Abstract
Vincristine is a common chemotherapeutic agent in cancer treatment, while it often causes chemotherapy-induced peripheral neuropathy(CIPN), which brings patients a great disease burden and associated economic pressure. The mechanism under CIPN remains mostly unknown. The previous study has shown that cell-type-specific spinal synaptic plasticity in the dorsal horn plays a pivotal role in neuropathic pain. Downregulation of GABA transmission, which mainly acts as an inhibitory pathway, has been reported in the growing number of research. Our present study found that GAD67, responsible for > 90% of basal GABA synthesis, is down-regulated, while its relative mRNA remains unchanged in vincristine-induced neuropathy. Considering microRNAs (miRNAs) as a post-transcription modifier by degrading targeted mRNA or repressing mRNA translation, we performed genome-wide miRNA screening and revealed that miR-30d might contribute to GAD67 down-regulation. Further investigation confirmed that miR-30d could affect the fluorescence activity of GAD67 by binding to the 3 'UTR of the GAD67 gene, and intrathecal injection of miR-30d antagomir increased the expression of GAD67, partially rescued vincristine-induced thermal hyperalgesia and mechanical allodynia. In summary, our study revealed the molecule interactions of GAD67 and miR-30d in CIPN, which has not previously been discussed in the literature. The results give more profound insight into understanding the CIPN mechanism and hopefully helps pain control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced peripheral neuropathy(CIPN), which presents as hyperalgesia or allodynia, is a common side effect of several chemotherapeutic agents and severely impacts a patient's cancer treatment, as well as the quality of life (QoL). However, current treatment options for CIPN are limited and inefficient, which deserve the new therapeutic strategy development. The precise mechanism involved in CIPN remains unclear mainly, making it more difficult to find a new treatment approach. Recent reports have strongly linked MicroRNAs (miRNAs) to nociceptive processing. MiRNAs are small endogenous RNAs that regulate gene expression post-transcriptionally. They consist of approximately 21–23 nucleotides and have uridine at their 5′-end, which partially complements the 3′-end untranslated regions of the messenger RNA (mRNA). miRNA also recruits Argonaute (AGO) protein complex to a complementary target mRNA, which results in translation repression or degradation or deadenylation of the mRNA [1]. Previous studies have demonstrated miRNA is involved in a variety of diseases, such as neurodegenerative diseases [2], cardiovascular diseases [3], or cancer [4]. In terms of neuropathic pain(NP), miRNAs also play a pivotal role. Zhiqiang Pan et al. [5] suggested that miRNA-23a is involved in NP pathology via targeting CXCR4. Mónica Tramullas et al. [6] found that miR-30c-5p contributes to allodynia development by inhibiting TGF-β1 expression. These results could help us to shed more light on the development of a new therapeutic strategy. Several miRNA treatments have already been conducted to phase II clinical trials [7].
Pain transmission is the integral step of the pain pathway related to dysregulation of the excitatory pathway and inhibitory system. The γ-Aminobutyric acid (GABAergic) circuit in the spinal cord is an essential component of the inhibitory system [8], and its effect is mediated through activation of ionotropic (i.e., GABA-A and GABA-C) and metabotropic (i.e., GABA-B) receptors [9]. The activated GABAA receptor, a chloride ion channel, can hyperpolarize the postsynaptic neuron or prevent depolarization caused by concurrent excitatory input [8]. The GABA-B receptors are metabotropic members of the seven-transmembrane G-protein coupled receptors superfamily; activation of GABA-B receptor at presynaptic sites can lead to inhibition of high voltage-gated Ca2+ channel activity and inhibits glutamate as well as substance P(SP) and Calcitonin Gene-Related Peptide(CGRP) release [9]. GABA neurotransmission is regulated at the level of interactions with GABA receptors and also by synthesis, vesicular release or reuptake mechanisms at the presynaptic site. GABA is synthesized from glutamate by the rate-limiting enzyme glutamate decarboxylase (GAD), which is consists of two isoforms, GAD67 and GAD65, and encoded by two separate genes, Gad1 and Gad2, respectively [10, 11]. Y Wang et al. [12]. suggested that promotion of GAD65 or GAD67 expression by vector-mediated gene transfer to dorsal root ganglion(DRG) can alleviate pain-related behaviors in models of painful diabetic neuropathy, and Kanao Megumi et al. [13]. also confirmed the effect of GAD67 transfection to spinal cord in decreasing pain in the HIV-related neuropathic pain model. These results imply that targeting GABA neurotransmission is a potential therapeutic strategy in pain management.
At present, whether GAD67 contributes to vincristine-induced neuropathic pain and the underlying mechanism remained unclear. The causal link between miR-30d and GABAergic dysfunction has not been determined. The objective of the study was to address the questions and to test if miR-30d mediated modulation of GAD67 and its impact on synaptic plasticity in rodent models with neuropathic pain induced by vincristine.
Methods and Materials
Animals
Male Sprague Dawley rats (180–230 g) were provided by the Animal Center of Naval military Medical University. All animals were kept in a temperature-controlled room (23 ± 1 °C) with a light / dark period of 12 h. All animal testing procedures are approved by the local animal protection and use committee and are carried out in accordance with the animal protection and ethical guidelines of the National Institutes of Health.
Drug Administration
Vincristine sulfate (Main Luck Pharmaceuticals Inc., China) dissolved in saline to a concentration of 50 μg/ml was intraperitoneally injected at a dose of 0.1 mg/kg daily for 10 consecutive days [14, 15]. The control group was injected intraperitoneally with the same dose of vehicle. In order to carry out in vivo experiments, cholesterol-coupled miR-30d antagomir and antagomir control were commercially obtained from RiboBio (RiboBio Co., Ltd. Guangzhou, China).
Intrathecal miRNA Modulator Application
The method of intrathecal injection of miR-30d antagomir(0.5 nmol/d) was referred to in our previous study [16]. In brief, a polyethylene-10 catheter was inserted into the subarachnoid space of rats through the spinal cord segment space between L5 and L6, with the tip at the level of the spinal cord segment of L5. MiR-30d antagomir(0.5 nmol/d) was administered intrathecally 30 min before the first dose of vincristine and continuously injected for 5 days.
Behavioral Test
Mechanical Allodynia
Von Frey filaments were used to implement mechanical stimulation to the hind paw's plantar surface to determine the withdrawal threshold of the hind paw. A 50% foot withdrawal threshold is defined as the lowest force that produces 5 or more foot withdrawal responses out of 10 stimuli.
Thermal Hyperalgesia
Thermal hyperalgesia was tested using the tester mentioned above (7370; Ugo Basile Plantar Test Apparatus) [17]. In short, the rats were placed on a glass plate under which a radiant heat source was aimed at the plantar surface of the hind paw. In each test phase, the foot's withdrawal latency was measured three times for each rear claw. The interval between three consecutive tests is > 5 min. The average data on each side determined the final result of withdrawal latency. In order to prevent tissue damage, the cut-off time of the radiation heat source was set as the 20 s. The experimenter who carried out the behavioral test was set blind to all treatment.
Application of miRNA Regulator in Glioma Cell Line C6
Malignant glioma cell line C6 was cultured in a conventional medium (DMEM containing 10% fetal bovine serum and 1% antibiotics). Then, according to the manufacturer's instructions, the 20 pmol of miR-30d mimic and scrambled control miRNA (Guangzhou RiboBio Co., Ltd.) were transfected into C6 cells in a 24-well plate with liposome 2000 (Invitrogen). One day after transfection, the expression of GAD67 was detected by Western blot.
Luciferase Reporter Vector Construction
The luciferase reporter vector (psi-CHECK2 vector), in which the 3ʹ-UTR of Gad1 was fused to the luciferase coding sequence, was constructed. The luciferase reporter vector and miR-30d mimic were cotransfected to human embryonic kidney 293 (HEK-293) cells, and the luciferase signal was recorded.
RNA Isolation and Microarray Experiments
The total RNA of the spinal dorsal horn was extracted by TRIzol reagent (Invitrogen). According to the previous research [18], RNA labeling and hybridization were performed on miRNA microarray chips. To put it simply, RNA was purified using a mirVANA miRNA separation kit (Applied Biosystems), then labeled with fluorescein, and hybridized on a miRNA microarray chip (miRNA microarray V4.0) containing 1965 probes. The microarray probe corresponds to the miRNA genes of 988 humans, 627 mice, and 350 rats designed according to miRbase Release 12.0. Three independent RNA samples from rat spinal dorsal horn treated with vincristine or excipient at different time points were hybridized with miRNA microarray. The hybridization strength value of a single sample is filtered and normalized to each chip's average value. The candidate miRNAs with intensity signal > 500 were regarded as positive.
Western Blot
In short, the protein extracted from the spinal dorsal horn or experimental cells was first separated by gel electrophoresis (SDS-PAGE) and transferred to the PVDF membrane. The cells were sealed at room temperature for 1 h and then incubated with GAD67 primary antibody (1:1000, Abcam) at 4 °C overnight. After the first antibody was incubated, Western blot was used to incubate with IgG secondary antibody bound to horseradish peroxidase. Finally, the immunocomplexes were detected by electrochemiluminescence (Pierce). The protein was quantified by a computer-aided analysis system (ImageJ).
Immunohistochemistry
In short, rats were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg) and perfused with 4% paraformaldehyde through the ascending aorta. The lumbar spinal cord was removed and fixed overnight in 4% paraformaldehyde. Frozen sections with a thickness of 16 μm were taken and stained with GAD67 (1:200; Santa Cruz Biotechnology) monoclonal antibody by immunohistochemical staining. After incubation overnight at 4 °C, Cy3 or fluorescein isothiocyanate was combined with a secondary antibody and incubated for 2 h at room temperature. Then, the slices were observed with a Leica (Leica, Oskar-Barnack, Germany) fluorescence microscope, and the images were taken with a Leica DFC350FX camera. The GAD67 positive immunoreactivity area was quantitatively analyzed by the Leica Qwin V3 image analysis system.
PCR Amplification of MiRNAs and mRNAs
TaqMan miRNA probe (biological system) was used to quantify the mature miRNAs by stem-loop qRT-PCR analysis. The specific conditions for real-time PCR, using TaqMan PCR kit and Biosystems 7500 sequence detection system are as follows: 95 °C for 10 min, 95 °C for 60 cycles, 15 s, and 60 °C for 1 min. All the samples involved in the reaction, including the no-template control, were in triplicate. The relative expression rate of miRNA in spinal cord tissue of rats was counted by the 2−ΔΔCT measuring method.
GAD67 mRNA detection: According to the manufacturer's protocol, we use SYBR Green qPCR SuperMix (Invitrogen) and the ABI PRISM7500 Sequence Detection System for real-time qRT-PCR. The PCR reaction conditions were as follows: incubation at 95 °C for 3 min, then thermal cycle for 40 cycles (95 °C 10 s, 58 °C 20 s, 72 °C 10 s). The primers used in PCR are shown in Table 1.
Spinal Cord Section Preparation and Patch-Clamp Recording
Male SD rats (25–35 days) were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg, ip). The spinal cord was quickly removed and put into the following cold culture medium (unit mM): 95 NaCl, 1.8 KCl, 1.2 KH2PO4, 0.5 CaCl2, 7 MgSO4, 26 NaHCO3, 15 glucose, 50 sucrose, oxygenated with 95% O2/5% CO2, the pH was adjusted to 7.4, and the osmotic pressure was 310 to 320 mOsm/L. The dura mater and ventral roots of the spinal cord were removed. The parasagittal slices with dorsal roots (10–15 mm long) were cut into 400–600 μm thick with a vibrating micro slicer (DTK-1000; Dosaka) and put into 33 °C incubation medium for at least 60 min. After incubation, a single slice attached to the dorsal root was transferred to the recording chamber, and oxygenated ACSF solution was continuously perfused at room temperature. The dorsal root is gently connected to the suction electrode. The neurons in the I–II lamina were observed with a 60-degree water immersion objective lens on an upright infrared Nikon microscope. The suction electrode (4–6 mΩ) consists of both the EPC-10 amplifier and the PULSE program (HEKA Electronics), internal solution containing the following (in mM): 120 CsSO4, 20 TEA-Cl, 2 MgCl2, 2 Na2ATP, 10 EGTA, 0.5 NaGTP, biocytin 5, 10 HEPES, pH 7.28, with CsOH at a measured osmolarity of 300 mOsm/L. The electrode stimulation signal stimulates the dorsal root of the spinal cord (A360; World Precision Instruments) with a pulse width of 0.1 ms. The clamping voltage of neurons was controlled at 0 mV to minimize NMDA and AMPA/ kainic acid receptors' effect. After incubation with strychnine at 1 μM, IPSCs was induced by inhaling electrode to stimulate the attached dorsal root. At the end of the experiment, bicuculline (10 μM) was immersed and IPSCs, were wholly blocked to prove that the recorded current was mainly mediated by the GABAA receptor. For GABA mIPSCs, the slices were incubated with glycine and glutamatergic ion transfer antagonists [1 μM strychnine, 50 μM Dink 2-amino-5-phosphopentanoic acid and 10 μM 6-cyano-7-nitroquinoxaline-2-diketone] and recorded in the presence of lidocaine (2 mM) to block the action potential dependence of cells. In all experiments, only one neuron was recorded in each spinal cord section.
C-Fiber-Evoked Field Potentials Recording
The rat sciatic nerve was electrical stimulated, and a glass microelectrode was inserted into the dorsal horn of the L4–L5 spinal cord to record the field potential. The glass microelectrode was inserted into the dorsal horn at a depth of about 50–500 μm and was driven by an electronically controlled micro-stepping motor (Narishige Scientific Instrument Laboratory, Japan). The field potential data were recorded and analyzed by the LTP program (http://www.ltp-program.com). Before recording the field potential, the rat's sciatic nerve was tested by putting single square pulses (0.5-ms duration, in 1-min intervals). The intensity of stimulation was adjusted to 1.5–2 times the threshold of C-fiber response. The length from the sciatic nerve stimulation site to the dorsal horn field potential recording point was about 10 cm, and the experiment was performed only once in each rat.
Statistical Analysis
All data were expressed as mean ± SD and analyzed by SPSS13.0 software. Two-way ANOVA analyzed Western blot, qRT-PCR and electrophysiological data, and then Tukey's post hoc test. The behavior data were analyzed by repeated measures of one-way or two-way ANOVA and then Tukey's post hoc test. The standard of statistical significance was p < 0.05. The sample size is based on our and others' experience in pain behavior studies to provide power analysis.
Results
Vincristine Administration Leads to Decreased GABAergic Synaptic Function
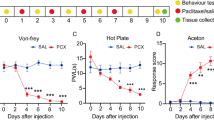
As our results show, vincristine administration (100 μg/kg·day, for consecutive 10 days) can induce marked mechanical allodynia (Fig. 1A) and thermal hyperalgesia (Fig. 1B) starting at day 3 since the first injection, compared with the saline vehicle group. The mechanical allodynia reached its peak on day 5. Both of them were persisting until the experimental endpoint, day 20. Next, we examined the alterations of electrophysiological character in superficial dorsal horn neurons after 10 days of vincristine administration. Our results suggested that the frequency of mIPSCs was significantly decreased in the vincristine group, while the amplitude was not altered (Fig. 1C). These results prompt that vincristine inhibits the presynaptic GABA releases in the spinal dorsal horn, and the impaired inhibitory synaptic connections in the dorsal horn may contribute to vincristine-induced hyperalgesia and allodynia.
The presynaptic GABAergic function is decreased in the vincristine induced neuropathic pain model. A Changes of mechanical hypersensitivity with time course.(**p < 0.01, ***p < 0.001 vs the vehicle group; n = 10 in each group.) B Time course of thermal hyperalgesia changes. (**p < 0.01 vs the vehicle group; n = 10 in each group.) C The traces represent mIPSCs alteration, and chart bars express its statistic counting between rvincristine and vehicle group. (**p < 0.01 vs the vehicle group; n = 45 cells in each group.)
The Protein of GAD67, But Not mRNA, is Significantly Decreased in the Vincristine Group
GAD is essential for converting glutamate to GABA, and GAD67 is the major component of GAD in developing animals [19]. To identify whether GAD67 is involved in vincristine-induced GABAergic electrophysiological character alterations, we conducted Western blot and revealed that GAD67 is markedly downregulated in the dorsal horn after 5 days of administration of vincristine (Fig. 2A). However, the qPCR result suggests that the mRNA level of GAD67 is not significantly changed in the vincristine group (Fig. 2B). In addition to this, the immunofluorescence result also pointed that GAD67 is downregulated on days 10 after vincristine injection (Fig. 2C). These results suggest that post-transcriptional epigenetic modification may contribute to GAD67 downregulation.
Vincristine leads to GAD67 protein, but not mRNA, downregulation. A Western blotting and histogram showed the changes of GAD67 protein with the time course of vincristine injection. (**p < 0.01 vs the vehicle group; n = 5 in each group.) B Histogram showed the time-course changes of GAD67 mRNA after vincristine administration. (n = 5 in each group.) C Immunofluorescence showed that the expression of GAD67 decreased after 10 days of vincristine injection. (**p < 0.01 vs the vehicle group; n = 5 in each group.)
MiR-30d Contribute to GAD67 Downregulation Through the Predicted Sites
MicroRNAs are small non-coding RNAs that regulate gene expression by translational inhibition or mRNA degradation [20]. Previous studies have already confirmed the role of miRNA in regulating neuropathic pain development [21, 22]. To explore the potential miRNA regulating GAD67 expression, we conducted genome-wide miRNA screening in the vincristine pain model and set at least 2.5-fold upregulation as the evaluation criterion. The result reveals that a total of 29 miRNAs were upregulated on days 4 and 10 after vincristine administration (Fig. 3A). Since we are exploring the miRNA involved in GAD67 expression, the potential binding sites of the 29 miRNAs were predicted using the databases of TargetScan and Miranda analysis. By collecting the predictions made on the databases, we finally screened 4 candidate miRNAs meeting the requirements, including miR-30d, miR-181a, miR-331, and miR-188 (Fig. 3A). Next, we performed qRT-PCR and revealed that only miR-30d, but not the other three miRNAs, was significantly increased on both 4 and 10 days after vincristine administration (Fig. 3B), implying its specific function in CIPN.
Vincristine can increase the expression of miR-30d, which further contributes to GAD67 downregulation. A Heatmap of miRNA microarray between vehicle and vincristine group, miRNA meet the criterion are highlighted in red. (Red means high miRNA expression, and blue means low miRNA expression.) B Histogram showing the time course of different miRNA expression after vincristine administration. (*p < 0.05, **p < 0.01 vs the vehicle group; n = 5 in each group.) C Histogram showing the relative luciferase activity of reporter containing Gad1 or mutated Gad1 and MiR-30d mimic. (##p < 0.01 versus the scramble group, **p < 0.01 vs the MiR-30d mimic group; n = 6 in each group.) D Western blotting and histogram showed the changes of GAD67 protein after transfecting miR-30d mimic. (**p < 0.01 vs the scramble group; n = 6 in each group.)
To further confirm miR-30d in regulating GAD67 expression, we first conducted the luciferase reporter assay to explore the interaction of miR-30d and GAD67 mRNA. As illustrated in Fig. 3C, the luciferase signal in 20 pmol or 50 pmol dose of the miR-30d group is significantly decreased compared with the scramble group, implying that miR-30d may regulate GAD67 expression by interacting with the 3′-UTR region of its mRNA. To confirm our conjecture, we then constructed a mutant vector of Gad1 in the putative binding site of miR-30d (Fig. 3C). Further luciferase reporter assay by transfecting the mutant vector and miR-30d mimic to HEK-293 cells showed that the suppression of luciferase signal is significantly reversed in the mutant vector group (Fig. 3C). Besides, we transfected the miR-30d mimic into the C6 cells that exhibited a detectable endogenous GAD67 expression. As shown in Fig. 3D, the miR-30d mimic could significantly downregulate GAD67 expression in C6 cells. These results suggested that miR-30d contributes to GAD67 downregulation by interacting with 3′-UTR of Gad1 mRNA.
The Involvement of miR-30d in the Behavioral Changes of Pain Induced by Vincristine in Rats is Related to Its Effect on C-Fiber-Evoked Field Potentials and the Expression of GAD67
In order to further clarify the mechanism of miR-30d involved in vincristine-induced hyperalgesia and allodynia, we intrathecally administrated miR-30d antagomir in rats to observe its effect on pain behavior. First of all, the intrathecal administration of miR-30d antagomir alone did not cause mechanical or thermal hyperalgesia, and control antagomir in the vincristine group had no significant effect on existing mechanical or thermal hyperalgesia. However, compared with the control antagomir administration in the vincristine group, miR-30d antagomir could significantly prevent mechanical and thermal hyperalgesia induced by vincristine injection (Fig. 4A, B). Besides, we also carried out Western blot experiments to detect the effect of antagomir on the expression of GAD67 at the general animal level. Since the use of miR-30d antagomir alone had no significant effect on pain behavior, the follow-up trial used miR-30d antagomir alone as the control group. As shown in Fig. 4C, the control antagomir didn't change the downregulated GAD67 protein expression in the vincristine group, supported by our previous experiments, while miR-30d antagomir administration could reverse the decreased GAD67 protein level in the vincristine induced pain model.
Intrathecal administration of miR-30d antagomir could alleviate vincristine induced neuropathic pain by upregulation GAD67 expression and inhibit dorsal horn's sensitivity to C-fiber evoked field potential. A Changes of mechanical hypersensitivity in different intervention groups.(**p < 0.01, ***p < 0.001 vs the vehicle + miR-30d antagomir group, #p < 0.05, ##p < 0.01 vs the vincristine + antagomir control group; n = 10 in each group.) B Thermal hyperalgesia changes in different intervention groups. (**p < 0.01 vs the vehicle + miR-30d antagomir group, ##p < 0.01 vs the vincristine + antagomir control group; n = 10 in each group.) C Western blotting and histogram showed the changes of GAD67 protein expression after intrathecal administration of miR-30d antagomir. (**p < 0.01 vs the vehicle + antagomir group, ##p < 0.01 vs the antagomir control + VCR group; n = 6 in each group.) D The traces represent the original recordings of the field potentials, and the line chart expresses the stimulus–response curves of C-fiber–evoked field potentials in different groups. (*p < 0.05, **p < 0.01 vs the vehicle + miR-30d antagomir group, #p < 0.05, ##p < 0.01 vs the vincristine + antagomir control group; n = 6 in each group.)
Further, we performed electrophysiology to study the effect of miR-30d on the nerve transmission function of the spinal dorsal horn in rats. Since the pathology of peripheral neuropathy that leads to neuropathic pain is mainly involved with unmyelinated C fibers [23]. We compared the strength of synaptic transmission mediated by C-fibers by recording spinal C-fiber–evoked field potentials in different intervention groups of rats 7 to 10 days after injection of vincristine or vehicle with or without miR-30d antagomir application. As shown in Fig. 4Da, the stimulus–response trace in the dorsal horn evoked by afferent C-fibers is significantly enhanced in the vincristine group, compared with the vehicle + miR-30d antagomir group. Intrathecal administration of miR-30d antagomir in the vincristine group could partially reverse the elevated field potential induced by vincristine, compared with antagomir control injection. In addition to this, with the stimulation intensity increasing, the magnitude of field potential elevation is significantly different in each group. The field potentials increasing in the vincristine and vincristine + antagomir control group were most robust, and miR-30d antagomir could substantially inhibit the field potentials change, especially in higher intensity of stimulation (Fig. 4Db). These results were suggesting that miR-30d may involve in vincristine induced neuropathic pain by downregulating GAD67 expression, and miR-30d antagomir attenuates the vincristine-induced LTP but does not affect basal synaptic transmission mediated by C-fibers.
Discussion
Peripheral neuropathy caused by chemotherapeutic drugs is a prevalent side effect in the process of tumor chemotherapy. Some commonly used chemotherapeutic drugs, such as vincristine, paclitaxel, oxaliplatin, bortezomib, et al., can cause peripheral neuropathy. Cancer patients often stop chemotherapy because they cannot tolerate CIPN, which brings significant challenges to tumor treatment and seriously affects patients' physical and mental health. However, there is no effective prevention and treatment for CIPN, and its specific pathophysiological mechanism is not very clear. Previous studies have shown that inhibitory and excitatory neurons at the spinal cord level are closely related to pain sensitization of neuropathic pain [24]. GABA neurons are one of the primary inhibitory neurons in the mammalian spinal cord, closely related to the development of neuropathic pain [25]. The GABA of embryonic medial ganglion eminence (MGE) can recover the spinal cord's GABA signal by transplanting the cortical precursor cells of embryonic medial ganglion eminence neurons into the spinal cord, which can reverse the mechanical hyperalgesia or hypersensitivity induced by paclitaxel [26]. At present, some drugs for the treatment of neuropathic pain, such as Carbemazepine, Clobezam and so on, can also relieve pain by activating or partially activating the function of GABA neurons [27]. Glutamate decarboxylase (GAD) is a key enzyme in GABA synthesis, in which GAD67 plays a major role [19]. There is also study have shown that transplanting GAD67 expression vector into DRG through human foamy virus (HFV) can relieve neuropathic pain caused by spinal cord injury [28]. Our study first confirmed that vincristine can cause mechanical and thermal hyperalgesia and reduce the frequency of GABA-inhibitive postsynaptic potentials. Further experiments showed that vincristine could reduce the expression of GAD67 protein, but had no significant effect on the amount of GAD67 mRNA. The difference in the amount of protein and mRNA makes us very interested in the upstream regulation mechanism of GAD67.
In recent years, there are accumulating studies on the mechanism of epigenetics in the occurrence and development of neuropathic pain. Epigenetics includes DNA methylation, histone modification, and the function of non-coding RNA. MicroRNA is a kind of non-coding RNA. Its main function is to regulate protein expression by inhibiting the translation of mRNA or degrading mRNA at the post-transcriptional level. Also, more and more studies have shown that some miRNA (miR-30c-5p, miR-17-92, miR-183, miR-7a, miR-21, miR-103) are closely related to the occurrence and development of neuropathic pain [6, 29,30,31,32,33]. Based on our previous study results, we speculate that the down-regulation of GAD67 may be caused by the role of miRNA. In order to confirm our hypothesis, we first carried out genome-wide miRNA screening of the vincristine pain model to find miRNA with differential expression, and then finally targeted miR-30d through binding site prediction and qRT-PCR. In previous studies, it has been found that miR-30d is closely related to pathophysiological processes such as myocardial remodeling [34], tumor invasion, and metastasis [35, 36], but its role in CIPN has not been studied. Our study suggests that miR-30d may interact with the specific region of GAD67 mRNA, which we identified as 3'UTR 1085–1109, to inhibit the expression of GAD67 and thus participate in the development of CIPN. Intrathecal administration of miR-30d antagonist can reverse the sensitivity of stimulus–response in spinal dorsal horn neurons to peripheral C-fibres evoked field potentials, upregulate the expression of GAD67 and alleviate the hyperalgesia and allodynia induced by VCR in rats.
At present, there is still no standard treatment for neuropathic pain caused by chemotherapeutic drugs. Gabapentin and pregabalin have partial GABA activation functions and are commonly used in the clinical treatment of CIPN [37, 38]. However, some studies have shown that the therapeutic effect of these two drugs on CIPN is not accurate, and there are side effects in the process of drug use [39, 40]. Although miRNA in neuropathic pain treatment is still in the experimental animal stage, some studies on tumor treatment have shown potential clinical value [41, 42]. There are two main strategies in applying miRNA in the treatment of neuropathic pain: one is the direct strategy, including the direct use of oligonucleotides or active viruses to inhibit the overexpression of miRNA or supplement the loss of miRNA expression. The other is the indirect strategy, including using drugs to regulate the expression of miRNA by acting on the transcription and processing of targeted miRNA[43]. In order to overexpress miRNA, miRNA mimics can be used as precursors or mature miRNA sequences[44]. The most common synthetic miRNA inhibitors are antagomirs, chemically modified for enhanced stability and cholesterol conjugated for high-efficiency delivery[45]. The miRNA mimics and miRNA antagomir were used in our experiment. The results showed that miRNA antagomir could prevent mechanical and thermal hyperalgesia and reverse the decrease of mIPSC frequency induced by CIPN, which to some extent suggested the feasibility of designing targeted drugs for miR-30d to prevent CIPN. In addition, some studies have shown that the expression of miR-30d in tumors is increased and related to tumor metastasis [35, 36]. These studies suggested that the prevention of CIPN against miR-30d does not affect the therapeutic effect of chemotherapeutic drugs on the tumor but may also be beneficial to tumor treatment. However, our experiment uses the direct intrathecal injection of miRNA mimics and antagomir, which is not a conventional way of drug use in the clinic, and whether drugs can pass through the blood–brain barrier in routine clinical use, such as oral administration and intravenous administration, need to be further studied in future experiments.
Our experiment also has some limitations: firstly, we did not do the co-localization of miR-30d and GAD67. On the other hand, we confirmed through cell experiments that miR-30d could act on GAD67 mRNA; Secondly. Genome-wide miRNA screening suggested that the expression of multiple miRNAs was increased. In our investigation, we finally determined the target miRNA as miR-30d by qRT-PCR, but the effect of other miRNAs is worth further exploration. Thirdly, this study only studied the pain model induced by vincristine, and the role of miR-30d in different pain models needs to be further explored. Last but not least, Our experiments did not identify the effect of miR-30d antagomir on spinal GABA tissue levels. The current experimental methods for identifying tissue GABA levels are mainly high-pressure liquid chromatography, and we were unable to perform this experiment due to experimental constraints. In previous studies on pain [46,47,48], spinal GABA levels have been closely associated with pain behavioral relief, and alterations in the levels of GAD65, one of the key enzymes for GABA synthesis, have also been confirmed to lead to GABA levels change [49]. Our experiments investigated the alterations of spinal GAD67 and pain behavior in the CIPN model or miR-30d antagomir application rats. Combined with previous experiments, we hypothesized that spinal GABA levels also have corresponding changes in these processes. In our subsequent experiments, we will also conduct more in-depth studies to explore the alterations of tissue GABA levels and provide our conclusions more definitive evidence.
In conclusion, Our study found for the first time that in vincristine-induced neuropathic pain, the down-regulated expression of GAD67 is related to the increased expression of miR-30d. MiR-30d affects its translation by acting on the 3'-UTR region of GAD67 mRNA, and then inhibits the spinal GABAergic synaptic function and affects the synaptic plasticity of peripheral C fibers transmitted to the center to participate in the development of neuropathic pain. However, our experiment needs further mechanism discussion and data analysis to confirm.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Vishnoi A, Rani S (2017) MiRNA biogenesis and regulation of diseases: an overview. Methods Mol Biol (Clifton, NJ) 1509:1–10
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells (Dayton, OH) 30:1556–1564
Zhao W, Zhao SP, Zhao YH (2015) MicroRNA-143/-145 in cardiovascular diseases. BioMed Res Int 2015:531740
van Schooneveld E, Wildiers H, Vergote I, Vermeulen PB, Dirix LY, Van Laere SJ (2015) Dysregulation of microRNAs in breast cancer and their potential role as prognostic and predictive biomarkers in patient management. Breast Cancer Res : BCR 17:21
Pan Z, Shan Q, Gu P, Wang XM, Tai LW, Sun M, Luo X, Sun L, Cheung CW (2018) miRNA-23a/CXCR4 regulates neuropathic pain via directly targeting TXNIP/NLRP3 inflammasome axis. J Neuroinflamm 15:29
Tramullas M, Francés R, de la Fuente R, Velategui S, Carcelén M, García R, Llorca J, Hurlé MA (2018) MicroRNA-30c-5p modulates neuropathic pain in rodents. Sci Transl Med 10:eaao6299
Rupaimoole R, Slack FJ (2017) MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 16:203–222
Price TJ, Prescott SA (2015) Inhibitory regulation of the pain gate and how its failure causes pathological pain. Pain 156:789–792
Goudet C, Magnaghi V, Landry M, Nagy F, Gereau RW, Pin JP (2009) Metabotropic receptors for glutamate and GABA in pain. Brain Res Rev 60:43–56
Modi JP, Prentice H, Wu JY (2015) Regulation of GABA neurotransmission by glutamic acid decarboxylase (GAD). Curr Pharmaceut Des 21:4939–4942
Bu DF, Erlander MG, Hitz BC, Tillakaratne NJ, Kaufman DL, Wagner-McPherson CB, Evans GA, Tobin AJ (1992) Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proc Natl Acad Sci USA 89:2115–2119
Wang Y, Nowicki MO, Wang X, Arnold WD, Fernandez SA, Mo X, Wechuk J, Krisky D, Goss J, Wolfe D, Popovich PG, Lawler S, Chiocca EA (2013) Comparative effectiveness of antinociceptive gene therapies in animal models of diabetic neuropathic pain. Gene Ther 20:742–750
Kanao M, Kanda H, Huang W, Liu S, Yi H, Candiotti KA, Lubarsky DA, Levitt RC, Hao S (2015) Gene transfer of glutamic acid decarboxylase 67 by herpes simplex virus vectors suppresses neuropathic pain induced by human immunodeficiency virus gp120 combined with ddC in rats. Anesth Analg 120:1394–1404
Siau C, Xiao W, Bennett GJ (2006) Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol 201:507–514
Xu T, Li D, Zhou X, Ouyang HD, Zhou LJ, Zhou H, Zhang HM, Wei XH, Liu G, Liu XG (2017) Oral application of magnesium-L-threonate attenuates vincristine-induced allodynia and hyperalgesia by normalization of tumor necrosis factor-alpha/nuclear factor-kappaB signaling. Anesthesiology 126:1151–1168
Li D, Huang ZZ, Ling YZ, Wei JY, Cui Y, Zhang XZ, Zhu HQ, Xin WJ (2015) Up-regulation of CX3CL1 via nuclear factor-κB-dependent histone acetylation is involved in paclitaxel-induced peripheral neuropathy. Anesthesiology 122:1142–1151
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88
Liu CG, Calin GA, Meloon B, Gamliel N, Sevignani C, Ferracin M, Dumitru CD, Shimizu M, Zupo S, Dono M, Alder H, Bullrich F, Negrini M, Croce CM (2004) An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc Natl Acad Sci USA 101:9740–9744
Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K (1997) Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA 94:6496–6499
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Kusuda R, Cadetti F, Ravanelli MI, Sousa TA, Zanon S, De Lucca FL, Lucas G (2011) Differential expression of microRNAs in mouse pain models. Mol Pain 7:17
Gong Q, Lu Z, Huang Q, Ruan L, Chen J, Liang Y, Wang H, Yue Y, Feng S (2015) Altered microRNAs expression profiling in mice with diabetic neuropathic pain. Biochem Biophys Res Commun 456:615–620
Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN (2017) Neuropathic pain Nat Rev Dis Primers 3:17002
Todd AJ (2010) Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11:823–836
Magoul R, Onteniente B, Geffard M, Calas A (1987) Anatomical distribution and ultrastructural organization of the GABAergic system in the rat spinal cord. An immunocytochemical study using anti-GABA antibodies. Neuroscience 20:1001–1009
Bráz JM, Wang X, Guan Z, Rubenstein JL, Basbaum AI (2015) Transplant-mediated enhancement of spinal cord GABAergic inhibition reverses paclitaxel-induced mechanical and heat hypersensitivity. Pain 156:1084–1091
Alles SRA, Smith PA (2018) Etiology and pharmacology of neuropathic pain. Pharmacol Rev 70:315–347
Liu W, Liu Z, Liu L, Xiao Z, Cao X, Cao Z, Xue L, Miao L, He X, Li W (2008) A novel human foamy virus mediated gene transfer of GAD67 reduces neuropathic pain following spinal cord injury. Neurosci Lett 432:13–18
Sakai A, Saitow F, Maruyama M, Miyake N, Miyake K, Shimada T, Okada T, Suzuki H (2017) MicroRNA cluster miR-17-92 regulates multiple functionally related voltage-gated potassium channels in chronic neuropathic pain. Nat Commun 8:16079
Peng C, Li L, Zhang MD, Bengtsson Gonzales C, Parisien M, Belfer I, Usoskin D, Abdo H, Furlan A, Häring M, Lallemend F, Harkany T, Diatchenko L, Hökfelt T, Hjerling-Leffler J, Ernfors P (2017) miR-183 cluster scales mechanical pain sensitivity by regulating basal and neuropathic pain genes. Science (New York, NY) 356:1168–1171
Sakai A, Saitow F, Miyake N, Miyake K, Shimada T, Suzuki H (2013) miR-7a alleviates the maintenance of neuropathic pain through regulation of neuronal excitability. Brain 136:2738–2750
Zhang ZJ, Guo JS, Li SS, Wu XB, Cao DL, Jiang BC, Jing PB, Bai XQ, Li CH, Wu ZH, Lu Y, Gao YJ (2018) TLR8 and its endogenous ligand miR-21 contribute to neuropathic pain in murine DRG. J Exp Med 215:3019–3037
Favereaux A, Thoumine O, Bouali-Benazzouz R, Roques V, Papon MA, Salam SA, Drutel G, Léger C, Calas A, Nagy F, Landry M (2011) Bidirectional integrative regulation of Cav1.2 calcium channel by microRNA miR-103: role in pain. EMBO J 30:3830–3841
Li J, Salvador AM, Li G, Valkov N, Ziegler O, Yeri AS, Xiao CY, Meechoovet B, Alsop E, Rodosthenous RS, Kundu P, Huan T, Levy D, Tigges JC, Pico AR, Ghiran I, Silverman MG, Meng X, Kitchen R, Xu J, Van Keuren-Jensen K, Shah RV, Xiao J, Das S (2020) Mir-30d regulates cardiac remodeling by intracellular and paracrine signaling. Circul Res 128:e1
Croset M, Pantano F, Kan CWS, Bonnelye E, Descotes F, Alix-Panabières C, Lecellier CH, Bachelier R, Allioli N, Hong SS, Bartkowiak K, Pantel K, Clézardin P (2018) miRNA-30 family members inhibit breast cancer invasion, osteomimicry, and bone destruction by directly targeting multiple bone metastasis-associated genes. Cancer Res 78:5259–5273
Lin ZY, Chen G, Zhang YQ, He HC, Liang YX, Ye JH, Liang YK, Mo RJ, Lu JM, Zhuo YJ, Zheng Y, Jiang FN, Han ZD, Wu SL, Zhong WD, Wu CL (2017) MicroRNA-30d promotes angiogenesis and tumor growth via MYPT1/c-JUN/VEGFA pathway and predicts aggressive outcome in prostate cancer. Mol Cancer 16:48
Tanabe M, Takasu K, Takeuchi Y, Ono H (2008) Pain relief by gabapentin and pregabalin via supraspinal mechanisms after peripheral nerve injury. J Neurosci Res 86:3258–3264
Fradkin M, Batash R, Elmaleh S, Debi R, Schaffer P, Schaffer M, Asna N (2019) Management of peripheral neuropathy induced by chemotherapy. Curr Med Chem 26:4698–4708
Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA, Warner DO, Novotny P, Kutteh LA, Wong GY (2007) Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer 110:2110–2118
Shinde SS, Seisler D, Soori G, Atherton PJ, Pachman DR, Lafky J, Ruddy KJ, Loprinzi CL (2016) Can pregabalin prevent paclitaxel-associated neuropathy?: an ACCRU pilot trial. Support Care Cancer 24:547–553
Beg MS, Brenner AJ, Sachdev J, Borad M, Kang YK, Stoudemire J, Smith S, Bader AG, Kim S, Hong DS (2017) Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig New Drugs 35:180–188
van Zandwijk N, Pavlakis N, Kao SC, Linton A, Boyer MJ, Clarke S, Huynh Y, Chrzanowska A, Fulham MJ, Bailey DL, Cooper WA, Kritharides L, Ridley L, Pattison ST, MacDiarmid J, Brahmbhatt H, Reid G (2017) Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol 18:1386–1396
Tan PH, Pao YY, Cheng JK, Hung KC, Liu CC (2013) MicroRNA-based therapy in pain medicine: current progress and future prospects. Acta Anaesthesiol Taiwan 51:171–176
Tsuda N, Mine T, Ioannides CG, Chang DZ (2009) Synthetic microRNA targeting glioma-associated antigen-1 protein. Methods Mol Biol (Clifton, NJ) 487:435–449
Zhou S, Wang Y, Meng Y, Xiao C, Liu Z, Brohawn P, Higgs BW, Jallal B, Jia Q, Qu B, Huang X, Tang Y, Yao Y, Harley JB, Shen N (2016) In vivo therapeutic success of microrna-155 antagomir in a mouse model of lupus alveolar hemorrhage. Arthritis Rheumatol (Hoboken, NJ) 68:953–964
Jiménez-Zárate BS, Piña-Leyva C, Rodríguez-Sánchez M, Florán-Garduño B, Jiménez-Zamudio LA, Jiménez-Estrada I (2021) Day-night variations in the concentration of neurotransmitters in the rat lumbar spinal cord. J Circadian Rhythms 19:9
Ryu SW, Kim YO, Kim HB, Oh SB, Choi JI, Yoon MH (2021) Antinociceptive effect of intrathecal P7C3 via GABA in a rat model of inflammatory pain. Eur J Pharmacol 899:174029
Maeda Y, Lisi TL, Vance CG, Sluka KA (2007) Release of GABA and activation of GABA(A) in the spinal cord mediates the effects of TENS in rats. Brain Res 1136:43–50
Shen X, Liu Y, Xu S, Zhao Q, Wu H, Guo X, Shen R, Wang F (2014) Menin regulates spinal glutamate-GABA balance through GAD65 contributing to neuropathic pain. Pharmacol Rep 66:49–55
Acknowledgements
This work has not been previously published and has not been submitted elsewhere for consideration. This study was funded by the National Natural Science Foundation of China (81600955, 81971048), Shanghai Pujiang Program (2020PJD059), "Deep Blue 123" Military Medical Research Special Key Research Project(2019YSL008), Natural Science Foundation of Shaanxi Province Department of Science and Technology (2018JM7052) and Scientific Research Fund Project of Shaanxi Province Department of Education (18JK0675).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures were approved by the Naval Medical University Animal Care and Use Committee and carried out following the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, H., Sun, Y., Wu, Y. et al. MiR-30d Participates in Vincristine-Induced Neuropathic Pain by Down-Regulating GAD67. Neurochem Res 47, 481–492 (2022). https://doi.org/10.1007/s11064-021-03462-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03462-3