, CD11b, CD45, CD73, CD90, CD105 and HLA-DR). In this study, the hUC-MSCs were suspended in saline for injection purposes.
Small Interfering RNA Transfection
Human UC-MSCs were transfected with 10 nM siRNA against tumor necrosis factor receptor 1 (labeled as siTNFR1-MSCs, Invitrogen), brain-derived neurotrophic factor (labeled as siBDNF-MSCs, Invitrogen), hepatocyte growth factor (labeled as siHGF-MSCs, Synbio China) or negative control siRNA (labeled as siNC-MSCs, Synbio China) for 24 h (Table 1). Transfection was performed using Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific, Massachusetts, USA) according to the manufacturer's instructions. The effect of siRNA on hUC-MSCs was verified using PCR (Supplementary Fig. 1). The primers were synthesized by Genewiz Biotechnology Co., Ltd (Suzhou, China) (Table 2). Subsequent assessments of cytodifferentiation, immunophenotype and cell proliferation of hUC-MSCs post-siRNA transfection were illustrated in Supplementary Fig. 2.
Rat Transient Middle Cerebral Artery Occlusion (MCAO) Model
Male Sprague–Dawley rats (7 weeks old, weighing 250–280 g) were obtained from Beijing SiPeifu Biotech Co., Ltd. (Beijing, China). The animals were treated humanely and maintained in a temperature and humidity-controlled room with a 12-h light/dark cycle, food and water available ad libitum. All experimental procedures involving animals were conducted by the national legislation and approved by the Laboratory Animal Ethics Committee (IACUC NO: IACUC20211123-04, IACUC20220408-04, IACUC20220408-03, Yugen Pharmaceutical Technology Co., Ltd, Tianjin, China).
Animals were allowed to acclimatize to the laboratory 1 week before experiments. Rats were anesthetized with isoflurane via a face mask and kept in the supine position. Throughout the surgical procedure, the body temperature was maintained at 37 °C. Middle cerebral artery occlusion (MCAO) and reperfusion surgery was conducted corresponding to previous report [28]. Rats in the sham group underwent the same procedure without MCAO. Following reperfusion for 24 h, a neurological function assessment was conducted to score the degree of neurological impairment based on predefined criteria. Rats exhibiting obvious neurological deficits (score ≥ 8) were selected for subsequent experiments in this study.
Neurological Severity Score Determination
Modified Neurological Severity Scores (mNSS) were used to assess the motor function, reflexes, and balance of rats (normal score: 0; maximal deficit score: 16). For the motor function test, each animal was lifted by its tail to observe and record specific behaviors. These included the flexion of the forelimb and hindlimb, the posture of head raising (0–3), and the gait observed after the animal was placed on the floor (0–3). The reflexes absent test (0–4) included evaluations of the pinna reflex, corneal reflex, panic reflex, and spasticity. Rats with total scores greater than or equal to 8 were considered to have obvious neurological deficits. The improvement rate of mNSS was calculated using the formula: (mNSS before treatment—mNSS after treatment)/mNSS before treatment × 100%.
Experiments Design
Animals with significant neurological deficits following MCAO (score ≥ 8) were randomly allocated into different groups (n = 10). At the end point of experiments, the animals were anesthetized with isoflurane and euthanized, and brain tissues were collected for experiments. Timeline were showed in corresponding Figures.
Experiment 1: Administration route study of hUC-MSCs for treating MCAO rats.
Sham group and model group treated with a cell solvent by intravenous injection (2 mL/kg); treatment groups received hUC-MSCs (5 × 106 cells/kg or 1 × 107 cells/kg) by intravenous injection (2 mL/kg, i.v.) or received hUC-MSCs (5 × 106 cells/kg) by intrathecal injection (80 μL/kg, i.t.) [24]. The treatment was performed 24 h post reperfusion. Modified NSS were performed before treatment and at 1, 3, 7, and 14 days after treatment.
Experiment 2: Therapeutic time window study of hUC-MSCs for treating MCAO rats.
Four treatment groups received hUC-MSCs—designated as hUC-MSCs-1, hUC-MSCs-2, hUC-MSCs-3, and hUC-MSCs-4. The treatment groups were administered a single intravenous injection of hUC-MSCs at a dose of 1 × 107 cells/kg (2 mL/kg) at different time points—3 h, 1 day, 3 days, or 7 days after MCAO and reperfusion, respectively. The sham and model groups received an equivalent volume of cell solvent after one day of reperfusion. Modified NSS were performed before treatment and at 1, 3, 7, 14, 21, and 28 days after treatment.
Experiment 3: Multiple-dose study of hUC-MSCs for treating MCAO rats.
The sham group and model group were treated with a cell solvent. Three groups received different doses of hUC-MSCs (5 × 106 cells/kg, 1 × 107 cells/kg, or 2 × 107 cells/kg) at 24 h after reperfusion with a single intravenous injection (4 mL/kg). A positive control group was treated with Edaravone (6 mg/kg, Simcere, Nanjing, China) immediately after reperfusion via intravenous injection (4 mL/kg) corresponding to previous reports [29, 30], once daily for three days. (1) Short-term study: mNSS were performed before treatment and at 1, 2, and 3 days after treatment. Blood samples were extracted from the jugular vein of each animal at 1 and 3 days after treatment. (2) Long-term study: mNSS were performed before treatment and at 7, 14, 21, and 28 days after treatment.
Experiment 4: Mechanism study of hUC-MSCs for treating MCAO rats.
The sham group and model group were treated with a cell solvent. The hUC-MSCs treatment group was divided into four subgroups: siHGF-MSCs, siTNFR1-MSCs, siBDNF-MSCs, and a negative control group (siNC-MSCs). Each subgroups received intravenous stem cell injections at a dosage of 2 × 107 cells/kg (2 mL/kg) at 24 h after reperfusion. (1) Short-term mechanism study: mNSS were performed before treatment and at 1, 2, and 3 days after treatment. (2) Long-term mechanism study: mNSS were performed before treatment and at 7, 14, 21, and 28 days after treatment. (3) This part consisted of sham group, model group, siRNA negative control group, and siRNA-TNFR1 group. Blood from the abdominal aorta were collected for experiments.
TTC Staining of Brain Tissue
Brain tissue was first thoroughly frozen at liquid nitrogen and transferred to − 20 °C. It was then cut into 2 mm slices and incubated in a 2% solution of 2,3,5-triphenyltetrazolium chloride (TTC) at 37 °C for 5 min. In the imaging, infarct areas appeared white, while non-infarct areas were red. Measuring the infarct volumes with image J software and calculating the percentage of infarct volumes of total brain area by an investigator who was blind to the groups. The formula used was: Infarct volumes percentage (%) = infarct volumes / total brain volumes × 100%.
Measurement of Malondialdehyde (MDA) and Total Superoxide Dismutase (T-SOD)
The hippocampus of the brain was homogenized with normal saline to make 10% tissue homogenate. After centrifugation at 3000 rpm for 10 min, the supernatant was collected to determine the content of MDA and T-SOD by using a commercial kit (A003-1-2 and A001-3-2, Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
Peripheral and Neural Inflammatory Factors Detection
Blood samples, collected from the jugular vein or abdominal aorta of rats, were centrifuged at 3000 rpm for 10 min to acquire blood serum and used to detect peripheral inflammatory factors. Brain tissue from rats was homogenized with PBS to prepare the 10% homogenate and used to detect neural inflammatory factors. The supernatant from jugular vein was analyzed to determine the levels of IL-1β, IL-6, TNF-α, IFN-γ, IL-10, VEGF, MCP-1, IL-17A, and IL-12 by using Bead-Based Multiplex Assays (Millipore, Massachusetts, USA) according to manufacturer's instruction. The level of TNF-α, IL-1β, and IL-6 in brain tissue homogenate and blood serum from abdominal aorta were measured using the Rat Quantikine ELISA Kit (R&D, Minneapolis, USA). Results were determined by a fluorescence bioreaction detection system based on Luminex technology (ThermoFisher, Massachusetts, USA).
Blood–Brain Barrier Permeability Test
One hour before the experiment’s endpoint, rats were administered 2% Evan’s Blue solution via tail vein injection at a dosage of 4 mL/kg. Subsequently, the rats were humanely euthanized and underwent cardiac perfusion with PBS to facilitate the collection of brain tissue samples. After preparing the homogenate by mixing brain tissue with formamide and incubating it at 37 °C for 24 h, the supernatant was obtained by centrifuging at 14,000 rpm for 30 min. The optical density of this supernatant was then measured at a wavelength of 630 nm. Simultaneously, Evan's Blue standard samples were also measured to construct a standard curve, which was used for calculating the content in the brain tissue.
Western Blotting Analysis
Total proteins (approximately 30–40 μg) were separated by 10% SDS-PAGE using TGX FastCast Acrylamide Solutions (Bio-Rad, California, USA) and transferred to PVDF membranes by using Trans-Blot Turbo Transfer Packs (Bio-Rad, California, USA) in the Trans-Blot® Turbo™ Transfer System (Bio-Rad, California, USA). After blocking for 2 h at room temperature, the membranes were incubated with the primary antibodies diluted in blocking buffer at 4 °C overnight. Antibodies against NQO-1 (1:2000, ab80588), HO-1 (1: 2000, ab189491), and β-actin (1: 2000, ab8227) were obtained from Abcam (Cambridge, United Kingdom). Antibody against ZO-1 (1: 2000, 21,773-1-AP) was obtained from Proteintech (Rosemont, USA). After incubation with Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (H + L) secondary antibody (1:5000, ab205718) that purchased from Abcam (Cambridge, United Kingdom), the target protein was detected using the BeyoECL Moon Extreme Hypersensitivity Chemiluminescent Kit (Beyotime, Shanghai, China) in the iBright FL1500 gel imaging analysis system (Invitrogen, California, USA). β-actin was used as an internal control to normalize the relative expression of each protein. The optical densities of the bands were quantified using Image J software.
Statistical Analysis
All data are presented as the Mean ± Standard Error of Mean (SEM). Experimental data with homogeneity of variance were analyzed by one-way or two-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test, otherwise, Kruskal–Wallis followed by uncorrected Dunn’s test was used to compare the difference between multiple groups by using GraphPad Prism software (Version 9.0, San Diego, CA). Statistical significance was considered at P < 0.05.
Results
Intravenous Injection of hUC-MSCs is More Effective in Treating Ischemic Stroke
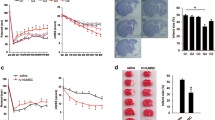
As shown in Fig. 1b, compared with the sham group, the mNSS of the model group was significantly increased after MCAO and reperfusion for 24 h and at 1, 3, 7, and 14 days after treatment (P < 0.001). The main manifestations of rats in the model group were forelimb and posterior limb flexion when lifting the tail, turning to the hemiparetic side during crawling, and falling off during the beam balance test. Compared with the model group, hUC-MSCs treatment (1 × 107 cells/kg) by intravenous injection remarkably decreased the mNSS at 3,7 and 14 days after treatment (P < 0.05 or P < 0.01). At 7 and 14 days after treatment, the hUC-MSCs group (5 × 106 cells/kg, i.v.) represented a noticeable decrease compared with the model group (P < 0.01). The significant difference between the intrathecal injection of hUC-MSCs (5 × 106 cells/kg, i.t.) group and the model group was only detected at 14 days after treatment (P < 0.01).
Administration route study of hUC-MSCs alleviated ischemic stroke induced brain damage in rats. a Timeline about experiment design. b Modified Neurological severity scores (mNSS) of rats at different time points after MCAO and reperfusion according to experiment design and improved rate (%) at different time points compared with pretreatment in rats (n = 10). c Coronal brain sections of rats stained with 2,3,5-triphenyltetrazolium hydrochloride at endpoint. Total cerebral infarction rate (%) and improved rate (%) compared with the model group in rats (n = 10). The infarction area presents white. Data are expressed as the mean ± standard error of mean (SEM). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test or Kruskal–Wallis followed by uncorrected Dunn’s test. ##p < 0.01, ###p < 0.001 compared with the sham group; *p < 0.05, **p < 0.01, ***p < 0.001 compared with the model group with MCAO
Furthermore, after treatment for 7 days, mNSS have been significantly ameliorated by intravenous injection of hUC-MSCs (5 × 106 cells/kg and 1 × 107 cells/kg) compared with the model group (P < 0.001), and all treatment groups have also appeared significant improvement rate at 14 days after treatment compared with the model group (P < 0.01 or P < 0.001).
The results of infarction rate in the brain are shown in Figs. 1c. Compared with the sham group, severe cerebral infarction has appeared in the model group (P < 0.001). Intravenous injection of hUC-MSCs (5 × 106 cells/kg and 1 × 107 cells/kg) significantly decreased infarction rate (P < 0.05 or P < 0.01), with improvements of 15.2% and 20.9%, respectively, in the brain affected by MCAO and reperfusion.
In summary, intravenous injection of hUC-MSCs appears to be an effective administration route for alleviating ischemic stroke induced by MCAO and reperfusion in rats.
24 Hours After Ischemic Stroke is the Best Time for hUC-MSCs Treatment
Compared with the sham group, mNSS of the model group showed significant differences at all time points (P < 0.001, Fig. 2b). However, there was a notable decrease of mNSS in the hUC-MSCs-2 and hUC-MSCs-3 groups (treated after reperfusion for 1 or 3 days) from 7 to 28 days post-treatment (P < 0.05 or P < 0.01). Furthermore, the mNSS of the hUC-MSCs-4 group (treated after reperfusion for 7 days) demonstrated a significant improvement from day 14 to 28 post-treatment (P < 0.05). Intriguingly, an earlier administration (hUC-MSCs-1 group) displayed limited effect.
The therapeutic time windows study of hUC-MSCs alleviated ischemic stroke induced brain damage in rats. a Timeline about experiment design. Timepoints of each groups conducting mNSS listed on the left side of the timeline. Timepoints about rats receiving treatment after MCAO and reperfusion listed on the right side of the timeline. b mNSS of rats at different time points after MCAO and reperfusion according to experiment design and c improved rate (%) at different time points compared with pretreatment in rats (n = 10). d Coronal brain sections of rats stained with 2,3,5-triphenyltetrazolium hydrochloride at endpoint. Total cerebral infarction rate (%) and improved rate (%) compared with the model group in rats (n = 10). The infarction area presents white. Data are expressed as the mean ± standard error of mean (SEM). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test or Kruskal–Wallis followed by uncorrected Dunn’s test. ###p < 0.001 compared with the sham group; *p < 0.05, **p < 0.01, ***p < 0.001 compared with the model group with MCAO
Similar to the significant difference of mNSS between the model group and treatment groups, the hUC-MSCs-2 and hUC-MSCs-4 groups showed a significant improvement in mNSS from day 3 to 28 compared to the severe neurological dysfunction in model group (P < 0.001, Fig. 2c). Similarly, the hUC-MSCs-3 group also exhibited a significant positive impact on the mNSS, with noticeable improvements observed at day 1 and persisting from day 7 to 28 post-treatment (P < 0.05, P < 0.01, or P < 0.001). Moreover, compared to the sham group, the model group exhibited significant cerebral infarction (P < 0.001, Figs. 2d). Additionally, the hUC-MSCs-2 and hUC-MSCs-3 groups significantly reduced the total cerebral infarction rate (P < 0.05), with improvement rates of 22.2% and 19.4%, respectively.
Altogether, our findings revealed that within 1 to 7 days after MCAO and reperfusion is the efficacious time of hUC-MSCs to alleviate ischemic stroke.
High-Dose hUC-MSCs Have Better Therapeutic Effects on Ischemic Stroke
Compared with the obvious neurological dysfunction in the model group (P < 0.001, Fig. 3b), the hUC-MSCs group (2 × 107 cells/kg) and edaravone group significantly decreased mNSS at 2 days after treatment (P < 0.05). The hUC-MSCs group (2 × 107 cells/kg) remarkably decreased mNSS (P < 0.05) with an improved rate of 20.0% at 3 days after treatment. Similarly, the positive control edaravone group (6 mg/kg) also significantly decreased mNSS (P < 0.001) with an improvement rate of 17.6%.
Short-term multiple-dose study of hUC-MSCs alleviated ischemic stroke induced brain damage in rats. a Timeline about experiment design. b mNSS of rats at different time points after MCAO and reperfusion according to experiment design and improved rate (%) 3 days after treatment compared with the model group in rats (n = 10). c Coronal brain sections of rats stained with 2,3,5-triphenyltetrazolium hydrochloride at endpoint (n = 10). The infarction area presents white. d Total cerebral infarction rate (%) and improved rate (%) compared with the model group in rats (n = 10). e Malondialdehyde content and f total superoxide dismutase activity in the hippocampus of the rats brain (n = 10). g Multiple factors concentration in blood serum from jugular vein of rats (n = 10). Data are expressed as the mean ± standard error of mean (SEM). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test or Kruskal–Wallis followed by uncorrected Dunn’s test. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the sham group; *p < 0.05, **p < 0.01, ***p < 0.001 compared with the model group with MCAO
Subsequently, we found that hUC-MSCs treatment groups decreased the total cerebral infarction rate of the model group that has significant differences from the sham group (P < 0.001, Fig. 3c and d). A single injection of hUC-MSCs (2 × 107 cells/kg) significantly alleviated the infarction rate (P < 0.05) with an improvement rate of 32.4% compared with the model group, which was higher than the positive control edaravone group (P < 0.05, 31.1% of improve rate).
Above all, our results indicated that the effect of hUC-MSCs on treating ischemic stroke is dose-dependent, with higher doses of hUC-MSCs (2 × 107 cells/kg) yielding more effective results. Furthermore, one single treatment of hUC-MSCs is better than a continuous injection of edaravone for 3 days.
hUC-MSCs Alleviate Oxidative Stress and Neuroinflammation of Ischemic Stroke
Compared with the sham group, the model group has a significant increase in MDA levels (P < 0.05, Fig. 3e) accompanied by a decreasing trend of T-SOD levels without significant difference (P > 0.05, Fig. 3f). Treatment with hUC-MSCs (5 × 106 cells/kg, 1 × 107 cells/kg, and 2 × 107 cells/kg) and edaravone significantly decreased MDA content (P < 0.05 or P < 0.01) and improved the level of T-SOD (P < 0.05).
Next, we determined the peripheral inflammatory factors levels in the blood serum from jugular vein of rats at 1-day and 3-day post-treatment (Fig. 3g). Compared with the sham group, the levels of TNF-α, IL-6, IL-12 p70, IL-17A, and MCP-1 were significantly increased (P < 0.05, P < 0.01, or P < 0.001) in the model group at 1 day after treatment. Treatment with hUC-MSCs (5 × 106 cells/kg, 1 × 107 cells/kg and 2 × 107 cells/kg) or edaravone have a significant effect ameliorated these changes, which remarkably decreased TNF-α (P < 0.05), IL-6 (P < 0.001), IL-12 p70 (P < 0.05 or P < 0.01), IL-17A (P < 0.05), and hUC-MSCs (2 × 107 cells/kg) notably increased VEGF (P < 0.01) that is the key of promoting angiogenesis.
These data suggested that the potential mechanisms by which hUC-MSCs alleviate ischemic stroke may include ameliorating oxidative stress and neuroinflammation, as well as inducing activation of vascular endothelial cells.
Powerful Long-Term Restorative Effect of hUC-MSCs on Ischemic Stroke
After treatment for 1, 2, 3, and 4 weeks, compared with the model group, hUC-MSCs groups (1 × 107 cells/kg and 2 × 107 cells/kg) significantly decreased mNSS (P < 0.05 ~ 0.001, Fig. 4b), and showed obvious neurological repairment (P < 0.001) with 21.6% and 25.5% improve rate at the end point of study, which was higher than the positive edaravone group (15.7%, Fig. 4b).
Long-term multiple-dose study of hUC-MSCs alleviated ischemic stroke induced brain damage in rats. a Timeline about experiment design. b mNSS of rats at different time points after MCAO and reperfusion according to experiment design and improved rate (%) 4 weeks after treatment compared with the model group in rats (n = 10). c Coronal brain sections of rats stained with 2,3,5-triphenyltetrazolium hydrochloride at endpoint. Total cerebral infarction rate (%) and improved rate (%) compared with the model group in rats (n = 10). The infarction area presents white. d Western blot of oxidative stress relative protein expression in the brain of rats (n = 10). Data are expressed as the mean ± standard error of mean (SEM). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test or Kruskal–Wallis followed by uncorrected Dunn’s test. ###p < 0.001 compared with the sham group; *p < 0.05, **p < 0.01, ***p < 0.001 compared with the model group with MCAO
In the same way, compared with the infarction rate in the brain of the model group (P < 0.001, Fig. 4c), hUC-MSCs groups (1 × 107 cells/kg and 2 × 107 cells/kg) have a significant ameliorating effect to ameliorate total cerebral infarction rate at 4 weeks after treatment (P < 0.05, P < 0.01) with the improvement rates of 37.9% and 43.6% respectively. The positive edaravone group also remarkably decreased brain infarction area (P < 0.05), but the improving outcome (38.5%) was lower than the hUC-MSCs group (2 × 107 cells/kg). Besides, oxidative stress relative protein detection results showed that even if 4 weeks after a single injection, hUC-MSCs (2 × 107 cells/kg) treatment remains significantly decreased HO-1 and NQO-1 protein expression (P < 0.05 and P < 0.01, Fig. 4d) compared with model group, whose HO-1 and NQO-1 expression was higher than sham group (P < 0.01 or P < 0.001).
Thus, the above data suggested that hUC-MSCs possess the effect of enduring prompting repair of neurological dysfunction induced by MCAO and reperfusion, and high-dose hUC-MSCs single injection is better than the continuous injection of edaravone.
Key Factors Secreted by hUC-MSCs Related to Their Therapeutic Effect for Ischemic Stroke
The short-term study revealed that, compared with the model group, the total cerebral infarction rate of the siNC-MSCs group has decreased remarkably (P < 0.05, Fig. 5b). However, the total cerebral infarction rate of siHGF-MSCs and siTNFR1-MSCs groups were significantly higher than siNC-MSCs group (P < 0.05).
Key secretory factors derived from hUC-MSCs ameliorated ischemic stroke induced brain damage of rats in the acute stage. a Timeline about experiment design. b Coronal brain sections of rats stained with 2,3,5-triphenyltetrazolium hydrochloride at endpoint and total cerebral infarction rate (%) in rats (n = 10). The infarction area presents white. c mNSS of rats at different time points after MCAO and reperfusion according to experiment design (n = 10). d Malondialdehyde content and total superoxide dismutase activity in the hippocampus of the rats brain (n = 10). Data are expressed as the mean ± standard error of mean (SEM). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test or Kruskal–Wallis followed by uncorrected Dunn’s test. ##p < 0.01, ###p < 0.001 compared with the sham group. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the model group with MCAO. &p < 0.05, &&p < 0.01 compared with the NC-hUC-MSC group with MCAO
The obvious elevating mNSS in the model group (P < 0.001, Fig. 5c) has been significantly alleviated at 2 and 3 days after treatment with siNC-MSCs (P < 0.05). But this effect was decreased in three genes silencing groups, and the mNSS of the siHGF-MSCs group remarkably increased compared with siNC-MSCs group on the third day after treatment (P < 0.05).
Besides, the MDA level was increased and the T-SOD level was decreased in the model group compared with the sham group (P < 0.001, Fig. 5d). The siNC-MSCs group showed a decreased MDA level (P < 0.001) and an improved T-SOD level (P < 0.01). But siHGF-MSCs and siTNFR1-MSCs groups significantly decreased the effect of hUC-MSCs in MDA (P < 0.01), and there was a significant difference between the gene silencing groups and sham group (P < 0.01 or P < 0.001). Correspondingly, the content of T-SOD in gene silencing groups was decreased but still had a significant difference compared with the sham group (P < 0.01 or P < 0.001).
We also conducted long-term study. The total cerebral infarction rate increased in the model group (P < 0.001, Fig. 6b), which could be significantly alleviated when treated with siNC-MSCs (P < 0.001). Similarly, three genes silencing groups could significantly reverse the effect of hUC-MSCs in total cerebral infarction rate (P < 0.05, P < 0.01, or P < 0.001). Also, hUC-MSCs significantly improved mNSS from day 7 to 28 (P < 0.05 or P < 0.01, Fig. 6c). But this improvement was notably reduced in the siHGF-MSCs group at 7 days post-treatment (P < 0.05).
Key secretory factors derived from hUC-MSCs ameliorated ischemic stroke induced brain damage of rats in the long-term study. a Timeline about experiment design. b Coronal brain sections of rats stained with 2,3,5-triphenyltetrazolium hydrochloride at endpoint and total cerebral infarction rate (%) in rats (n = 10). The infarction area presents white. c mNSS of rats at different time points after MCAO and reperfusion according to experiment design (n = 10). Data are expressed as the mean ± standard error of mean (SEM). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test or Kruskal–Wallis followed by uncorrected Dunn’s test. ###p < 0.001 compared with the sham group. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the model group with MCAO. &p < 0.05, &&p < 0.01, &&&p < 0.001 compared with the NC-hUC-MSC group with MCAO
These findings indicated that HGF and TNFR1 were crucial genes in hUC-MSCs for mitigating brain damage caused by ischemic stroke. Additionally, BDNF from hUC-MSCs may also play a therapeutic role in the recovery phase.
TNFR1 of hUC-MSCs Regulates Inflammation and Oxidative Stress
First, day 3 after treatmen, the results of blood supernatant from abdominal aorta and brain homogenate showed that peripheral and neural inflammatory factors induced by ischemic strokes, such as TNF-α, IL-1β, and IL-6, significantly improved in the model group (P < 0.05, P < 0.01 or P < 0.001, Fig. 7b), while siNC-MSCs significantly decreased the levels of peripheral inflammatory factors (P < 0.05 or P < 0.01). In particular, IL-6 in the blood supernatant of siTNFR1-MSCs has a significant increase compared with that of siNC-MSCs group (P < 0.05).
TNFR1 derived from hUC-MSCs maybe the key of regulating inflammation, oxidative stress and blood–brain barrier permeability in ishchemic stroke induced brain damage of rats. a Timeline about experiment design. b The concentration of peripheral inflammatory factors in blood supernatant from abdominal aorta and neural inflammatory factors in brain tissue of rats (n = 10). c Malondialdehyde content and total superoxide dismutase activity in the hippocampus of the rats brain (n = 10). d Western blot of oxidative stress and blood–brain barrier relative protein expression in the brains of rats (n = 10). e Evan’s blue content in the brains of rats (n = 10). Data are expressed as the mean ± standard error of mean (SEM). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test or Kruskal–Wallis followed by uncorrected Dunn’s test. #p < 0.05, ##p < 0.01, ###p < 0.001 compared with the sham group. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the model group with MCAO. &p < 0.05, &&p < 0.01 compared with the NC-hUC-MSC group with MCAO
Second, MCAO and reperfusion resulted in MDA improvement and T-SOD decrease (P < 0.001 and P < 0.05, Fig. 7c), which could be remarkably reversed by treatment with siNC-MSCs (P < 0.001). Accordingly, the MDA content of the siTNFR1-MSCs group was significantly higher than the siNC-MSCs group (P < 0.01). Compared with the sham group, the model group significantly improved the HO-1 and NQO-1 levels (P < 0.01 and P < 0.001, Fig. 7d), which are the key proteins of oxidative stress. Noticeably, the silencing of TNFR1 expression in hUC-MSCs reversed hUC-MSCs’ effect (P < 0.05) in decreasing NQO-1 expression (P < 0.01).
Third, the results of Evan’s blue test demonstrated a significant improvement in BBB permeability in the model group even at 4 days after treatment (P < 0.001 Fig. 7e), while the siNC-MSCs group had the effect of repairing the BBB permeability (P < 0.01). More important is that siTNFR1-MSCs group remarkably reversed the treatment effect of hUC-MSCs (P < 0.05). Likewise, the expression of the BBB-related protein ZO-1 significantly diminished in the model group (P < 0.01). However, hUC-MSCs treatment (specifically of siNC-MSCs) led to an increase in ZO-1 levels (P < 0.05), although this increase was less pronounced in the siTNFR1-MSCs group.
The above data indicated that TNFR1 was the key molecule of hUC-MSCs involved in alleviating MCAO and reperfusion-induced brain injury, including inflammation, oxidative stress, and BBB permeability improvement.
Discussion
This study showed that the effectiveness of hUC-MSCs in treating ischemic stroke depends on the dosage, with a higher dose of hUC-MSCs (2 × 107 cells/kg) yielding more effective results. We also observed that a single treatment of hUC-MSCs had a greater effect compared to a continuous injection of edaravone for 3 days. Further investigation revealed that treatment with hUC-MSCs showed significant improvement in reducing oxidative stress and neuroinflammation, which are two major mechanisms of ischemic stroke. Interestingly, long-term experiments demonstrated remarkable reparative effect of hUC-MSCs. Of particular importance, this study provided the first evidence that HGF, BDNF, and especially TNFR1 play a key role in the treatment of ischemic stroke using hUC-MSCs.
Stem cell therapy has rapidly emerged as a prominent research field in recent years, garnering significant attention in the life sciences. Recent reports indicated that MSCs derived from bone marrow (BM-MSCs) are particularly effective in ameliorating damage to the brain and spinal marrow, adipose-derived MSCs (AD-MSCs) show greater efficacy in treating ovarian injuries and promoting skin regeneration, and UC-MSCs demonstrate superior efficacy in alleviating pulmonary diseases and acute respiratory distress syndrome [12]. In numerous animal models of ischemic stroke, the transplantation of MSCs has shown remarkable treatment effects [31, 32]. Furthermore, the use of three-dimensional (3D) spheroid-cultured MSCs has demonstrated enhanced reparative effects on ischemic stroke by inhibiting the activation of microglia [33]. Studies have reported that intravenous injection of BM-MSCs resulted in reduced infarct volumes and improved neurological function through their migration to the ischemic border zone of the brain and subsequent differentiation into neurons and astrocytes [34]. AD-MSCs alleviate ischemic stroke through their immunomodulatory properties on microglia [35]. However, systemic administration of hUC-MSCs in treating ischemic stroke have not been systematically studied.
In this study, we investigated the protective and restorative effects of hUC-MSCs on an ischemic stroke rat model induced by MCAO and reperfusion. We also investigated critical aspects including the route of administration, timing of delivery, and optimal dosage. While systemic and local administration have been explored as methods for MSC delivery, intrathecal delivery of MSCs may be considered as an alternative to direct injection, allowing for better accumulation of stem cells. However, it is important to note that this invasive method may come with inevitable complications [36, 37]. In comparison, intravenous injection of MSCs has been widely used in preclinical and clinical research on stroke, showing promising therapeutic potential in improving neurological recovery [38,39,40]. Similarly, our findings indicated that intravenous injection is a more effective route for administering hUC-MSCs to alleviate ischemic stroke in rats. However, it is important to note that this method may induce side effects in certain individuals, such as anaphylaxis accompanied by symptoms similar to fever [41, 42].
Clinical research has demonstrated that the narrow therapeutic time window of traditional methods, specifically only 4.5 h, restricts the number of patients who can receive timely treatment with rt-PA [43]. Additionally, the risk of recurrent stroke increases over time [44]. In contrast, our study overcomes this limitation by using hUC-MSCs to effectively alleviate brain injury caused by ischemic stroke, even within 24 h of onset. And the preferable time of hUC-MSCs to alleviate ischemic stroke is within 1 to 7 days after MCAO and reperfusion (acute stage), and treatment with hUC-MSCs at the hyperacute stage has shown limited effectiveness, which may subvert the conventional wisdom that the sooner get treatment after ischemic stroke the better recovery. Furthermore, we found that the beneficial treatment and reparative effects of hUC-MSCs persisted for a long time, even up to day 28, surpassing the effects of edaravone administered consecutively for 3 days. Compared to the limitations of traditional methods, such as their narrow therapeutic time window, poor prognosis, and associated side effects, this novel therapeutic strategy presents a viable option for patients who cannot receive prompt drug intervention for brain damage caused by ischemic stroke. It offers a sustained repair effect and minimizes secondary damage often seen with conventional treatments. Additionally, this study identifies an optimal dosage of hUC-MSCs at 2 × 107 cells/kg for clinical use.
The pathological mechanisms underlying ischemic stroke encompass a range of molecular processes, such as oxidative stress, inflammation, calcium overload, and apoptosis [45]. Oxidative stress and neuroinflammation are important pathological mechanisms involved in ischemic stroke-induced brain damage [46]. Oxidative stress, considered a major mechanism in ischemic stroke, has been extensively studied. During the early stage of ischemic stroke, the intracellular concentration of Ca2+ remarkably increases due to reduced blood flow in the brain, leading to the activation of oxidative stress [47]. In central nervous system disorders, a notable correlation exists between the excessive production of reactive oxygen species (ROS) and the increased expression of NADPH oxidase (NOX) in cells. This process plays a crucial role in causing oxidative stress [48,49,50]. Thus, inhibiting the production of ROS or enhancing the scavenging activity of ROS are effective strategies for treating ischemic stroke [51]. SOD, an antioxidant enzyme, can efficiently lower ROS levels, similar to MDA, which indicates the extent of lipid peroxidation. Both SOD and MDA serve as crucial biomarkers for oxidative stress and indirectly signify the levels of ROS [52, 53]. Thus, regulating oxidative stress is an essential aspect of treating ischemic stroke. Our study found that hUC-MSCs effectively ameliorated oxidative stress in the hippocampus by reducing MDA levels and increasing T-SOD levels. These results indicated that the antioxidant capacity of hUC-MSCs plays a crucial role in combating oxidative stress in ischemic stroke.
Numerous reports have demonstrated the significant impact of neuroinflammation on ischemic stroke [54,55,56]. Microglia, a vital immunocyte in the central nervous system, undergo rapid activation following an ischemic stroke and release proinflammatory factors, notably IL-6, IL-1β and TNF-α, which contribute to brain damage by causing neuronal cell death and disrupting the integrity of the vascular endothelial cells in the BBB [57,58,59]. This inflammatory signaling is primarily regulated by the NF-κB pathway, which plays a key role in activating microglial cells and inducing neuroinflammation [60,61,62,63]. A recent report has shown that apoptosis induced by ischemic stroke can be alleviated through co-culture with MSCs, resulting in reduced inflammation [58]. Similarly, hUC-MSCs reduced inflammatory factors, including TNF-α, IL-10 and IL-17, in peripheral blood serum from mice after stroke [64]. This study was consistent with previous researches, hUC-MSCs demonstrate excellent anti-inflammatory activity in vivo by detecting peripheral and neural inflammatory factors, especially in the decrease of IL-6. The combination of anti-inflammation and anti-oxidative stress capabilities in hUC-MSCs plays a crucial role in ameliorating damage and promoting long-term repair in rats with ischemic stroke induced by MCAO and reperfusion.
Based on the aforementioned study, we conducted additional research to explore the potential molecular mechanisms through which hUC-MSCs alleviate brain injury caused by MCAO and reperfusion. Previous reports have shown that BDNF, a neurotrophin primarily secreted by astrocytes and vital for neuron function, plays a neuroprotective role in ischemic brain injury by regulating various molecular signaling pathways, with its binding to the TrkB receptor being particularly important [65,66,67,68]. BDNF is a well-known regulatory factor in the treatment of stroke for its ability to ameliorate oxidative stress [69]. Furthermore, it's noted that HGF performs diverse regulatory roles in various cell types by activating its receptor, the tyrosine kinase cMet [70]. Mechanically, HGF is key in mitigating damage caused by hypoxia, which is closely associated with oxidative stress, a frequent trait of ischemic stroke [71]. Furthermore, this study not only observed a marked increase in inflammation, but other research has also demonstrated that TNF-α, a key proinflammatory cytokine in the intricate inflammation network, can initiate pro-survival signaling pathways by binding to its receptors like TNFR1 [72, 73]. Previous research has shown that the activated TNFR1 pathway can protect rats from liver injury caused by LPS by reducing oxidative stress [74]. Thus, we hypothesized that BDNF, HGF, and TNFR1 are the possible key molecules in alleviating brain injury caused by ischemic stroke, therefore we conducted experiments to verify the involvement of these molecules. First, our findings revealed that HGF and TNFR1 are particularly significant in regulating the treatment of acute ischemic stroke using hUC-MSCs, which was based on the results of the total cerebral infarction rate, mNSS scores, and the regulation of oxidative stress by reducing MDA content. Subsequent experiments demonstrated that BDNF and TNFR1 exhibited superior therapeutic and repair effects in long-term studies. All three molecules derived from hUC-MSCs displayed remarkable abilities to ameliorate brain injury caused by MCAO and reperfusion at various stages of ischemic stroke.
Building upon the above findings, we conducted additional experiments to further investigate the underlying mechanism of TNFR1 in mitigating ischemic stroke. Consistent with previous reports, we discovered that the anti-inflammatory effect of hUC-MSCs was associated with TNFR1, particularly the concentration of IL-6 in blood, which plays a crucial role in the TNF-α induced inflammatory cascade reaction. This can be attributed to the competitive inhibitory effect of TNFR1 derived from hUC-MSCs, which hinders the binding of TNF-α to TNFR1 on cells, thereby alleviating inflammation.
Furthermore, oxidative stress plays a central role in brain injury caused by ischemic stroke and is closely related to the levels of ROS in the brain. The Nrf2 signaling pathway, which is a vital system for combating oxidative stress in the body, effectively reduces apoptosis and oxidative stress induced by ischemic stroke by regulating the transcriptional expression of various antioxidant proteins, including NQO-1 and HO-1 [75]. Recent reports have demonstrated that MSCs reduce apoptosis through the Nrf2 pathway, and TNF-α plays a key role in regulating this pathway in cardiomyocytes [76, 77]. Therefore, the TNF-α/TNFR1 pathway may be the crucial link connecting inflammation and oxidative stress. Our results confirmed the aforementioned hypothesis that TNFR1 from hUC-MSCs has a significant regulatory effect on oxidative stress, as evidenced by the changes in MDA content and NQO-1 expression.
Additionally, it is widely recognized that the BBB plays a crucial role in protecting the central nervous system, and damage to the BBB is a key factor contributing to secondary brain injury following central nervous system diseases like stroke [78]. Recent research has reported that necroptosis of cells in BBB, a passive form of cell death caused by overwhelming inflammation or injury stress, was the primary mechanism of brain injury induced by ischemic stroke [79, 80]. A previous report has demonstrated that glucose oxygen deprivation and reperfusion (OGD/R) significantly induce the secretion of TNF-α in microglia is considered a critical factor that triggers necroptosis of endothelial cells in in vitro experiments, which in turn leads to the disruption of the BBB [81]. Reports have indicated that MSCs can protect BBB stability from stroke by regulating inflammatory factors [58, 82]. Consistent with the above reports, our study found that TNFR1 derived from hUC-MSCs protects the permeability and integrity of BBB from damage caused by MCAO and reperfusion, which can be confirmed by the expression of ZO-1, a biomarker of BBB. Above findings indicated that TNFR1 derived from hUC-MSCs is the most critical molecule in alleviating MCAO and reperfusion-induced brain injury by modulating inflammation and oxidative stress and repairing the BBB to maintain brain homeostasis.
Taken together, this study has shown that intravenous injection of hUC-MSCs at 2 × 107 cells/kg following MCAO and 24-h reperfusion effectively reduced brain injury from ischemic stroke in rats. Additionally, our findings suggested that the reduction of neuroinflammation and oxidative stress, along with the repair of the BBB, were potential mechanisms by which hUC-MSCs alleviated ischemic stroke. Key molecules in this process include HGF, TNFR1, and BDNF, with TNFR1 being particularly significant. Overall, this study indicated that hUC-MSCs could represent a novel approach for treating brain injury in ischemic stroke and offered important insights for developing clinical protocols for future trials involving hUC-MSCs.
Data Availability
All authors have confrmed that all data and materials support their published claims and comply with feld standards.
References
Shinozuka K et al (2013) Stem cells for neurovascular repair in stroke. J Stem Cell Res Ther 4(4):12912
Cao W et al (2015) Effectiveness and safety of autologous bone marrow stromal cells transplantation after ischemic stroke: a meta-analysis. Med Sci Monit 21:2190–2195
Roy-O’Reilly M et al (2014) Sex differences in stroke: the contribution of coagulation. Exp Neurol 259:16–27
Hankey GJ (2017) Stroke. Lancet 389(10069):641–654
Kleindorfer DO et al (2021) 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the american heart association/american stroke association. Stroke 52(7):e364–e467
Phipps MS, Cronin CA (2020) Management of acute ischemic stroke. BMJ 368:l6983
Guo X et al (2023) Ischemia reperfusion injury induced blood brain barrier dysfunction and the involved molecular mechanism. Neurochem Res 48(8):2320–2334
Zhu H et al (2022) Interleukins and ischemic stroke. Front Immunol 13:828447
Sun MS et al (2018) Free Radical Damage in Ischemia-Reperfusion Injury: An Obstacle in Acute Ischemic Stroke after Revascularization Therapy. Oxid Med Cell Longev 2018:3804979
Su Z et al (2022) Pathophysiology of ischemic stroke: noncoding RNA role in oxidative stress. Oxid Med Cell Longev 2022:5815843
Kleindorfer D et al (2004) Eligibility for recombinant tissue plasminogen activator in acute ischemic stroke: a population-based study. Stroke 35(2):e27–e29
Hoang DM et al (2022) Stem cell-based therapy for human diseases. Signal Transduct Target Ther 7(1):272
Doeppner TR et al (2015) Extracellular vesicles improve post-stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Transl Med 4(10):1131–1143
Hughey CC et al (2013) Mesenchymal stem cell transplantation for the infarcted heart: therapeutic potential for insulin resistance beyond the heart. Cardiovasc Diabetol 12:128
Schmehl J et al (2021) Intravascular application of labelled cell spheroids: an approach for ischemic peripheral artery disease. Int J Mol Sci 22(13):6831
Wang LQ et al (2014) Timing and dose regimens of marrow mesenchymal stem cell transplantation affect the outcomes and neuroinflammatory response after ischemic stroke. CNS Neurosci Ther 20(4):317–326
Shinozuka K et al (2013) Stem cell transplantation for neuroprotection in stroke. Brain Sci 3(1):239–261
Ishizaka S et al (2013) Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 44(3):720–726
Guillamat-Prats R (2021) The role of MSC in wound healing scarring and regeneration. Cells 10(7):1729
Shen Z et al (2021) Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front Immunol 12:749192
Chang D et al (2021) Application of mesenchymal stem cell sheet to treatment of ischemic heart disease. Stem Cell Res Ther 12(1):384
Psaraki A et al (2022) Extracellular vesicles derived from mesenchymal stem/stromal cells: the regenerative impact in liver diseases. Hepatology 75(6):1590–1603
Lin Y et al (2022) Huc-MSC-derived exosomes modified with the targeting peptide of aHSCs for liver fibrosis therapy. J Nanobiotechnology 20(1):432
Liu G et al (2022) Therapeutic efficacy of human mesenchymal stem cells with different delivery route and dosages in rat models of spinal cord injury. Cell Transpl 31:9636897221139734
Huang J et al (2022) Mesenchymal stem cells-derived exosomes ameliorate ischemia/reperfusion induced acute kidney injury in a porcine model. Front Cell Dev Biol 10:899869
Wang XX et al (2022) NADPH is superior to NADH or edaravone in ameliorating metabolic disturbance and brain injury in ischemic stroke. Acta Pharmacol Sin 43(3):529–540
Röther J et al (2013) Thrombolytics in acute ischaemic stroke: historical perspective and future opportunities. Cerebrovasc Dis 35(4):313–319
Chen J et al (2001) Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32(4):1005–1011
Liu W et al (2022) Edaravone ameliorates cerebral ischemia-reperfusion injury by downregulating ferroptosis via the Nrf2/FPN pathway in rats. Biol Pharm Bull 45(9):1269–1275
Kikuchi K et al (2009) Edaravone attenuates cerebral ischemic injury by suppressing aquaporin-4. Biochem Biophys Res Commun 390(4):1121–1125
Li Y et al (2021) Strategies to improve the efficiency of transplantation with mesenchymal stem cells for the treatment of ischemic stroke: a review of recent progress. Stem Cells Int 2021:9929128
Guo Y et al (2021) Progress in mesenchymal stem cell therapy for ischemic stroke. Stem Cells Int 2021:9923566
Li Y et al (2021) Three-dimensional cultured mesenchymal stem cells enhance repair of ischemic stroke through inhibition of microglia. Stem Cell Res Ther 12(1):358
Song M et al (2017) Human dental pulp stem cells are more effective than human bone marrow-derived mesenchymal stem cells in cerebral ischemic injury. Cell Transpl 26(6):1001–1016
Sánchez-Castillo AI et al (2022) Switching roles: beneficial effects of adipose tissue-derived mesenchymal stem cells on microglia and their implication in neurodegenerative diseases. Biomolecules 12(2):219
Deng L et al (2019) Intrathecal injection of allogenic bone marrow-derived mesenchymal stromal cells in treatment of patients with severe ischemic stroke: study protocol for a randomized controlled observer-blinded trial. Transl Stroke Res 10(2):170–177
Ran Y et al (2022) Mesenchymal stem cell aggregation mediated by integrin α4/VCAM-1 after intrathecal transplantation in MCAO rats. Stem Cell Res Ther 13(1):507
Chen Y et al (2023) A comparative study of different doses of bone marrow-derived mesenchymal stem cells improve post-stroke neurological outcomes via intravenous transplantation. Brain Res 1798:148161
Ghazavi H et al (2017) Fibroblast growth factor type 1 (FGF1)-overexpressed adipose-derived mesenchaymal stem cells (AD-MSCFGF1) induce neuroprotection and functional recovery in a rat stroke model. Stem Cell Rev Rep 13(5):670–685
Bang OY et al (2022) Circulating extracellular vesicles in stroke patients treated with mesenchymal stem cells: a biomarker analysis of a randomized trial. Stroke 53(7):2276–2286
Meng F et al (2020) Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther 5(1):172
Lu J et al (2021) One repeated transplantation of allogeneic umbilical cord mesenchymal stromal cells in type 1 diabetes: an open parallel controlled clinical study. Stem Cell Res Ther 12(1):340
Wang X et al (2008) Targeting extracellular matrix proteolysis for hemorrhagic complications of tPA stroke therapy. CNS Neurol Disord Drug Targets 7(3):235–242
Mohan KM et al (2011) Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke 42(5):1489–1494
Huang Y et al (2021) CUEDC2 ablation enhances the efficacy of mesenchymal stem cells in ameliorating cerebral ischemia/reperfusion insult. Aging (Albany NY) 13(3):4335–4356
Chamorro Á et al (2016) Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol 15(8):869–881
Ludhiadch A et al (2022) Role of calcium homeostasis in ischemic stroke: a review. CNS Neurol Disord Drug Targets 21(1):52–61
Barua S et al (2019) The role of NOX inhibitors in neurodegenerative diseases. IBRO Rep 7:59–69
Su XT et al (2020) Mechanisms of acupuncture in the regulation of oxidative stress in treating ischemic stroke. Oxid Med Cell Longev 2020:7875396
Lou Z et al (2018) Upregulation of NOX2 and NOX4 mediated by TGF-β Signaling pathway exacerbates cerebral ischemia/reperfusion oxidative stress injury. Cell Physiol Biochem 46(5):2103–2113
Li G et al (2022) Oxidative injury in ischemic stroke: a focus on NADPH oxidase 4. Oxid Med Cell Longev 2022:1148874
Mao L et al (2020) Low expression of miR-532-3p contributes to cerebral ischemia/reperfusion oxidative stress injury by directly targeting NOX2. Mol Med Rep 22(3):2415–2423
Samarghandian S et al (2017) Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother 87:223–229
Dziedzic T (2015) Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother 15:523–531
Pluta R et al (2021) Neuroinflammation in post-ischemic neurodegeneration of the brain: friend, foe, or both? Int J Mol Sci 22:1–16
Nakamura K, Shichita T (2019) Cellular and molecular mechanisms of sterile inflammation in ischaemic stroke. J Biochem 165:459–464
Kim E, Cho S (2016) Microglia and monocyte-derived macrophages in stroke. Neurotherapeutics 13(4):702–718
Dabrowska S et al (2019) Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation 16(1):178
Picascia A et al (2015) Innate and adaptive immune response in stroke: focus on epigenetic regulation. J Neuroimmunol 289:111–120
Guo H et al (2020) Selective activation of estrogen receptor β alleviates cerebral ischemia neuroinflammatory injury. Brain Res 1726:146536
Harari OA, Liao JK et al (2010) NF-kappaB and Innate Immunity in Ischemic Stroke. Ann N Y Acad Sci 1207:32–40
Dong L et al (2016) The E3 ubiquitin ligase c-Cbl inhibits microglia-mediated CNS inflammation by regulating PI3K/Akt/NF-kappaB pathway. CNS Neurosci Ther 22:661–669
Patel H et al (2017) Anti-inflammatory effects of astroglial alpha7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-kappaB pathway and activation of the Nrf2 pathway. J Neuroinflammation 14:192
Cheng Q et al (2015) Human umbilical cord mesenchymal stem cells protect against ischemic brain injury in mouse by regulating peripheral immunoinflammation. Brain Res 1594:293–304
Liu W et al (2020) Brain-derived neurotrophic factor and its potential therapeutic role in stroke comorbidities. Neural Plast 2020:1969482
Choo M et al (2017) Retrograde BDNF to TrkB signaling promotes synapse elimination in the developing cerebellum. Nat Commun 8(1):195
Li C et al (2020) Cholic acid protects in vitro neurovascular units against oxygen and glucose deprivation-induced injury through the BDNF-TrkB signaling pathway. Oxid Med Cell Longev 2020:1201624
Ya BL et al (2018) Uric acid protects against focal cerebral ischemia/reperfusion-induced oxidative stress via activating Nrf2 and regulating neurotrophic factor expression. Oxid Med Cell Longev 2018:6069150
González-Rodríguez P et al (2019) Brain-derived neurotrophic factor alleviates the oxidative stress induced by oxygen and glucose deprivation in an ex vivo brain slice model. J Cell Physiol 234(6):9592–9604
Zhang Y et al (2015) Hepatocyte growth factor suppresses hypoxia/reoxygenation-induced XO activation in cardiac microvascular endothelial cells. Heart Vessels 30(4):534–544
Guo Y et al (2014) The synergistic therapeutic effect of hepatocyte growth factor and granulocyte colony-stimulating factor on pulmonary hypertension in rats. Heart Vessels 29(4):520–531
Brenner D et al (2015) Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol 15(6):362–374
Feltham R et al (2018) Mind Bomb regulates cell death during TNF signaling by suppressing RIPK1’s cytotoxic potential. Cell Rep 23(2):470–484
Zhao S et al (2019) Lipopolysaccharide protects against acetaminophen-induced hepatotoxicity by reducing oxidative stress via the TNF-α/TNFR1 pathway. Biochem Biophys Res Commun 513(3):623–630
Liu D et al (2020) Protective effects of chlorogenic acid on cerebral ischemia/reperfusion injury rats by regulating oxidative stress-related Nrf2 pathway. Drug Des Devel Ther 14:51–60
Chen X et al (2020) BM-MSC transplantation alleviates intracerebral hemorrhage-induced brain injury, promotes astrocytes vimentin expression, and enhances astrocytes antioxidation via the Cx43/Nrf2/HO-1 axis. Front Cell Dev Biol 8:302
Shanmugam G et al (2016) A biphasic effect of TNF-α in regulation of the Keap1/Nrf2 pathway in cardiomyocytes. Redox Biol 9:77–89
Do PT et al (2021) Mesenchymal stem/stromal cell therapy in blood-brain barrier preservation following ischemia: molecular mechanisms and prospects. Int J Mol Sci 22(18):10045
Han F et al (2019) Therapeutic potential of a TrkB agonistic antibody for ischemic brain injury. Neurobiol Dis 127:570–581
Lalaoui N et al (2015) The molecular relationships between apoptosis, autophagy and necroptosis. Semin Cell Dev Biol 39:63–69
Chen AQ et al (2019) Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis 10(7):487
Chi L et al (2018) Tail vein infusion of adipose-derived mesenchymal stem cell alleviated inflammatory response and improved blood brain barrier condition by suppressing endoplasmic reticulum stress in a middle cerebral artery occlusion rat model. Med Sci Monit 24:3946–3957
Acknowledgements
We would like to thank MedSci (www.medsci.cn) for English language editing.
Funding
This study was funded by Beijing Municipal Science and Technology Project (No. Z221100007922028 and Z211100002521006).
Author information
Authors and Affiliations
Contributions
All authors contributed to the idea and design of this study. Material preparation, experiment performance, data collection and analysis were performed by GYL, DHW, JRJ, CHH and QGG. The first draft of the manuscript was written by GYL and DHW. LQX, CLZ, XL and YM provided support for animal studies. HRW, LM, YYC, JWZ and XDX participated in data analysis and interpretation. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical Approval
All animals were maintained in SPF conditions and treated humanely. All experimental procedures involving animals were conducted by the national legislation and approved by the Laboratory Animal Ethics Committee, Yugen Pharmaceutical Technology Co., Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11064_2024_4212_MOESM1_ESM.tif
Supplementary file1 (TIF 37326 KB)—(a) The expression and inhibitory rate of HGF, BDNF, and TNFR1 in hUC-MSCs after siRNA transfection (n=6). (b) PCR of gene expression in hUC-MSCs after siRNA transfection. Data are expressed as the mean ± standard error of mean (SEM). Statistical analyses were performed using unpaired t-tests. ###p <0.001 compared with the siNC-MSCs group.
11064_2024_4212_MOESM2_ESM.tif
Supplementary file2 (TIF 48661 KB)—(a) The representative diagram of osteogenesis, chondrogenesis and lipogenesis in hUC-MSCs after siRNA transfection. (b) Cell viability (%) of hUC-MSCs detected by CCK-8 assay after siRNA transfection (n=4). (c) The immunophenotype of hUC-MSCs after siRNA transfection detected by flow cytometry. Data are expressed as the mean ± standard error of mean (SEM). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, G., Wang, D., Jia, J. et al. Neuroprotection of Human Umbilical Cord-Derived Mesenchymal Stem Cells (hUC-MSCs) in Alleviating Ischemic Stroke-Induced Brain Injury by Regulating Inflammation and Oxidative Stress. Neurochem Res 49, 2871–2887 (2024). https://doi.org/10.1007/s11064-024-04212-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-024-04212-x