Abstract
Evidence shows that miRNAs are deeply involved in nervous system diseases, but whether miRNAs contribute to the bortezomib (BTZ)-induced neuropathic pain remains unclear. We aimed to investigate whether miRNAs contribute to bortezomib (BTZ)-induced neuropathic pain and explore the related downstream cascades. The level of miRNAs in the spinal dorsal horn was explored using miRNA microarray and PCR. MiR-672-5p was significantly downregulated in dorsal horn neurons in the rats with BTZ treatment. Intrathecal injection of miR-672-5p agomir blunted the increase of the amplitude and frequency of sEPSCs in dorsal horn neurons and mechanical allodynia induced by BTZ. In addition, the knockdown of miR-672-5p by intrathecal injection of antagomir increased the amplitude and frequency of sEPSCs in dorsal horn neurons and decreased the mechanical withdrawal threshold in naïve rats. Furthermore, silico analysis and the data from subsequent assays indicated that REEP6, a potential miR-672-5p-regulating molecule, was increased in the spinal dorsal horn of rats with BTZ-induced neuropathic pain. Blocking REEP6 alleviated the mechanical pain behavior induced by BTZ, whereas overexpressing REEP6 induced pain hypersensitivity in naïve rats. Importantly, we further found that miR-672-5p was expressed in the REEP6-positive cells, and overexpression or knockdown of miR-672-5p reversely regulated the REEP6 expression. Bioinformatics analysis and double-luciferase reporter assay showed the existence of interaction sites between REEP6 mRNA and miR-672-5p. Overall, our study demonstrated that miR-672-5p directly regulated the expression of REEP6, which participated in the neuronal hyperexcitability in the spinal dorsal horn and neuropathic pain following BTZ treatment. This signaling pathway may potentially serve as a novel therapeutic avenue for chemotherapeutic-induced mechanical hypersensitivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer-related chronic pain, as a common and distressing symptom, includes tumor-induced pain and chemotherapy/radiotherapy-induced pain. Bortezomib (BTZ), as a selective proteasome inhibitor, is widely used for the treatment of multiple myeloma and other hematological neoplasms [1, 2]. However, BTZ-induced neuropathy, which manifests as mechanical allodynia and spontaneous pain, serves as the common reason for treatment discontinuation or dose reduction [3, 4]. Although many studies have been made to understand the mechanism of BTZ-induced neuropathy, there is no well-established treatment to prevent or minimize this neuropathy.
miRNAs, as the evolutionarily conserved noncoding RNAs, participate in multiple physiological processes such as cell death, survival and pluripotency, et al. [5,6,7]. Recent reports have intensively linked miRNAs to nociceptive processing, including the development and maintenance of hyperalgesia and/or allodynia induced by peripheral inflammation or nerve injury [8, 9]. For instance, the downregulation of miR-194 relived neuropathic pain induced by sciatic nerve injury via preventing neuroinflammation [10], and clinical and animal studies showed that the miR-132-3p was associated with chronic neuropathic pain [11]. In addition, studies suggested that miRNAs may be also involved in chemotherapeutic drug-induced neuropathic pain. For example, the blocking miR-155 in the spinal dorsal horn attenuated the oxaliplatin-induced mechanical allodynia in rats [12]. In this study, miR-672-5p was profiled via miRNA chip assay. A recent study showed that miR-672-5p was significantly downregulated in rats DRG following nerve injury [13]. Furthermore, miR-672-5p was reported to be involved in different pathophysiological process. M2 microglial exosomes rich in miR-672-5p could suppress the AIM2/ASC/Caspase-1 signaling pathway, and consequently inhibit neuronal pyroptosis [14]. MiR-672 was significantly downregulated in the DRGs of the rats with entrapment neuropathy [15]. However, whether miR-672-5p contributes to the BTZ-induced neuropathic pain is elusive.
Evidence showed that microRNAs (miRNAs) regulate gene expression by interacting with the 3′-UTR of target mRNAs [16, 17]. In this study, REEP6 was found, via online prediction tools, to be one of the downstream molecule of miR-672-5p. REEP6, as an important member of the receptor expression enhancing protein (REEP) family, was initially identified in retinal ganglion cells [18]. It functions to enhance the expression of cell surface receptors and shape endoplasmic reticulum (ER) membrane [19, 20]. REEP6 polymorphisms are functionally associated with colon cancer and inflammatory bowel disease [21]. Furthermore, REEP6 is indispensable for the development of retinal photoreceptors, a class of polarized post-mitotic sensory neurons [22]. However, little is known about REEP6 function in neuropathic pain. Moreover, it is still unknown whether and how miR-672-5p regulates the expression of REEP6 following BTZ application.
Materials and Methods
Animals
Male Sprague–Dawley rats (180–230 g) were obtained from the Institute of Experimental Animals of Sun Yat-Sen University. Rats were kept in the separated cages with ad libitum access to food and water at room temperature with 50–60% humidity. All of the experimental procedures were approved by the Sun Yat-Sen University Animal Care and Use Committee and were performed in accordance with the guidelines of the National Institutes of Health on animal care and the ethical criterion.
Drug Administration and Behavioral Test
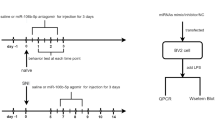
Bortezomib (BTZ) (Haoran Biological Technology Co, Shanghai, China) was intraperitoneally injected at 0.2 mg/kg once per day for consecutive 5 days. The equivalent vehicle was injected into the rats in the control group. MiR-672-5p agomir (3 nmol/day, 10 µl, for consecutive 5 days, Ribobio, Guangzhou, China), antagomir (3 nmol/day, 10 µl, for consecutive 5 days, Ribobio), REEP6 siRNA (1 nmol, 10 µl, for consecutive 5 days, Ribobio) or scramble (1 nmol, 10 µl, for consecutive 5 days, Ribobio) were intrathecally injected 30 min before application of BTZ.
Intrathecal injection is in accordance with the described method [23]. Briefly, the L5 vertebra laminectomy was performed after the injection of sodium pentobarbital (50 mg/kg, i.p.). A polyethylene-10 catheter was inserted into the L5/L6 intervertebral subarachnoid space, and the localization of catheter tip was placed between the L4–L6 spinal segmental. The rats were recovered for 7 days after surgery prior to subsequent drug injection. The rats exhibiting hind limb paralysis or paresis were exclusive of the study.
For intraspinal injection of the AAV virus, the L4–L5 vertebrae were exposed following the fixation of vertebral column with a stereotaxic frame. A slight laminotomy was performed, and the dura was incised to expose the spinal cord. 600 nl of AAV-REEP6-EGFP (OBIO Technology Company Ltd, Shanghai, China) was injected into the bilateral superficial spinal dorsal horn in rats at 4 injection sites (150 nl of AAV was injected at each site). The micropipette was withdrawn 10 min after viral injection.
The 50% mechanical withdrawal threshold were assessed by using von Frey hairs [24]. Briefly, each animal was placed in a plastic box for adaptation for 3 consecutive days (15 min/day) before testing. Mechanical withdrawal threshold was examined by using von Frey hairs of different bending force. A nociceptive response was defined as a brisk paw withdrawal or flinching of the paw following von Frey hair application. In the absence of a withdrawal response to the initially selected hair, a stronger stimulus was presented; otherwise, the weaker stimulus was chosen in next testing. Optimal threshold calculation by this method required 5 responses in the immediate vicinity of the 50% threshold. The behavioral test was conducted blind to the experimenters.
Spinal Cord Slice and Patch-Clamp Recording
Male Sprague Dawley rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.). The L4–L6 spinal cord was quickly removed and transferred to oxygenated (95% O2 and 5% CO2) ice-cold slice solution containing (in mM): 240 mM sucrose, 25 mM NaHCO3, 2.5 mM KCl, 1.25 mM NaH2PO4, 0.5 mM CaCl2, and 3.5 mM MgCl2, pH 7.4. 400-µm thick spinal cord slices were cut on a vibratome (Leica VT-1000S) and kept in the incubation solution (ACSF: 126 mM NaCl, 3 mM KCl, 1.3 mM MgCl2, 2.5 mM CaCl2, 26 mM NaHCO3, 1.25 mM NaH2PO4, and 11 mM glucose, pH 7.3) at 33 °C for at least 30 min. Following the incubation, the slices were transferred to the recording chamber, which was continuously perfused with pre-heated ACSF at a rate of 2 ml/min. Superficial dorsal horn neurons were visualized using a x40 water-immersion objective on an upright infrared Nikon microscope (Nikon, Tokyo, Japan). An EPC-10 amplifier and PULSE program (HEKA Electronics, Lambrecht, Germany) were used with a pipette (4–6 MΩ) containing the internal solution as follows (in mM): 135 mM K-gluconate, 5 mM KCl, 0.5 mM CaCl2, 2 mM MgCl2, 5 mM EGTA, 5 mM Hepes, and 5 mM Mg-adenosine triphosphate. sEPSCs were recorded at the holding potential of − 70 mV in the presence of 10 μM picrotoxin, and the frequency and amplitude were analyzed using Clampfit10.4 (Axon Instruments Inc., USA). The analysis was performed from the fifth to sixth minutes in a single experiment for the determination of frequency but excluded ‘bursts’ with highly superimposed events in the determination of amplitudes. Three neurons per slice were sampled for electrophysiological recording [25].
RNA Extraction and Real-Time qPCR
Trizol was used to extract total RNA from dorsal horn tissues. The reverse transcription was performed according to the manufacturer’s protocol of PCR production kit. The primer sequences for all targeted mRNAs are presented in Supplementary Table 1. qPCR was performed using SYBR Green qPCR SuperMix (Invitrogen). The cycles of reaction were 40 times at 95 °C for 3 min, and the condition of thermal cycling is 10 s at 95 °C, 20 s at 58 °C, and 10 s at 72 °C. The ratio of mRNA expression in the dorsal horn tissues was analyzed by the − 2△△CT method.
miRNA Microarray Experiments
Trizol was used to extract total RNA from dorsal horn tissues. The quality of RNA was checked by agarose gel electrophoresis and RNA absorbance detection. RNA labeling and hybridization on miRNA microarray chips were conducted as described previously [26]. Briefly, the purified RNA was labeled with fluorescein, and hybridization was performed on miRNA microarray chips (miRNA microarray V4.0; CapitalBio). RNA samples on different time points from spinal dorsal horn tissue in the rats treated with BTZ were hybridized with miRNA microarrays separately. Hybridization intensity values from individual samples were filtered and normalized to per-chip mean values. The differential gene expression analysis of the miRNA chip was performed via EdgeR package. The dispersion value (BCV) was 0.01. Benjamini–Hochberg analysis was performed for the correction for the number of statistical tests. The miRNA with the criteria of P < 0.05 and FC > 2 or < 0.5 was considered to be differentially changed. The differential analysis software used in this approach has limitation to analyze the microarray data without duplicate, therefore the results were validated with further real-time qPCR.
Western Blot
Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) at different time points. L4–L6 spinal cord was removed and sectioned in a cryostat. Dorsal horn tissues were taken with a 15-gauge cannula and homogenized on ice in 15 mmol/l Tris buffer containing a cocktail of proteinase inhibitors and phosphatase inhibitors. Protein samples were separated by SDS-PAGE and transfected onto a PVDF membrane. The membrane was incubated with primary antibody against REEP6 (1:600, Affinity) or GAPDH (1:2000, CST) after blocking with the block buffer for 1 h. Then the horseradish peroxidase-conjugated secondary antibodies were used to incubate the blots, and the immune complex was detected using ECL (Pierce). The intensity of bands was explored with a computer-assisted imaging analysis system (Tanon biology). The blot images suggest that the antibody for REEP6 is not specific, which was validated via the blockage and overexpression experiments.
FISH and Immunohistochemistry
Perfusion was performed through the ascending aorta with 4% paraformaldehyde following the intraperitoneal injection of sodium pentobarbital at 50 mg/kg dose. The L4–L6 segments of spinal cord were removed and placed into the same paraformaldehyde for 3 h, and stored in 30% DEPC-sucrose overnight. For FISH analysis, cryostat sections at 25 µm thickness were cut, and the sections were incubated with the 3′ and 5′-TYE665-labeled miR-672-5p probe 5′-CACACACAGTACACCAACCTCA-3′ and REEP6 antibody (1:200, Affinity), NeuN (1:500, Chemicon), Iba1 (1:250, Chemicon) and GFAP (1:600, Chemicon). For immunohistochemistry, the sections were incubated with primary antibody for REEP6 (1:200, Affinity), NeuN (1:500, Chemicon), Iba1 (1:250, Chemicon) and GFAP (1:600, Chemicon) for overnight at 4 ℃. Then, the sections were incubated with cy3-conjugated or fluorescein isothiocyanate-conjugated secondary antibodies for 1 h. Nikon fluorescence microscope with a digital camera was used to examine and capture the image.
Dual-Luciferase Reporter Assay
A luciferase reporter assay was performed to test the binding of miR-672-5p to its target gene REEP6. A 1489 bp segment of rat REEP6 3′-UTR containing a presumed miR-672-5p complementary sites was amplified by PCR and constructed into the 3′-UTR of pMIR-Report Luciferase vector. 293 T cells were co-transfected with a mixture of 200 ng pMIR-Report Luciferase vector, miR-672-5p mimic (OBIO Technology Company Ltd, Shanghai, China) and scramble control miRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. To test the binding specificity, the seed sequence of miR-672-5p was mutated from CAACCTCA to GTTGGAGT in the REEP6 3′-UTR (Fig. 4f). After 48 h, the luciferase activity of the cells was measured using a dual-luciferase reporter assay kit (Promega). The result was normalized to the ratio between Firefly activity and Renilla luciferase activity.
Statistical Analysis
The data were shown as mean ± SEM, and analyzed using SPSS 25. The data were analyzed using the two independent samples t test or one-way ANOVA followed by Tukey’s post hoc test. When tests of normality were not satisfied, the permutation test was substituted. The criterion of statistical significance was 0.05. Although no power analysis was performed, the sample size was determined according to peer’s and our previous publication in behavioral and pertinent molecular studies [24].
Results
BTZ Treatment Induced the Central Sensitization and Mechanical Allodynia
Here, we first explored the mechanical withdrawal threshold in the rats following BTZ application. The results showed that BTZ treatment (0.2 mg/kg, for 5 continuous days, i.p.) significantly decreased the withdrawal threshold on day 4, 7, which maintained to the end of the experiment (day 10) (Fig. 1a). Accumulate evidence shows that central sensitization in spinal dorsal horn plays an important role in the development and maintenance of neuropathic pain [27]. To determine whether BTZ induces central sensitization in dorsal horn, we examined the change of sEPSCs in the rats’ superficial dorsal horn neurons following BTZ treatment. The results showed that the amplitude and frequency of sEPSC were significantly increased in the superficial dorsal horn neurons on day 4 and 10 following BTZ treatment (Fig. 1b, c). These results suggested that BTZ treatment may enhance the excitability of dorsal horn neurons and induce the neuropathic pain, while the underlying mechanism was still unclear.
BTZ treatment mediated central sensitization and mechanical allodynia. a Paw withdrawal threshold of rats was significantly reduced on day 4, 7 and 10 after BTZ treatment (**p < 0.01 versus corresponding vehicle group, n = 6 rats for each group). b, c The application of BTZ increased the amplitude and frequency of sEPSCs in dorsal horn neurons in rats (*p < 0.05, **p < 0.01 versus corresponding vehicle group, n = 26, 21 or 16 neurons in different groups)
miR-672-5p Contributed to the BTZ-Induced Neuropathic Pain in Rats
In the present study, we explored the miRNAs expression in spinal dorsal horn through miRNA sequencing on day 4 and 10 after BTZ treatment. We found 93 miRNAs changed on day 4 and 113 miRNAs changed on day 10 following BTZ application, respectively (P < 0.05, FC > 2 or < 0.5, Fig. 2a). Among these changed miRNAs, the expression of 51 miRNAs showed a consistent dysregulated on day 4 and 10 after BTZ treatment (Fig. 2a, Supplementary Fig. 1). Next, the top three downregulated miRNAs including miR-672-5P, miR-488-3p and miR-598-5p were selected as the candidates (Fig. 2b). PCR analyses further showed that miR-672-5p significantly decreased on day 4, 7 and 10 following BTZ treatment (Fig. 2c), and the time course of miR-672-5p expression was consistent with that of mechanical allodynia. However, miR-488-3p and miR-598-5p only decreased on day 4 and 7 after BTZ treatment (Fig. 2c). FISH staining showed that the expression of miR-672-5p was colocalized with the NeuN-positive cells, but not GFAP-positive cells or Iba1-positive cells in spinal dorsal horn (Fig. 2d). Next, we assessed the involvement of miR-672-5p in the central sensitization and neuropathic pain by the studies with the loss and gain of function. The results showed that intrathecal injection of miR-672-5p agomir (3 nmol/day for consecutive 5 days) significantly inhibited the increase of frequency and amplitude of sEPSC in dorsal horn neurons (Fig. 2e), and attenuated the mechanical allodynia in the BTZ rats, when compared with the BTZ + Scramble group (Fig. 2f). Furthermore, suppression of miR-672-5p by intrathecal injection of antagomir significantly increased the frequency and amplitude (Fig. 2g) of sEPSC in dorsal horn neurons and induced mechanical allodynia in naïve rats (Fig. 2h). These results suggested that miR-672-5p contributed to the BTZ-induced neuropathic pain through regulating the central sensitization of dorsal horn neurons.
The decreased miR-672-5p was involved in the BTZ-induced neuropathic pain in rats. a The venn diagram showed the dysregulated miRNAs on day 4 and 10 following BTZ treatment. b The top three miRNAs were miR-672-5P, miR-488-3p and miR-598-5p. c The expression of miR-672-5P, miR-488-3p and miR-598-5p on different time points after BTZ treatment (*p < 0.05, **p < 0.01 versus corresponding vehicle group, n = 3 or 4 rats in different groups). d FISH showed that miR-672-5p was co-expressed in NeuN-positive cells (a neuronal marker), but not GAFP-positive cells (an astrocyte marker) or Iba1-positive cells (a microglial marker). e Intrathecal injection of miR-672-5p agomir inhibited the increase of amplitude and frequency of sEPSC in dorsal horn neurons (*p < 0.05, **p < 0.05 versus corresponding vehicle group, n = 22 or 27 neurons in different groups). f miR-672-5p agomir treatment increased the withdrawal threshold to mechanical stimuli in the BTZ-treated rats (*p < 0.05, **p < 0.05 versus corresponding BTZ group, n = 8 or 6 rats in different group). g Intrathecal injection of miR-672-5p antagomir significantly increased the amplitude and frequency of sEPSC in naïve rats (**p < 0.05 versus corresponding vehicle group, n = 16 or 20 neurons in different groups). h Application of miR-672-5p antagomir induced mechanical allodynia in naïve rats (*p < 0.05, **p < 0.05 versus corresponding BTZ group, n = 6 rats for each group)
REEP6 was Involved in BTZ-Induced Neuropathic Pain
MiRNAs regulate gene expression at the posttranscriptional level through interacting with a target mRNA [28]. To find the downstream molecule of miR-672-5p, we predicted the target gene of miR-672-5p using two online databases, TargetScan and miRDB. The silico analysis obtained 6 common potential targets with top 5% score from two online databases, and PCR results showed that only the level of REEP6 mRNA significantly increased on day 10 following BTZ treatment (Fig. 3a). Next, we examined the time course of REEP6 expression following BTZ treatment. We found that the REEP6 mRNA levels were significantly increased on day 4, 7 and 10 after BTZ treatment (Fig. 3b). Similarly, the REEP6 protein was also up-regulated on day 4, 7 and 10 after BTZ treatment accordingly (Fig. 3c). Double immunofluorescence revealed that the REEP6 was expressed in neurons, but not astrocyte or microglia (Fig. 3d). Furthermore, behavioral test showed that pre-intrathecal injection of REEP6 siRNA (1 nmol/10 µl for consecutive 5 days) inhibited the upregulation of REEP6 and ameliorated the mechanical allodynia induced by BTZ (Fig. 3e, f). Moreover, intraspinal injection of AAV-REEP6-EGFP significantly enhanced the REEP6 expression of dorsal horn in naïve rats (Fig. 3g). Behavioral test showed that the overexpression of REEP6 by AAV-REEP6-EGFP induced the mechanical allodynia in naïve rats (Fig. 3h). These results indicated that the upregulation of REEP6 participated in the BZT-induced neuropathic pain.
The role of REEP6 in the neuropathic pain following BTZ treatment. a The expression of REEP6 mRNA was significantly increased among 6 common potential miR-672-5p binding mRNAs on day 10 following BTZ treatment (*p < 0.05 versus corresponding vehicle group, n = 4 rats for each group). b The expression of REEP6 mRNA was examined on day 4, 7, and 10 after BTZ treatment in the spinal dorsal horn (*p < 0.05 versus corresponding vehicle group, n = 3 rats for each group). c The expression of REEP6 protein was significantly increased following BTZ application (*p < 0.05 versus day 0 group, n = 3 rats for each group). d The REEP6 was expressed on the neurons, but not astrocyte and microglia, in the spinal dorsal horn (n = 3). e, f Intrathecal injection of REEP6 siRNA inhibited the upregulation of REEP6 and attenuated the mechanical allodynia induced by BTZ (*p < 0.05, **p < 0.01 versus corresponding vehicle group, n = 5 or 6 rats in different group). g, h Injection of AAV-CMV-REEP6-EGFP (Intraspinal) increased the REEP6 expression and induced the mechanical allodynia in naïve rats (**p < 0.01 versus corresponding vehicle group, n = 3 rats for WB analysis, n = 7 rats for each group in behavioral test)
Spinal miR-672-5p Negatively Regulates REEP6 Expression in BTZ-Induced Neuropathic Pain
Next, we verified the interaction between miR-672-5p and REEP6. FISH results showed that REEP6-positive cells were colocalized with miR-672-5p-positive cells in the dorsal horn (Fig. 4a). Intrathecal injection of miR-672-5p agomir significantly decreased the expression of REEP6 mRNA and protein in the spinal dorsal horn on day 10 after BTZ treatment (Fig. 4b, c). Moreover, miR-672-5p antagomir treatment (i.t.) significantly increased the level of REEP6 mRNA and protein in the dorsal horn of naïve rats (Fig. 4d, e). To explore the functional association between miR-672-5p and REEP6, we constructed a luciferase reporter vector (pMIR-REPORT vector) in which the 3′-UTR regions of REEP6 gene was fused to the luciferase coding sequence. We then transfected 293T cells with luciferase reporter vector and miR-672-5p mimic. The results showed that the luciferase activities in 293T cells were significantly suppressed by miR-672-5p mimic at the concentration of 100 nM (Fig. 4g). Moreover, we mutated the miR-672-5p binding sites (CAACCTCA) in the 3′-UTR region of REEP6 and constructed Luc-REEP6 3′UTRmut vector (Fig. 4f). The results showed that mutation of predicted site in the 3′-UTR region of REEP6 significantly abolished the suppression of luciferase signal induced by miR-672-5p mimic (Fig. 4g). Together, these results indicated that miR-672-5p may negatively regulate the expression of REEP6 via the binding site in REEP6 3′-UTR.
Spinal miR-672-5p regulates REEP6 expression. a miR-672-5p was colocalized with REEP6-positive cells in the dorsal horn. b, c The application of miRNA-672-5p agomir significantly decreased the level of REEP6 mRNA and protein in BTZ rats (*p < 0.05 versus corresponding scramble group, n = 3 or 4 rats in different groups). d, e The inhibition of miRNA-672-5p by using antagomir significantly increased the level of REEP6 mRNA and protein in the dorsal horn of naïve rats (*p < 0.05 versus corresponding scramble group, n = 3 rats for each group). f 6 putative miR-672-5p binding sites (CAACCTCA) located in the 3′-UTR regions of REEP6 gene. The mutated REEP6 sites in Luc-REEP6 3′UTRmut vectors were shown as red bases. g Luciferase activity of reporter constructs containing either the 3′-UTR of REEP6 or mutated REEP6 sites was co-transfected with REEP6 miR-672-5p mimic (**p < 0.01 versus corresponding control group, n = 3 replicates for each group)
Discussion
Accumulating evidence has shown the role of noncoding RNAs such as miRNA in neuropathic pain [29]. The current study illustrated a novel molecular mechanism involving miR-672-5p/REEP6 pathway, which critically contributed to the central sensitization and mechanical allodynia in the setting of BTZ-induced neuropathic pain. In addition, in vitro study further showed that the sequence of CAACCTCA in 3′-UTR region of REEP6 might be the critical binding site for miR-672-5p to regulate the expression of REEP6. The present study significantly advanced our understanding on the role of miRNA in the pathogenesis of bortezomib-induced chronic pain.
REEP6 was initially discovered in retinal ganglion cells [18], which served as an adaptor protein to mediate the process of shaping tubular organelles such as the ER and Golgi [30]. In addition, REEP6 is expressed in other tissues and cells as well [31]. To our knowledge, the present study was the first to validate the expression of REEP6 in spinal dorsal horn neurons, which was significantly increased following the induction and maintenance of BTZ-induced neuropathic pain. Moreover, inhibition of REEP6 by intrathecal injection of REEP6 siRNA ameliorated the mechanical allodynia induced by BTZ, and overexpression of REEP6 in spinal dorsal horn induced the pain response to mechanical stimulus in naïve rats. Accumulative research showed the role of inflammatory responses on the neuropathic pain induced by various factors [32]. For example, inhibition of CXCR1/2 pathways can reduce the paclitaxel-induced neuropathic pain [33], and IL-8 level was significantly increased in rat model of neuropathic pain following sciatic nerve injury [34]. A recent study reported that overexpression of REEP6 enhanced IL-8-stimulated cellular responses [31], and previous study showed that IL-8 application increased the synaptic transmission by the presynaptic mechanism [35]. In addition, REEP6, as an important member of the receptor expression enhancing protein (REEP) family, was involved in the process of receptor trafficking into membrane [19, 36]. Therefore, we speculate that REEP6 could mediate the trafficking of glutamate receptors into cell membranes, thereby facilitating glutamatergic transmission via postsynaptic mechanism. The present study showed that REEP6 can regulate the excitability of spinal neurons following the BTZ treatment, which was consistent with peer’s study that REEP6 can affect the electrical activity of retina [37]. However, the detailed mechanism underlying REEP6-mediated sEPSC adaptation need to be further verified. Altogether, it was postulated that REEP6 likely promoted the inflammatory response or the pain-related receptor trafficking to enhance the glutamatergic transmission, by which it contributed to the BTZ-induced neuropathic pain.
Recently, many studies have showed the dysregulation of miRNA expression in the spinal cord during the progression of neuropathic pain [26, 38]. For example, inhibition of miR-155 mitigates neuropathic pain induced by BTZ via modulating neuroinflammation in spinal dorsal horn [39]. Of note, miR-672-5p is associated with cancer [40], and BTZ is a first-line anticancer drug [41]. It prompted us to study the role of miR-672-5p in the BTZ-induced neuropathy. Here, although miRNA profiling assay showed a downregulation of miR-672-5p, miR-488-3p and miR-598-5p in dorsal horn on day 7 and 10 after BTZ treatment, PCR and behavior test further revealed that only the time course of miR-672-5p downregulation was consistent with that of mechanical allodynia. In addition, FISH staining showed that the miR-672-5p was colocalized with neurons in spinal dorsal horn. Moreover, intrathecal injection of miR-672-5p agomir attenuated BTZ-induced central sensitivity and neuropathic pain, and miR-672-5p antagomir induced the sensitization and mechanical hypersensitivity in naïve rats. These results suggested that the downregulation of miR-672-5p was involved in the BTZ-neuropathic pain, which was consistent with the previous finding that miR-672-5p was significantly decreased in rats DRG following nerve injury [13]. Furthermore, we presented the morphological evidence for the distribution of miR-672-5p in REEP6-positive cells in the dorsal horn, and further identified the machinery for the direct binding of miR-672-5p in the critical sequence of the REEP6 mRNA. Importantly, we found that activation of miR-672-5p by agomir remarkably attenuated the REEP6 increase in the BTZ-treated rats, and intrathecal injection of miR-672-5p antagomir enhanced the REEP6 expression in the dorsal horn of naïve rats. Together, the present results presented a miR-672-5p-involved mechanism to regulate REEP6 expression in dorsal horn, which contributed to the induction of neuropathic pain in a chemotherapeutic BTZ-treated rodent model.
Data Availability
Enquiries about data availability should be directed to the authors.
References
Curran MP, McKeage K (2009) Bortezomib a review of its use in patients with multiple myeloma. Drugs 69:859–888
Tobinai K (2007) Proteasome inhibitor, bortezomib, for myeloma and lymphoma. Int J Clin Oncol 12:318–326
Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, Singhal S, Siegel DS, Irwin D, Schuster M, Srkalovic G, Alexanian R, Rajkumar SV, Limentani S, Alsina M, Orlowski RZ, Najarian K, Esseltine D, Anderson KC, Amato AA (2006) Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol 24:3113–3120
Dougherty PM (2016) Is chemotherapy-induced peripheral neuropathy more than just a peripheral nervous system disorder? Anesthesiology 124:992–993
Lewis MA, Steel KP (2010) MicroRNAs in mouse development and disease. Semin Cell Dev Biol 21:774–780
Irminger-Finger I, Thomson JM, Kim JK (2010) MicroRNAs, a superimposed regulatory network important for development and disease. Int J Biochem Cell Biol 42:1234–1235
Tarakcioglu E, Tastan B, Arioz BI, Tufekci KU, Genc S (2022) Melatonin alters the miRNA transcriptome of inflammasome activation in murine microglial cells. Neurochem Res
Elramah S, Landry M, Favereaux A (2014) MicroRNAs regulate neuronal plasticity and are involved in pain mechanisms. Front Cell Neurosci 8:31
Sengupta JN, Pochiraju S, Kannampalli P, Bruckert M, Addya S, Yadav P, Miranda A, Shaker R, Banerjee B (2013) MicroRNA-mediated GABA Aalpha-1 receptor subunit down-regulation in adult spinal cord following neonatal cystitis-induced chronic visceral pain in rats. Pain 154:59–70
Zhang X, Chen Q, Shen J, Wang L, Cai Y, Zhu KR (2020) miR-194 relieve neuropathic pain and prevent neuroinflammation via targeting FOXA1. J Cell Biochem 121:3278–3285
Leinders M, Uceyler N, Pritchard RA, Sommer C, Sorkin LS (2016) Increased miR-132-3p expression is associated with chronic neuropathic pain. Exp Neurol 283:276–286
Miao F, Wang R, Cui G, Li X, Wang T, Li X (2019) Engagement of MicroRNA-155 in exaggerated oxidative stress signal and TRPA1 in the dorsal horn of the spinal cord and neuropathic pain during chemotherapeutic oxaliplatin. Neurotox Res 36:712–723
Chang HL, Wang HC, Chunag YT, Chou CW, Lin IL, Lai CS, Chang LL, Cheng KI (2017) miRNA expression change in dorsal root ganglia after peripheral nerve injury. J Mol Neurosci 61:169–177
Zhou Z, Li C, Bao T, Zhao X, Xiong W, Luo C, Yin G, Fan J (2022) Exosome-shuttled miR-672-5p from anti-inflammatory microglia repair traumatic spinal cord injury by inhibiting AIM2/ASC/Caspase-1 signaling pathway mediated neuronal pyroptosis. J Neurotrauma
Rau CS, Jeng JC, Jeng SF, Lu TH, Chen YC, Liliang PC, Wu CJ, Lin CJ, Hsieh CH (2010) Entrapment neuropathy results in different microRNA expression patterns from denervation injury in rats. BMC Musculoskelet Disord 11:181
O’Brien J, Hayder H, Zayed Y, Peng C (2018) Overview of MicroRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol 9:402
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Sato H, Tomita H, Nakazawa T, Wakana S, Tamai M (2005) Deleted in polyposis 1-like 1 gene (Dp1l1): a novel gene richly expressed in retinal ganglion cells. Invest Ophthalmol Vis Sci 46:791–796
Bjork S, Hurt CM, Ho VK, Angelotti T (2013) REEPs are membrane shaping adapter proteins that modulate specific g protein-coupled receptor trafficking by affecting ER cargo capacity. PLoS ONE 8:e76366
Mainland J, Matsunami H (2012) RAMP like proteins : RTP and REEP family of proteins. Adv Exp Med Biol 744:75–86
Wellmann A, Fogt F, Hollerbach S, Hahne J, Koenig-Hoffmann K, Smeets D, Brinkmann U (2010) Polymorphisms of the apoptosis-associated gene DP1L1 (deleted in polyposis 1-like 1) in colon cancer and inflammatory bowel disease. J Cancer Res Clin Oncol 136:795–802
Hao H, Veleri S, Sun B, Kim DS, Keeley PW, Kim JW, Yang HJ, Yadav SP, Manjunath SH, Sood R, Liu P, Reese BE, Swaroop A (2014) Regulation of a novel isoform of Receptor Expression Enhancing Protein REEP6 in rod photoreceptors by bZIP transcription factor NRL. Hum Mol Genet 23:4260–4271
Xu T, Zhang XL, Ou-Yang HD, Li ZY, Liu CC, Huang ZZ, Xu J, Wei JY, Nie BL, Ma C, Wu SL, Xin WJ (2017) Epigenetic upregulation of CXCL12 expression mediates antitubulin chemotherapeutics-induced neuropathic pain. Pain 158:637–648
Zhang SB, Lin SY, Liu M, Liu CC, Ding HH, Sun Y, Ma C, Guo RX, Lv YY, Wu SL, Xu T, Xin WJ (2019) CircAnks1a in the spinal cord regulates hypersensitivity in a rodent model of neuropathic pain. Nat Commun 10:4119
Wang Z, Jiang C, He Q, Matsuda M, Han Q, Wang K, Bang S, Ding H, Ko MC, Ji RR (2020) Anti-PD-1 treatment impairs opioid antinociception in rodents and nonhuman primates. Sci Transl Med 12
Huang ZZ, Wei JY, Ou-Yang HD, Li D, Xu T, Wu SL, Zhang XL, Liu CC, Ma C, Xin WJ (2016) mir-500-mediated GAD67 downregulation contributes to neuropathic pain. J Neurosci 36:6321–6331
Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152:S2–S15
Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10:126–139
Song G, Yang Z, Guo J, Zheng Y, Su X, Wang X (2020) Interactions among lncRNAs/circRNAs, miRNAs, and mRNAs in neuropathic pain. Neurotherapeutics 17:917–931
Agrawal SA, Burgoyne T, Eblimit A, Bellingham J, Parfitt DA, Lane A, Nichols R, Asomugha C, Hayes MJ, Munro PM, Xu M, Wang K, Futter CE, Li Y, Chen R, Cheetham ME (2017) REEP6 deficiency leads to retinal degeneration through disruption of ER homeostasis and protein trafficking. Hum Mol Genet 26:2667–2677
Park CR, You DJ, Park S, Mander S, Jang DE, Yeom SC, Oh SH, Ahn C, Lee SH, Seong JY, Hwang JI (2016) The accessory proteins REEP5 and REEP6 refine CXCR1-mediated cellular responses and lung cancer progression. Sci Rep 6:39041
Malcangio M (2019) Role of the immune system in neuropathic pain. Scand J Pain 20:33–37
Brandolini L, Benedetti E, Ruffini PA, Russo R, Cristiano L, Antonosante A, d’Angelo M, Castelli V, Giordano A, Allegretti M, Cimini A (2017) CXCR1/2 pathways in paclitaxel-induced neuropathic pain. Oncotarget 8:23188–23201
Khan J, Hassun H, Zusman T, Korczeniewska O, Eliav E (2017) Interleukin-8 levels in rat models of nerve damage and neuropathic pain. Neurosci Lett 657:106–112
Cui GB, An JZ, Zhang N, Zhao MG, Liu SB, Yi J (2012) Elevated interleukin-8 enhances prefrontal synaptic transmission in mice with persistent inflammatory pain. Mol Pain 8:11
Saito H, Kubota M, Roberts RW, Chi Q, Matsunami H (2004) RTP family members induce functional expression of mammalian odorant receptors. Cell 119:679–691
Arno G, Agrawal SA, Eblimit A, Bellingham J, Xu M, Wang F, Chakarova C, Parfitt DA, Lane A, Burgoyne T, Hull S, Carss KJ, Fiorentino A, Hayes MJ, Munro PM, Nicols R, Pontikos N, Holder GE, Asomugha C, Raymond FL, Moore AT, Plagnol V, Michaelides M, Hardcastle AJ, Li Y, Cukras C, Webster AR, Cheetham ME, Chen R (2016) Mutations in REEP6 cause autosomal-recessive retinitis pigmentosa. Am J Hum Genet 99:1305–1315
Tang S, Jing H, Song F, Huang H, Li W, Xie G, Zhou J (2021) MicroRNAs in the spinal microglia serve critical roles in neuropathic pain. Mol Neurobiol 58:132–142
Duan Z, Zhang J, Li J, Pang X, Wang H (2020) Inhibition of microRNA-155 reduces neuropathic pain during chemotherapeutic bortezomib via engagement of neuroinflammation. Front Oncol 10:416
Teng Y, Ren Y, Hu X, Mu J, Samykutty A, Zhuang X, Deng Z, Kumar A, Zhang L, Merchant ML, Yan J, Miller DM, Zhang HG (2017) MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat Commun 8:14448
Velasco R, Alberti P, Bruna J, Psimaras D, Argyriou AA (2019) Bortezomib and other proteosome inhibitors-induced peripheral neurotoxicity: from pathogenesis to treatment. J Peripher Nerv Syst 24(Suppl 2):S52–S62
Acknowledgements
This study was funded by National Natural Science Foundation of China (Grant No. 31970936), Natural Science Foundation of Guangdong (2019A1515010871, 2019A1515011447, 2022A1515012259), Guangzhou Science and Technology Plan Project (202206060004).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection were performed by Yang Sun, Li Chen, and Ting Xu. Data analysis were performed by Bo Gou, Jing-Wen Mai and Jia-Yan Wu. The first draft of the manuscript was written by Wen-Jun Xin and Jia-Yan Wu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, Y., Chen, L., Xu, T. et al. MiR-672-5p-Mediated Upregulation of REEP6 in Spinal Dorsal Horn Participates in Bortezomib-Induced Neuropathic Pain in Rats. Neurochem Res 48, 229–237 (2023). https://doi.org/10.1007/s11064-022-03741-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03741-7