Abstract
Temporal lobe epilepsy (TLE) is a complex neurological disease, and its occurrence and development are closely related to the autophagy signaling pathway. However, the mechanism by which electroacupuncture (EA) affects the regulation of autophagy has not been fully elucidated. TLE gene chip dataset GSE27166 and data from rats without epilepsy (n = 6) and rats with epilepsy (n = 6) were downloaded from Gene Expression Omnibus. The differentially expressed genes (DEGs) in the TLE and control groups were identified with the online tool GEO2R. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) databases were used to analyse the functional and pathway enrichment of genes in the most important modules. A rat model of TLE induced by lithium–pilocarpine treatment was established. EA treatment at DU20 and DU14 in TLE rats was performed for 2 weeks. Neuronal regeneration was determined using immunofluorescence staining. The protein levels of AKT/mTOR signaling pathway and autophagy markers were detected through western blotting and immunohistochemistry. This study identified 1837 DEGs, including 798 upregulated genes and 1039 downregulated genes. GO enrichment and KEGG analyses were performed on DEGs and revealed functional enrichment mainly in the mTOR signaling pathway and autophagy-animal. Furthermore, the number of mature neurons was significantly increased upon coexpressing BrdU/NeuN in TLE rats treated with EA. Western blotting and immunohistochemistry results showed significantly decreased levels of the phosphorylated-AKT and p-mTOR in the hippocampal CA3 and DG regions of TLE rats with EA treatment. And increased p-ULK1/ULK1, LC3-II/LC3-I and p62 levels in TLE rats with EA stimulation. Therefore, this study suggested that EA promoted autophagy in hippocampal neurons during the onset of epilepsy by regulating the AKT/mTOR signaling pathway to treat epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temporal lobe epilepsy (TLE), a neurological disease, often presents as disturbances in consciousness, seizures, extensive neurocognitive dysfunction, and a decreased quality of life. It is the most common type of partially complex epileptic seizure in the clinical environment [1,2,3]. TLE is also the most common refractory focal epilepsy, which occurs in 40% of patients with epilepsy [4], and 20–30% of patients with TLE have poor seizure outcomes after treatment with antiepileptic drugs [5]. Its pathogenesis has always been a hot spot in epilepsy research. TLE is characterized by neurogenesis and angiogenesis, and its pathological manifestations are mainly changing in the hippocampal dentate gyrus (DG), neuron loss in CA1 and CA3 areas, compensatory gliosis, formation of new granule cells, and synaptic reconstruction, as well as the formation of abnormal neural circuits [6]. During the process of epilepsy, new hippocampal DG neurons extend dendrites into the molecular layer of the CA3 area to form new synaptic connections with pyramidal cell dendrites, thereby forming new, abnormal neural circuits, which result in spontaneous epilepsy at the outbreak point [6].
mTOR is a highly conserved serine/threonine protein kinase and one member of the phosphatidylinositol kinase-related protein kinase family. mTOR functions as a central signal regulator in the cell. It responds to a variety of stimuli and plays important roles in regulating cell growth, proliferation, apoptosis, autophagy, protein translation and immunosuppression. From hereditary epilepsy (epilepsy-related ganglion glioma and hyperamniotic fluid megacranial symptomatic epilepsy syndrome) [7] to acquired epilepsy (kainic acid model [8], PILO model and traumatic brain injury models) [9], the mTOR signaling pathway has been confirmed to be overactivated. Intervention with the mTOR inhibitor rapamycin not only reduces the number of seizures but also prevents or reverses the histopathological changes that lead to epilepsy, such as abnormal hypertrophy of neuronal cells in hereditary epilepsy and hippocampal neurons in acquired epilepsy. Abnormal activation of mTOR and a high incidence of epilepsy have been observed in rodent TSC1 and phosphatase gene (PTEN) knockout models and human patients with TSC. Hyperactivation of mTOR is a recognized potential mechanism of epileptic seizures [10]. Increasing the activity of mTOR may lead to epileptic discharge and subsequent epileptic diseases and even affect the development of epilepsy and changes in social behaviour [11]. The treatments for epilepsy currently mainly include pharmacological and nonpharmacological methods. Among them, the pharmacological effects of many antiepileptic drugs are derived from the enhanced inhibitory effect mediated by gamma-aminobutyric acid (GABA). GABA is the main inhibitory neurotransmitter in the adult central nervous system, and changes in the function of γ-aminobutyric acid receptors are related to TLE [12]. However, approximately 20–40% of patients with epilepsy may have drug-resistant epilepsy (DRE). In these patients, antiepileptic drugs do not effectively control seizures [13]. In recent years, nonpharmacological methods have received increasing attention as treatments for epilepsy. Nonpharmacological methods mainly include diet therapy and neuromodulation therapy. Auricular acupuncture has been shown to effectively reduce the occurrence of late-stage traumatic epilepsy, low-frequency electrical acupuncture to stimulate the Fengchi acupoint ameliorates epilepsy and sleep disorders caused by epilepsy [14], and acupuncture at Zusanli (St36), Dazhui (Du14), Baihui (Du20) and other specific acupoints effectively treats epilepsy [15, 16]. Although EA exerts a clear effect on the clinical treatment of epilepsy, its internal mechanism lacks in-depth research. Recent research indicates that autophagy is closely associated with epilepsy that lack of autophagy in neurons results in a distinct neurodegeneration and epileptic disorders [17]. And AKT/mTOR signaling plays key roles in regulating autophagy activity. Current therapeutic strategy for various diseases is targeting AKT/mTOR-mediated autophagy [18]. In view of the nonpharmacological, seizure and treatment mechanism results, the mechanism by which EA affects the regulation of autophagy in TLE has not been fully elucidated. In this study, the hippocampal CA3 and DG areas of epileptic rats were used as the main research areas, and the rat model of lithium chloride–pilocarpine-induced TLE, which has similar clinical and pathological features to humans with TLE, was used as a model [19]. Acupuncture at Baihui and Dazhui points was administered for treatment. Then, changes in the levels of p-AKT, p-mTOR, in hippocampal CA3 and DG areas were determined, the effect of EA at Du14 and Du20 acupoints on the levels of AKT/mTOR signaling pathway and autophagy markers in TLE rats were analysed, and the underlying mechanism of the treatment of epilepsy by acupuncture and moxibustion was explored.
Methods
Data Source and Differential Expression Analysis
The GSE27166 dataset consists of 12 microarray expression profiles and was downloaded from the Gene Expression Omnibus Comprehensive Website (GEO, http://www.ncbi.nlm.nih.gov/geo). Sample data were obtained from 6 rats with epilepsy (TLE samples) and 6 rats without epilepsy (control samples). The platform of the dataset was GPL2896 (GE Healthcare/Amersham Biosciences CodeLink™ Rat Whole Genome Bioarray). The differentially expressed genes in the TLE and control groups were analysed using the GEO2R tool, and were selected according to an at least 1.5-fold difference.
GO Analysis
The GO terminology was analysed to obtain the genes associated with TLE and to classify their cellular components (CC), biological processes (BP), and molecular functions (MF). The GO terminology for the GO enrichment analysis and EC analysis was retrieved from DAVID (http://david.abcc.ncifcrf.gov/). For the GO enrichment analysis, the significant differences in enriched GO terms in DEGs were compared with a background set consisting of 1837 DEGs, including 798 upregulated genes and 1039 downregulated genes. Without multiple test correlations, a BP P-value < 0.01 and MF P-value < 0.1 were set as significance thresholds.
Rat Epilepsy Model
Thirty male SD rats (purchased from Vital River Laboratory Animal Technology, Beijing, China) were randomly selected as the control group for epilepsy modelling. Among them, twenty rats were intraperitoneally injected with LiCl (Boehringer Mannheim, USA) 3 mEq/kg (approximately 125 mg/kg). Eighteen hours later, the rats were intraperitoneally injected with PILO (Sigma, USA), 10 mg/kg each injection, once every 30 min, until status eplepticus (SE) appeared. Thirty minutes later, the seizures were stopped with 10% chloral hydrate, and the degree of seizures was graded according to the standards established by Racine [20]. All animal experiments were carried out in accordance with the Chinese governing law on the use of medical laboratory animals (authorization no. 551998, 2013, by the Ministry of Health).
EA Treatment
The rat was anaesthetized by ether inhalation, and then the head of the rat was fixed on a stereotaxic device (SA301, China). One-inch needles were used to puncture Baihui and oblique Dazhui acupoints, approximately 7 mm. A G6805-2 electroacupuncture treatment instrument was used for EA. EA adopts sparse and dense waves, dense wave: 6.25 Hz, intensity: 1.0 mA, time: 2.08 s; sparse wave: 3.85 Hz, intensity: 1.0 mA, time: 1.28 s, lasting 30 min, 1 time/day continuous acupuncture. After 2 weeks, all rats were sacrificed with intraperitoneal injection of Nembutal (100 mg/kg).
Immunofluorescence Staining
Immunofluorescence staining of rat hippocampal sections was performed as previously described [21]. The sections were incubated with primary antibodies against 5-bromodeoxyuridine (BrdU, 1:200; ab8152, Abcam) and neuronal nuclei (NeuN, 1:200; ab104224, Abcam) at 4 °C overnight, followed by an incubation with fluorescently labelled secondary antibodies. The sections were examined using a Leica confocal microscope (LEICA TCS SP5).
Quantitative Real-Time PCR (qRT-PCR)
Rat hippocampus tissues were grinded in liquid nitrogen. Next, 800 μl of TRIzol reagent (T9429, Sigma, US) was added to each sample and repeatedly pipetted to dislodge all adherent cells, and the cells were transferred to 1.5 ml EP tubes. The total RNA of each sample was extracted according to the manufacturer’s instructions and reverse transcribed with the PrimeScript™ RT Reagent Kit (TaKaRa, Japan). qRT-PCR was performed with SYBR Green Detection Mix (TaKaRa, Japan). The relative expression levels of genes in this study were normalized to actin expression, analysed by the 2−ΔΔCt method, and summarized from separately harvested PMC samples.
Western Blot Analysis
Rat hippocampus tissue frozen in liquid nitrogen were lysed in radioimmunoprecipitation assay (RIPA) lysis buffer and transferred to a tube followed by the addition of 10 μl phenylmethylsulphonyl fluoride (PMSF). To remove the cell debris, lysates were centrifuged at 12,000 rpm for 10 min, and the supernatant was collected in a new tube. The bicinchoninic acid (BCA) protein quantification method was used to ensure that the concentration of each sample was basically equal. Protein samples were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes with electrophoresis systems (Tanon VE180 and Tanon VE186, Shanghai). The PVDF membranes were blocked with 5% (w/v) skim milk powder for 2 h and incubated at 4 °C overnight with the following primary antibodies (1:1000): rabbit anti-p-AKT (ab38449, Abcam), AKT (ab8805, Abcam), p-mTOR (ab109268, Abcam), mTOR (ab134903, Abcam), p-ULK1 (ab203207, Abcam), ULK1 (ab167139, Abcam), LC3 (ab48394, Abcam) and p62 (ab240635, Abcam). After three washes with 1 × PBS (Sangon, Shanghai), the membranes were incubated with HRP-labelled goat anti-rabbit IgG secondary antibodies (1:5000) (Abcam, UK). Immunoreactivity was determined with enhanced chemiluminescence (ECL) reagent (Thermo Fisher, US). A gel imaging system (Bio-Rad Gel Doc XR + , US) and software (Bio-Rad Image Lab Software, version 5.1 and SPSS 20.0) were used for imaging and statistical analysis. β-Actin (ab8227, Abcam) was used as an internal control to ensure equal protein loading.
Immunohistochemistry
Paraffin-embedded blocks of rat hippocampus tissues were cut into 4-μm-thick sections, and the sections were dewaxed and hydrated. Then, the sections were immersed in distilled water containing 3% hydrogen peroxidase twice to reduce endogenous oxidase activity. Next, the tissue sections were incubated with an anti-p-AKT antibody (ab38449, Abcam) and anti-mTOR antibody (ab32028, Abcam) for 2 h at room temperature, and subsequently, a goat-anti-rabbit antibody was applied to the cells and incubated at room temperature for 40 min. The degree of staining was determined by staining sections with the diaminobenzidine (DAB) chromogen (Bio-Rad, Inc., CA, USA). Subsequently, the tissue sections were dehydrated and sealed with gum. Five random fields of view at 100× magnification were imaged with a camera attached to a microscope (Olympus, Japan), and the mean microvessel count was recorded as the microvessel density.
Statistical Analysis
SPSS 22.0 (IBM Corporation, USA) and GraphPad Prism 5.0 (GraphPad Inc., USA) were employed for statistical analyses. All data are presented as the means ± SD (standard deviation). Independent group comparisons were performed using Student’s t-test or one-way ANOVA with Bonferroni’s post hoc test. A value of P < 0.05 was considered statistically significant.
Results
The AKT/mTOR Signaling Pathway was Involved in TLE in Rats
After data pre-processing, the expression matrix of 15739 genes was obtained from 12 samples. At a threshold of |log2FC|≥ 0.5, 1837 DEGs were selected for subsequent analysis, including 798 upregulated and 1039 downregulated DEGs, which were considered significant and were shown in a heatmap (Fig. 1a; Supplemental Table 1) and volcano plots (Fig. 1b). Based on these results, the DEGs from the two samples were distinguished from each other.
Transcriptomic analysis of TLE rats. a Heatmap of differentially expressed genes in control rats and TLE rats. X axis: sample name; Y axis: gene name. b Volcano plot of differentially expressed genes. X-axis: log2FC; Y-axis: −log10 (FDR). Red represents upregulated genes, and blue represents downregulated genes
Furthermore, a KEGG pathway analysis was performed on the 1837 DEGs, and DEGs belonging to the mTOR signaling pathway and autophagy-animal were enriched (Fig. 2a; Supplemental Table 2). By performing a GO analysis of the genes in this module, we were surprised to find that neuronal cell bodies, brain development, regulation of apoptotic processes and regulation of autophagy were significantly enriched (Fig. 2b; Supplemental Table 3).
Gene expression profile of TLE rats. a The canonical pathways related to the mTOR signaling pathway, mitophagy-animal, neurotrophin signaling pathway and autophagy-animal enriched in the upregulated and downregulated genes, as determined by the KEGG pathway analysis. b The analysis of GO terms enriched in MF, CC and BP for the DEGs. The negative logarithm of the P-value (x-axis) indicates the significance of the gene set belonging to predefined categories for the DEGs. The y-axis represents each GO category. The gene number and EC value of the category in the subnetwork are shown in brackets. c A heat map depicting the gene expression profiles of the mTOR signaling pathway and autophagy-animal in TLE rats. Red: high expression; yellow: intermediate expression; blue: low expression. d The expression levels of eight upregulated genes (Irs1, Ulk1, Gats3, Rho, AKT1s1, Fnip1, cab39 and Strada) were analysed using qRT-PCR. e The expression levels of eleven downregulated genes (Ikbkb, Depdc5, Tsc2, Fzd10, Dvl2, Wnt2b, AKT1, AKT2, Wdr24, Mlst8 and Rragc) were analysed using qRT-PCR
The heatmap depicting DEGs in TLE rats indicated that eight genes (Irs1, Ulk1, Gats3, Rho, AKT1s1, Fnip1, cab39 and Strada) participating in the AKT signaling pathway were significantly increased, and eleven genes in the mTOR signaling pathway (Ikbkb, Depdc5, Tsc2, Fzd10, Dvl2, Wnt2b, AKT1, AKT2, Wdr24, Mlst8 and Rragc) were significantly decreased (Fig. 2c; Supplemental Fig. S1). qRT-PCR confirmed these findings (Fig. 2d, e). These results indicated that the AKT/mTOR signaling pathway was closely related to the functions of TLE rats.
EA Protected Against Hippocampal Neuron Damage in TLE Rats
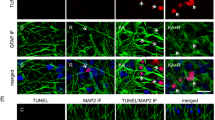
The intervention effect of EA stimulation was evaluated by observing the degree of hippocampal neurogenesis in TLE rats. Immunofluorescence staining was performed on the mature neuron markers, BrdU and NeuN protein. Results showed that decreased BrdU/NeuN signal in the TLE rats, and the BrdU/NeuN signal was increased in TLE rats stimulated with EA (Fig. 3a, c). In the TLE group, large areas of pyramidal cells in the hippocampal CA1 area were missing, the cell arrangement was sparse, the intercellular space was enlarged, the apical dendrites of neurons were shortened and disappeared, and the Nissl bodies in the cytoplasm were reduced or disappeared. However, in the TLE + EA group, a significant increase in the number of pyramidal cells was observed, apical dendrites of neurons were arranged in a concentrated and orderly manner, and Nissl bodies were abundant and obvious in the cytoplasm (Fig. 3b). A subsequent cell count analysis also confirmed that the number of cells increased significantly in the EA stimulation group compared to the simple epilepsy group (Fig. 3d). Therefore, EA stimulation significantly ameliorated the damage to hippocampal neurons after TLE.
EA reduced neuronal damage in TLE rats. a Immunofluorescence staining showing BrdU-positive cells/sections and neurons (NeuN) in hippocampal sections. b Nissl staining showing neurons in CA1 sections. c Quantitative analysis of BrdU-positive cells/NeuN-positive cells (%) in hippocampal sections. d Quantitative analysis of neuronal cells in CA1 sections. *p < 0.05 and **p < 0.01. Columns represent the means (n = 6); bars represent the SD; *p < 0.05 and **p < 0.01
EA Regulated the AKT/mTOR Signaling Pathway in DG and CA3 Region of TLE Rats
To investigate the relationship between EA treatment and the AKT/mTOR signalling pathway in TLE rats. Immunohistochemistry was performed to assess the levels of the classical factors in the AKT/mTOR signaling pathway: p-AKT and p-mTOR in DG and CA3 regions of TLE rats. The results demonstrated that p-AKT-positive cells and p-mTOR-positive cells were mainly observed in TLE rats (Fig. 4a), and p-AKT and p-mTOR immunostaining seemed to be increased in both the DG (Fig. 4b, d) and CA3 (Fig. 4c, e) regions of TLE rats compared to the controls, but reduced with EA treatment compared to the TLE groups (Fig. 4).
Effects of EA on the protein expression in the hippocampal DG and CA3 regions of TLE rats. a The expression of p-AKT and p-mTOR in the DG and CA3 of control rats, TLE rats and TLE rats treated with EA was determined using immunohistochemistry. b–e Quantitative analysis of the average optical density of A. Columns represent the means (n = 6); bars represent the SD; *p < 0.05 and **p < 0.01
EA Promoted Expression of Autophagy-Related Proteins in TLE Rats
To further investigate whether autophagy was involved in EA treatment through AKT/mTOR signaling pathway, we performed a western blot analysis to examine the expression of p-AKT/AKT, p-mTOR/mTOR and autophagy marker: p-ULK1/ULK1, LC3-II/I, p62 protein in the hippocampus of TLE rats. Western blot analysis showed significantly increased levels of the p-AKT/AKT, p-mTOR/mTOR in TLE rats, and significantly decreased levels after EA stimulation compared to the TLE groups (Fig. 5a–c). However, the p-ULK1/ULK1, LC3-II/I and p62 levels exhibited opposite trends that they all decreased significantly in TLE rats, but increased after EA stimulation (Fig. 5a, d, and f). Taken together, EA might prevent the phosphorylation of AKT/mTOR proteins to activate autophagy in TLE rats.
Effects of EA on protein expression in the hippocampal DG and CA3 regions in vitro. a The protein levels of p-AKT, AKT, p-mTOR, mTOR, p-ULK1 and ULK1, LC3-II, LC3-I and p62 were analyzed in the TLE group and the TLE plus EA group through western blot. b–f The quantitative analysis of p-AKT/ AKT, p-mTOR/mTOR, p-ULK1/ULK1, LC3-II/LC3-I, and p62/Actin ratio. Columns represent the means (n = 3); bars represent the SD; *p < 0.05 and **p < 0.01
Discussion
TLE is a clinically common type of complex epilepsy, and its pathogenesis is the focus of epilepsy research. The rat model of TLE induced by lithium chloride–pilocarpine is similar to human TLE [19], and it is an important tool for studying the mechanism of TLE. TLE pathology mainly manifests as the loss of neurons in the hippocampal DG, CA1 and CA3 areas, compensatory gliosis, the formation of new granule cells, the reconstruction of synapses, and the formation of abnormal neural circuits [22]. When epilepsy occurs, the dendrites of new hippocampal DG neurons extend into the molecular layer of the CA3 area to form a new synaptic connection with the dendrites of pyramidal cells, thereby resulting in spontaneous seizures [22]. The CA3 and DG areas of the hippocampus are important participants in TLE [23].
As a representative nonpharmacological treatment method, EA has been used in the clinic to treat patients with epilepsy. Research by Dos et al. showed that EA at ST36 prevented the atrophy of some border structures and improved the cognitive deficits of model rats with epilepsy induced by pilocarpine [24]. After EA treatment, the reduction in nitric oxide synthase expression [25] and the increase in melatonin levels [26] contribute to the antiepileptic effect. In the present study, a decrease in the BrdU/NeuN signal was observed in the TLE rats, which was increased in TLE + EA rats. In addition, EA stimulation significantly increased pyramidal cell numbers, rearranged the apical dendrites of neurons, and enhanced Nissl bodies in the cytoplasm. Taken together, these results suggested that EA stimulation improved of hippocampal neuron damage in TLE, which confirmed the previous findings that EA was an efficient clinical method for treating TLE.
The PI3K/AKT signalling pathway is an important pathway that regulates the activities of nerve cells. This pathway plays an important role in the repair of nervous system damage [27]. Recent experimental studies have revealed a significant decrease in the number of hippocampal CA3 pyramidal neurons in the model group after the injection of a specific antagonist into the lateral ventricle of epileptic rats to block the PI3K/AKT signalling pathway, and the number of hippocampal CA3 pyramidal cells was increased significantly at 4 h and 24 h after the epileptic seizure in the acupuncture group, indicating that the PI3K/AKT signalling pathway correlated with the process by which EA antagonizes epileptic brain injury [28]. The effect of EA on the PI3K/AKT signalling pathway is thought to inhibit hippocampal neuron apoptosis and improve related defects [29]. mTOR is a downstream serine/threonine kinase of the PI3K/AKT pathway. Animal experiments have shown that mTOR inhibitors effectively prevent seizures [30], significantly reduce the frequency of seizures, and reduce the severity of seizures [31]. In this study, immunohistochemistry showed that the protein levels of p-AKT and p-mTOR were significantly increased in the DG and CA3 zones of TLE rats. However, the EA treatment significantly reduced these expression levels. In addition, western blotting analysis of the p-AKT/AKT and p-mTOR/mTOR levels further confirmed the immunohistochemistry findings. Thus, these results suggested that EA at the DU14 and DU20 points effectively reduced the protein expression of AKT/mTOR proteins in rats with epilepsy, thereby exerting a certain antiepileptic effect.
The PI3K/AKT/mTOR pathway is involved in several normal cellular processes, such as autophagy, which is modulated by aberrant regulation [32]. The decreased expression levels of p-AKT and p-mTOR in EA-treated TLE rats suggested that autophagy activation might account for the reduction in neuronal cell damage observed after long-term TLE following EA administration. In this study, p-ULK1/UKL1, as key mediators of mTOR signalling in autophagy, were significantly increased after EA treatment compared to those in TLE rats. LC3-II is closely associated with the formation of autophagosomes [33] and p62 is a prototypical autophagy receptor [34]. The LC3-II/LC-I ratio and p62 level are widely used to monitor autophagy. In this study, the LC3-II/LC-I ratio and p62 level were similar to those of p-ULK1/UKL1. Thus, these results suggested that EA promoted autophagy in hippocampal neurons by inhibiting AKT/mTOR signalling.
In conclusion, this study demonstrated that EA protected against hippocampal neuron damage by accelerating autophagy through regulating the PI3K/AKT/mTOR signalling pathway in TLE rats. These data support the use of EA treatment for epilepsy and provide theoretical evidence for studying the antiepileptic mechanism of EA. More investigations are needed in the future to explore the underlying mechanisms further.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
De Cooman T et al (2018) Adaptive heart rate-based epileptic seizure detection using real-time user feedback. Physiol Meas 39:014005
Sj E et al (2014) Can temporal lobe epilepsy surgery ameliorate accelerated long-term forgetting? Neuropsychologia 53:64–74
Ren Y et al (2020) EEG source estimation in a rare patient with cold-induced reflex epilepsy. Epileptic Disord 22:489–493
Allone C et al (2017) Neuroimaging and cognitive functions in temporal lobe epilepsy: a review of the literature. J Neurol Sci 381:7–15
Johnson EL (2019) Seizures and epilepsy. Med Clin North Am 103:309–324
Shu Y et al (2016) The Ephrin-A5/EphA4 interaction modulates neurogenesis and angiogenesis by the p-AKT and p-ERK pathways in a mouse model of TLE. Mol Neurobiol 53:561–576
Callenbach PM, Brouwer OF (1997) Hereditary epilepsy syndromes. Clin Neurol Neurosurg 99:159–171
Gano LB et al (2018) Altered mitochondrial acetylation profiles in a kainic acid model of temporal lobe epilepsy. Free Radic Biol Med 123:116–124
Phillips KF, Deshpande LS, DeLorenzo RJ (2018) Hypothermia reduces mortality, prevents the calcium plateau, and is neuroprotective following status epilepticus in rats. Front Neurol 9:438
Limanaqi F et al (2020) mTOR-related cell-clearing systems in epileptic seizures, an update. Int J Mol Sci 21:1642
Schneider M et al (2017) mTOR inhibitor reverses autistic-like social deficit behaviours in adult rats with both Tsc2 haploinsufficiency and developmental status epilepticus. Eur Arch Psychiatry Clin Neurosci 267:455–463
Pavlov I, Walker MC (2013) Tonic GABA(A) receptor-mediated signalling in temporal lobe epilepsy. Neuropharmacology 69:55–61
Ulamek-Koziol M et al (2019) Ketogenic diet and epilepsy. Nutrients 11:2510
Shen CC, Jiang JF (2019) Auricular electroacupuncture for Late posttraumatic epilepsy after severe brain injury: a retrospective study. Evid Based Complement Altern Med 2019:5798912
Sun ZG et al (2019) Effect of acupuncture at ST36 on motor cortical excitation and inhibition. Brain Behav 9:e01370
Tian H et al (2012) Acupuncture activates signal transduction pathways related to brain-tissue restoration after ischemic injury. Neural Regen Res 7:1866–1872
Bejarano E, Rodriguez-Navarro JA (2015) Autophagy and amino acid metabolism in the brain: implications for epilepsy. Amino Acids 47:2113–2126
Xu Z et al (2020) Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl Microbiol Biotechnol 104:575–587
Wu XL et al (2015) Reduced Pumilio-2 expression in patients with temporal lobe epilepsy and in the lithium–pilocarpine induced epilepsy rat model. Epilepsy Behav 50:31–39
Racine RJ (1972) Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Linnoila J et al (2019) Mouse model of anti-NMDA receptor post-herpes simplex encephalitis. Neurol Neuroimmunol Neuroinflamm 6:e529
Biran I et al (2020) Interaction of temporal lobe epilepsy and posttraumatic stress disorder: network analysis of a single case. Front Psychol 11:1010
Mueller SG et al (2012) Different structural correlates for verbal memory impairment in temporal lobe epilepsy with and without mesial temporal lobe sclerosis. Hum Brain Mapp 33:489–499
Dos Santos JG Jr. et al (2005) Electroacupuncture prevents cognitive deficits in pilocarpine-epileptic rats. Neurosci Lett 384:234–238
Cha MH et al (2010) Acute electroacupuncture inhibits nitric oxide synthase expression in the spinal cord of neuropathic rats. Neurol Res 32(Suppl 1):96–100
Dong SA et al (2020) The Role of melatonin in electroacupuncture alleviating lung injury induced by limb ischemia-reperfusion in rabbits. Med Sci Monit 26:e922525
Yan T, Zhang Z, Li D (2020) NGF receptors and PI3K/AKT pathway involved in glucose fluctuation-induced damage to neurons and alpha-lipoic acid treatment. BMC Neurosci 21:38
Dai H et al (2019) Dynorphin activation of kappa opioid receptor protects against epilepsy and seizure-induced brain injury via PI3K/AKT/Nrf2/HO-1 pathway. Cell Cycle 18:226–237
Wang MM et al (2020) Electroacupuncture inhibits neuronal autophagy and apoptosis via the PI3K/AKT pathway following ischemic stroke. Front Cell Neurosci 14:134
Tang L et al (2020) Electroacupuncture upregulated ghrelin in rats with functional dyspepsia via AMPK/TSC2/Rheb-mediated mTOR inhibition. Dig Dis Sci 65:1689–1699
Curatolo P et al (2018) mTOR dysregulation and tuberous sclerosis-related epilepsy. Expert Rev Neurother 18:185–201
Fattahi S et al (2020) PI3K/AKT/mTOR signaling in gastric cancer: epigenetics and beyond. Life Sci 262:118513
Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3:542–545
Jiang T et al (2015) p62 links autophagy and Nrf2 signaling. Free Radic Biol Med 88:199–204
Acknowledgements
This work was supported by grants from the Medical and health science and technology project of Suzhou High-tech Zone (key project) (Grant No. 2018Z002); 2019 Suzhou Livelihood Science and Technology Guidance Project (Grant No. SYSD2019078), Scientific Innovation Fund Project of Suzhou High-tech Zone People’s Hospital (Grant No. SGY2018B01).
Funding
The Medical and health science and technology project of Suzhou High-tech Zone (key project) (2018Z002); 2019 Suzhou Livelihood Science and Technology Guidance Project (SYSD2019078), Scientific Innovation Fund Project of Suzhou High-tech Zone People’s Hospital (SGY2018B01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors confirm that there are no conflict of interest.
Ethical Approval
This study was approved by the Ethics Committee of People’s Hospital of Suzhou New District. All animal experiments were carried out in accordance with the Chinese governing law on the use of medical laboratory animal.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, D., Ma, L., Xie, Y. et al. Electroacupuncture Promotes Autophagy by Regulating the AKT/mTOR Signaling Pathway in Temporal Lobe Epilepsy. Neurochem Res 47, 2396–2404 (2022). https://doi.org/10.1007/s11064-022-03634-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03634-9