Abstract
As the elderly population rapidly increases worldwide, the onset of cognitive dysfunction is expected to increase. Although neuronal plasticity, neurogenesis, and mitochondrial dysfunction have been reported to be involved in cognitive function, the detailed mechanism of cognitive impairment accompanied by aging is poorly understood as there are many confounding factors associated with aging. Therefore, effective treatments for aging have not yet been developed, and the establishment of therapeutic strategies has not progressed accordingly. We have previously found a decline of cognitive function in the developmental stage in mice who lack the expression of Shati/Nat8l, an N-acetyl transferase However, the contribution of Shati/Nat8l to cognitive impairment in aged mice has not yet been investigated. In this study, we aimed to investigate the role of Shati/Nat8l in cognitive function during aging. We observed a reduction in Shati/Nat8l mRNA expression in the dorsal hippocampus of mice as a result of their aging. Moreover, the cognitive dysfunction observed in aged mice was reversed by Shati/Nat8l overexpression in the dorsal hippocampus. Shati/Nat8l overexpression in the dorsal hippocampus of mice did not alter the expression of neurotrophic factors or mitochondrial function-related genes, including Bdnf or Pgc-1α, which are suggested to be downstream genes of Shati/Nat8l. Decreased N-acetyl aspartate (NAA) in aged mice was upregulated by Shati/Nat8l overexpression, suggesting that the Shati/Nat8l-NAA pathway determines cognitive function with aging. Taken together, Shati/Nat8l and NAA in the dorsal hippocampus may be novel targets for the treatment of cognitive impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, improvements in healthcare have increased life expectancy, thereby rapidly increasing the aging population worldwide [1]. Aging is characterized by the impairment of neuronal and motor functions in humans [2] and is a major risk factor for the development of neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease [3, 4]. Although deficits in motor performance are among the more severe symptoms induced by aging, a decline in cognitive function is more common, thereby influencing life activities in several domains (memory, learning, comprehension, and judgment) in elderly humans [5]. In 2015, approximately 47 million people developed dementia, which is defined as aging-induced impairment of cognitive function, and this number is estimated to exceed 131 million by 2050 [6]. Owing to the many risk factors associated with aging, the mechanisms underlying aging-induced cognitive dysfunction are poorly understood; thus, effective treatments have not been established till date. Therefore, the discovery of novel anti-aging targets and strategies for medical treatment is desired accordingly. Understanding the underlying regulatory mechanisms of the aging process in the central nervous system could offer insights into the neuropathogenesis of aging-induced dysfunctions, including cognitive decline, resulting in more effective and promising approaches for treatment in humans.
As mentioned above, cognitive impairment is one of the most common dysfunctions accompanied by aging [7, 8]. The hippocampus is widely involved in brain networks supporting cognitive functions, including encoding, consolidation, and retrieval of memory, and it determines episodic memory, pattern discrimination, novelty detection, spatial navigation, and binding between spatially and temporally distributed representations [9, 10]. A decreased hippocampal volume has been observed in aged humans without any disease [11, 12]. Golomb et al. also reported a correlation between reduction in hippocampal volume and cognitive impairment in aged humans [13]. Several theories have been proposed in cognitive impairment related to changes in the hippocampus, suggesting decline in neuronal plasticity and neurogenesis, neuronal death, and mitochondrial dysfunction as plausible explanations [14,15,16].
We previously identified Shati/Nat8l, an N-acetyl transferase, in the brains of mice exposed to repeated methamphetamine administration [17]. Shati/Nat8l is involved in the reward system [18], depression [19], and the stress response [20]. In addition, Shati/Nat8l in the hippocampus is involved in cognitive, learning, and memory functions [21], and Shati/Nat8l knockout mice show cognitive impairment [22]. The expression of Shati/Nat8l, which we focused on in this study, increased in the whole brain following the maturation of mice from day 15 to 56 after birth [22]. All these studies have reported the contribution of Shati/Nat8l in the developmental stages of mice. However, the function of Shati/Nat8l in aged mice has not yet been investigated. Considering that hippocampal Shati/Nat8l regulates cognitive function in the juvenile to maturation period, it could also play a role in cognitive impairment associated with aging.
To investigate the mechanisms underlying aging-induced cognitive-behavioral alterations involving Shati/Nat8l expression in the dorsal hippocampus, we injected Shati/Nat8l cDNA-encoding adeno-associated virus (AAV) vectors into mice to induce Shati/Nat8l overexpression. Our results revealed that Shati/Nat8l overexpression in the dorsal hippocampus suppressed the decline in cognitive function following aging, which suggests that N-acetyl aspartate (NAA) regulated by Shati/Nat8l in the dorsal hippocampus is involved in pathogenesis. To the best of our knowledge, this study is the first to report a relationship between Shati/Nat8l and aging.
Methods
Animals and Environments
Male C57Bl/6 J mice at 8 weeks of age (Nihon SLC, Hamamatsu, Japan) and at 78 weeks of age (Nihon Charles River Laboratories, Kanagawa, Japan) were used in this study. All mice were housed in a regulated environment (temperature: 25 ± 1 °C, humidity: 50 ± 5%) with a 12-h light/dark cycle (lights were turned on at 7:00 am) and ad libitum access to food and water.
Production and Microinjection of AAV Vectors
The production and microinjection of AAV vectors were performed as reported previously [23, 24]. Briefly, an expression cassette that included the CMV promoter and cDNA-encoding or non-encoding Shati/Nat8l sequence was contained in the AAV-Shati/Nat8l and AAV-Mock vectors, respectively. After anesthetizing the mice with a combination of anesthetics (medetomidine [0.3 mg/kg], midazolam [4.0 mg/kg], and butorphanol [5.0 mg/kg]), the AAV-Shati/Nat8l or Mock vectors (1 × 1010–1012 units) were injected into their bilateral dorsal hippocampi (AP − 1.6 mm; ML ± 1.0 mm; DV 1.5 mm) using the mouse brain atlas [25]. Mice were used for the experiments 4 weeks after microinjection. This study was performed with permission from the Board of Safety Committee for Recombination DNA Experiments of the University of Toyama (G2020PHA-5).
Real-time RT-PCR Analysis
RT-PCR assays were performed as described previously [26]. Briefly, tissue sections were collected using mouse brain matrix (Brain Science Idea, Osaka, Japan). Total RNA was extracted and converted to cDNA using the Prime Script RT reagent kit (Takara, Otsu, Japan). The mRNA levels were quantified using the Thermal Cycler Dice Real-Time System (Takara) with Thunderbird SYBR qPCR Mix (Toyobo, Osaka, Japan). 36B4 was used as an internal control. The primer sequences used were designed using Primer BLAST as follows:
Shati/Nat8l (NM_001001985.3):
forward, 5′- GTGATTCTGGCCTACCTGGA-3′;
reverse, 5′-CCACTGTGTTGTCCTCCTCA-3′;
Bdnf2 (NM_001048139.1):
forward, 5′-CCATCCACACGTGACAAAAC-3′;
reverse, 5′-GGTGCTGAATGGACTCTGCT-3′;
Bdnf6 (NM_001048142.1):
forward, 5′-GACCAGAAGCGTGACAACAA-3′;
reverse, 5′-AGGGTCCACACAAAGCTCTC-3′;
Ngf (NM_013609.3):
forward, 5′-TGTGCCTCAAGCCAGTGAAA -3′;
reverse, 5′-CACTGAGGTGAGCTTGGGTC-3′;
Nt-3 (NM_001164034.1):
forward, 5′-GGCGAGACTGAATGACCGAA-3′;
reverse, 5′-TGGACATCACCTTGTTCACCT-3′;
Ppargc1a (NM_008904.2):
forward, 5′-CCCCAAGGGTTCCCCATTTGA-3′;
reverse, 5′-TGAAAGGGTTATCTTGGTTGGCT-3′;
Tfam (NM_009360.4):
forward, 5′-TGTTTTTCCAGCATGGGTAGC-3′;
reverse, 5′-CCACAGGGCTGCAATTTTCC-3′;
36B4 (NM_0087475.5):
forward, 5′- ACCCTGAAGTGCTCGACATC-3′;
reverse, 5′- AGGAAGGCCTTGACCTTTTC-3′.
Behavioral Tests
Locomotor Activity Test
Locomotor activity tests were performed as described previously [27]. Mice were placed in a Plexiglas box (40 × 40 × 30 cm), and their locomotion was measured using the SCANET MV-40AQ (MELQUEST, Toyama, Japan) for 60 min.
Y-Maze Test
The Y-maze test was performed as described in previous study [28]. The alteration ratio was defined as follows: (number of alternations) / (total number of arm entries—2) × 100.
Novel Object Recognition Test
The novel object recognition test was performed as described previously [28]. Three days after habituation, the mice were allowed to explore two objects (A and B) in a Plexiglas box (30 × 30 × 35 cm) for 10 min as a familiar process (pre-test). Twenty-four hours after the pre-test, familiar objects A and novel object C were set in the box, and the mice were allowed to explore the two objects (A and C) in a Plexiglas box for 10 min as a novel process (post-test). The exploratory preference percentage was defined as follows: (approach time to object B or C)/(approach time to object B or C + approach time to object B or C) × 100.
Measurement of NAA and N-acetyl-aspartyl-glutamate (NAAG)
The measurement of NAA and NAAG by HPLC was performed as described previously [29]. A homogenized sample solution containing perchloric acid for HPLC was applied to Bond Elut SAX anion exchange columns (Agilent Technologies, Santa Clara, CA, USA), followed by extraction with phosphoric acid (85%). The samples were injected into an HPLC system (LC-2010CHT, Shimadzu, Japan) and analyzed using LC solution software (Shimadzu Corporation, Kyoto, Japan).
Statistical Analysis
All data are presented as mean ± standard error of the mean. Data were analyzed using Prism version 5. The Student’s t-test was performed to analyze the data between the two groups. One-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test was performed to analyze the data between multiple groups. Two-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test was performed to analyze the data between the two factors.
Results
Alteration of Shati/Nat8l Expression in the Brain with Aging
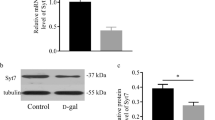
Shati/Nat8l mRNA levels were assessed in various regions of the mouse brain at 12, 26, and 78 weeks of age. Shati/Nat8l in the medial prefrontal cortex (mPFC) [30] and hippocampus [21] regulates memory, and the control of social interaction behaviors by Shati/Nat8l in the dorsal striatum has been reported previously [31]. Therefore, the role of Shati/Nat8l in these regions related to cognitive function was focused on and investigated. Shati/Nat8l mRNA levels in the dorsal hippocampus were lower in 78-week-old mice than in 12-week-old mice (Fig. 1a) (F2,12 = 5.019, p = 0.026; one-way ANOVA). However, Shati/Nat8l mRNA levels in the ventral hippocampus, mPFC, and dorsal striatum did not significantly change with age (Fig. 1b–d). These results suggest that Shati/Nat8l in the dorsal hippocampus is involved in the age-related decline in cognitive function.
Shati/Nat8l in the dorsal hippocampus, but not in other regions, decreased with aging; (a–d) Shati/Nat8l mRNA levels were measured in the dorsal hippocampus (dHIP) (a), ventral hippocampus (vHIP) (b), mPFC (c), and dorsal striatum (dSTR) (d). A significant reduction of Shati/Nat8l mRNA levels in the dorsal hippocampus was observed in 78-week-old mice (F2,12 = 5.019, p = 0.026). Dorsal hippocampus: 12 weeks, n = 5; 26 weeks, n = 6; 78 weeks, n = 4; ventral hippocampus: 12 weeks, n = 6; 26 weeks, n = 5; 78 weeks, n = 6; mPFC: 12 weeks, n = 6; 26 weeks, n = 5; 78 weeks, n = 6; dorsal striatum: 12 weeks, n = 5; 26 weeks, n = 6; 78 weeks, n = 6; *p < 0.05 vs 12-week-old mice (one-way ANOVA with Bonferroni post hoc tests)
Shati/Nat8l Overexpression in the Dorsal Hippocampus Reversed the Cognitive Impairment in Aged Mice
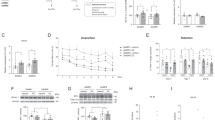
We generated Shati/Nat8l overexpression in the dorsal hippocampus of mice (dHIP-Shati mice) by microinjection of AAV-Shati/Nat8l [18, 26, 27, 29] in young (8-week-old) and old (78-week-old) mice. We also microinjected AAV-Mock into the dorsal hippocampus as a control (dHIP-Mock mice). A significant increase in Shati/Nat8l mRNA levels in the dorsal hippocampus was confirmed in dHIP-Shati mice compared to that in mock mice (Fig. 2a) (t10 = 3.754, p = 0.004; Student’s t-test). A series of behavioral tests to assess cognitive function, including the Y-maze and novel object recognition tests, was performed using these mice according to their schedules (Fig. 2b). First, we measured the locomotor activity of these mice as the effect of microinjection on motor activity in the behavioral test had to be considered. The locomotor activity test confirmed that microinjection did not influence motor function between young dHIP-Mock and -Shati mice or old dHIP-Mock and -Shati mice (Fig. 2c) (main effect of virus: F1,20 = 2.549, p = 0.1260; main effect of aging: F1,20 = 22.77, p = 0.0001; interaction effect: F1,20 = 0.774, p = 0.3894; two-way ANOVA). Although Shati/Nat8l in the dorsal hippocampus did not affect working memory in the Y-maze test (Fig. 2d), long-term memory in the novel object recognition test yielded different results. As shown in Fig. 2e, while there was no difference in exploratory preference among the mice in the pre-test, the impaired cognitive function exhibited in old dHIP-Mock mice was not observed in old dHIP-Shati mice in the post-test (main effect of virus: F1,20 = 0.458, p = 0.507; main effect of aging: F1,20 = 15.86, p = 0.0007; interaction effect: F1,20 = 23.66, p < 0.0001; two-way ANOVA). Therefore, it can be concluded that Shati/Nat8l in the dorsal hippocampus suppresses aging-induced cognitive impairment.
Overexpression of Shati/Nat8l in the dorsal hippocampus suppressed the decline in cognitive function in aged mice; a Shati/Nat8l mRNA levels in the dorsal hippocampus increased in the dHIP-Shati mice compared with those in the dHIP-Mock mice (t10 = 3.754, p = 0.004). dHIP-Mock, n = 6; dHIP-Shati, n = 6; ***p < 0.005 vs dHIP-Mock mice (Student’s t-test) b The timeline of experiments. Four weeks after microinjection, the behavioral tests were performed. c There were no differences in basic activity in locomotor activity test. Young dHIP-Mock, n = 6; Young dHIP-Shati, n = 6; Old dHIP-Mock, n = 5; Old dHIP-Shati, n = 7 (two-way ANOVA with Bonferroni post hoc tests). d There were no differences in working memory in the Y-maze test. Young dHIP-Mock, n = 6; Young dHIP-Shati, n = 6; Old dHIP-Mock, n = 5; Old dHIP-Shati, n = 7 (two-way ANOVA with Bonferroni post hoc tests). e Old dHIP-Mock mice showed the cognitive impairment compared with Young dHIP-Mock mice. Old dHIP-Shati mice did not show the cognitive impairment (main effect of virus: F1,20 = 0.458, p = 0.507; main effect of aging: F1,20 = 15.86, p = 0.0007; interaction effect: F1,20 = 23.66, p < 0.0001). Young dHIP-Mock, n = 6; Young dHIP-Shati, n = 6; Old dHIP-Mock, n = 5; Old dHIP-Shati, n = 7; ***p < 0.005 vs Old dHIP-Mock; ###p < 0.005 vs Old dHIP-Shati (two-way ANOVA with Bonferroni post hoc tests)
Shati/Nat8l in the Dorsal Hippocampus Did Not Affect the Expression of Neurotrophic Factors and Mitochondrial Function
Expression of various neurotrophic factors changes in the aged brain, contributing to cognitive dysfunction mediated by synaptic plasticity and neurogenesis [32, 33]. Brain-derived neurotrophic factor (BDNF) is involved in cognitive function [34, 35], and decreased BDNF expression is observed in aged individuals with cognitive decline [36]. Furthermore, BDNF upregulation in the brain prevents the onset of cognitive impairment [37]. Shati/Nat8l conditional knockout mice showed decreased Bdnf mRNA and protein levels [31], suggesting that overexpression of Shati/Nat8l upregulates BDNF expression. Bdnf has various promoters, and it is specifically regulated by different stimuli, followed by the production of multiple variants [38]. Bdnf II and VI, but not the others, are the most well-characterized Bdnf transcripts in aging, and their levels are decreased in aged brains [39]. We confirmed a decrease in Bdnf II and VI mRNA levels in the dorsal hippocampus of old dHIP-Mock mice compared with those in young dHIP-Mock mice, whereas the mRNA levels in the dorsal hippocampus were not changed by Shati/Nat8l overexpression in aged mice (Fig. 3a, b) (Bdnf II: F2,14 = 3.226, p = 0.0704, Bdnf VI: F2,15 = 5.824, p = 0.0134; one-way ANOVA). Other neurotrophic factors, Ngf and Nt-3, also suggest the involvement of cognitive function in promoting the neurogenesis-mediated cholinergic system or interaction with BDNF, respectively [40, 41]. While Ngf and Nt-3 mRNA levels significantly decreased with age, there were no differences in Ngf and Nt-3 mRNA levels in the dorsal hippocampus of old dHIP-Shati mice compared to those of dHIP-Mock mice (Fig. 3c, d) (Ngf: F2,15 = 12.17, p = 0.0007, Nt-3: F2,15 = 6.396, p = 0.0098; one-way ANOVA).
No effect of Shati/Nat8l overexpression in the dorsal hippocampus to neurotrophic factors; (a-d) Bdnf II a and VI b, Ngf c, and Nt-3 d mRNA levels in the dorsal hippocampus of old dHIP-Shati mice were measured. While these neurotrophic factors are decreased with aging, overexpression of Shati/Nat8l in the dorsal hippocampus were not induced alteration of these expression (Bdnf II: F2,14 = 3.226, p = 0.0704; Bdnf VI: F2,15 = 5.824, p = 0.0134; Ngf: F2,15 = 12.17, p = 0.0007; Nt-3: F2,15 = 6.396, p = 0.0098). Bdnf II: Young dHIP-Mock, n = 6; Old dHIP-Mock, n = 5; Old dHIP-Shati, n = 6; Bdnf VI: Young dHIP-Mock, n = 6; Old dHIP-Mock, n = 5; Old dHIP-Shati, n = 7; Ngf: Young dHIP-Mock, n = 6; Old dHIP-Mock, n = 5; Old dHIP-Shati, n = 7; Nt-3: Young dHIP-Mock, n = 6; Old dHIP-Mock, n = 5; Old dHIP-Shati, n = 7; **p < 0.01, *p < 0.05 vs Old dHIP-Mock (one-way ANOVA with Bonferroni post hoc tests)
Aging is also characterized by mitochondrial dysfunction [42, 43]. Reduction in mitochondria-related gene expression with aging contributes to decreased synaptic plasticity, resulting in cognitive impairment [44]. The upregulation of peroxisome proliferator-activated receptor-gamma coactivator-alpha (PGC-1α) by Shati/Na8l overexpression in brown adipocytes has been reported previously [45]. PGC-1α mediates the upregulation of mitochondrial biosynthesis via the regulation of mitochondrial transcription factor A (TFAM) expression [46]. However, decreased Ppargc1a and Tfam mRNA levels in the dorsal hippocampus with aging did not recover in dHIP-Shati mice (Fig. 4a, b) (Ppargc1a: F2,15 = 7.073, p = 0.0069, Tfam: F2,13 = 4.572, p = 0.0314; one-way ANOVA).
No effect of Shati/Nat8l overexpression in the dorsal hippocampus on mitochondria function; (a, b) Ppargc1a (a) and Tfam (b) mRNA levels in the dorsal hippocampus of old dHIP-Shati mice were measured. Decreased expression of Ppargc1a and Tfam mRNA with aging were not elevated by over expression of Shati/Nat8l in the dorsal hippocampus (Ppargc1a: F2,15 = 7.073, p = 0.0069; Tfam: F2,13 = 4.572, p = 0.0314). Pgc-1α: Young dHIP-Mock, n = 6; Old dHIP-Mock, n = 5; Old dHIP-Shati, n = 7, Tfam: Young dHIP-Mock, n = 6; Old dHIP-Mock, n = 4; Old dHIP-Shati, n = 7; *p < 0.05 vs Old dHIP-Mock (one-way ANOVA with Bonferroni post hoc tests)
Shati/Nat8l Overexpression in the Dorsal Hippocampus Increased NAA
Shati/Nat8l is an N-acetyl transferase responsible for the synthesis of NAA from acetyl-coenzyme A and aspartate [47]. NAA is then converted to NAAG, which functions as a highly selective agonist of the metabotropic glutamate type 3 receptor [48]. Considering that NAA levels decrease in the brains of patients with cognitive impairment [49], the NAA and NAAG levels in the dorsal hippocampus were measured. Decreasing NAA content in the dorsal hippocampus of old dHIP-Mock mice compared with that of young dHIP-Mock mice was recovered by Shati/Nat8l overexpression in the dorsal hippocampus of aged mice (Fig. 5a) (F2,15 = 13.72, p = 0.0004; one-way ANOVA). In contrast, aging or Shati/Nat8l overexpression did not alter the NAAG content in the dorsal hippocampus (Fig. 5b), suggesting that NAA, but not NAAG, in the dorsal hippocampus suppressed cognitive impairment with aging.
Overexpression of Shati/Nat8l increased NAA contents in the dorsal hippocampus; a NAA contents in the dorsal hippocampus decreased with aging. Reduction of NAA contents with aging increased in old dHIP-Shati mice (F2,15 = 13.72, p = 0.0004; one-way ANOVA). Young dHIP-Mock, n = 6; Old dHIP-Mock, n = 5; Old dHIP-Shati, n = 7; **p < 0.001 vs Old dHIP-Mock; ###p < 0.005 vs Old dHIP-Shati (one-way ANOVA with Bonferroni post hoc tests). b There were no differences in NAAG contents in the dorsal hippocampus. Young dHIP-Mock, n = 6; Old dHIP-Mock, n = 5; Old dHIP-Shati, n = 7 (one-way ANOVA with Bonferroni post hoc tests)
Discussion
In this study, we found evidence that aging is accompanied by a decrease in Shati/Nat8l levels in the dorsal hippocampus. Mice overexpressing dHIP-Shati/Nat8l were generated to investigate the role of Shati/Nat8l in cognitive function during aging. Shati/Nat8l overexpression in the dorsal hippocampus prevents cognitive impairment in old mice. To reveal the underlying mechanisms of the regulation of cognitive ability by Shati/Nat8l in the dorsal hippocampus, we also investigated the cognitive function-related genes downstream of Shati/Nat8l, including BDNF, PGC-1α, and NAA. Our results demonstrate that decline in NAA levels with aging is upregulated by Shati/Nat8l overexpression in the dorsal hippocampus, suggesting its involvement in aging-induced cognitive function.
The hippocampus strongly contributes to cognitive function including memory formation [50, 51]. Some studies have shown that mice with lesions of the dorsal hippocampus induced by microinjection of colchicine have impaired the long-term object recognition [52]. Another study using the DREADD system reported that chemogenetic inactivation of excitatory neurons in the dorsal hippocampus disrupts object recognition memory [53]. Structural alterations in the CNS, especially in the dorsal hippocampus, are observed in aged individuals with cognitive impairment, even in the absence of neurodegenerative diseases [54, 55]. There are many reports that the volume of the hippocampus, including the dorsal hippocampus, decreases with age, even in the absence of any illnesses [12, 56]. A separate analysis of the dorsal and ventral hippocampus using manganese-enhanced magnetic resonance imaging showed a significant correlation between the volume of the dorsal hippocampus and cognitive dysfunction in aging; however, no such differences were observed in the ventral hippocampus [57]. This finding is consistent with our results, which showed that Shati/Nat8l mRNA levels were altered in the dorsal hippocampus, but not in the ventral hippocampus, with aging (Fig. 1a, b). We had previously reported that phosphorylation of cAMP response element-binding protein (CREB) induced Shati/Nat8l expression, suggesting that altered Shati/Nat8l levels are dependent on CREB activity [58]. The total CREB protein and phosphorylated CREB levels in the dorsal hippocampus decreased in aged mice [59, 60], and the cognitive impairment observed in aged mice was ameliorated by CREB overexpression in the dorsal hippocampus [61]. Considering the contribution of CREB in the dorsal hippocampus to cognitive decline in aged mice, CREB activity might explain the brain-specific downregulation of Shati/Nat8l levels following aging. We also reported that Shati/Nat8l in the mPFC is involved in cognitive function [30]. However, no significant changes in Shati/Nat8l mRNA levels were observed in the mPFC (Fig. 1c). Notably, our results are in agreement with those of previous reports. In the present study, we investigated the role of Shati/Nat8l in age-related cognitive impairments. Both Shati/Nat8l in the dorsal hippocampus and mPFC regulate cognitive function via different mechanisms, and the Shati/Nat8l pathway in the dorsal hippocampus is thought to mediate aging-induced cognitive impairment.
As mentioned above, cognitive impairment associated with aging is strongly related to neurogenesis and mitochondrial dysfunction [14,15,16]. In particular, the involvement of BDNF in the brain has been reported [34,35,36,37]. BDNF in the dorsal hippocampus plays an important role in neuronal plasticity and in the regulation of cognitive memory [62]. Shati/Nat8l in the dorsal striatum regulates BDNF expression via epigenetic regulation of histone acetylation [31]. As shown in Fig. 3a and b, the expression of Bdnf II and VI mRNA, which are transcripts characterized by aging [39], in the dorsal hippocampus are not changed in dHIP-Shati mice. In the mPFC, BDNF is reportedly not dominantly regulated by Shati/Nat8l [31]. BDNF expression in the dorsal hippocampus is also controlled by other relational mechanisms, including histone and DNA methylation [63, 64], suggesting that Shati/Nat8l in the dorsal hippocampus hardly contributes to BDNF expression in the dorsal hippocampus as with in mPFC. Mitochondrial dysfunction has also been observed during aging [65]. Mitochondrial function-related genes, including PGC-1α and TFAM, are downregulated in the aging brains of mice [44]. TFAM expression is regulated by PGC-1α and it contributes to mitochondria biogenesis [46]. As Shati/Nat8l overexpression induces the upregulation of PGC-1α in brown adipocytes [45], these two genes were investigated. However, Ppargc1a and Tfam mRNA levels were not altered by the overexpression of Shati/Nat8l in the dorsal hippocampus (Fig. 4a, b), and our results are not consistent with those of previous studies. Shati/Nat8l has been reported to exist mainly in neuronal cells [66]. Differences in cell types may explain this contradiction.
NAA is synthesized by Shati/Nat8l, which converts it into NAAG [47, 48]. We demonstrated that the NAA content increased in dHIP-Shati mice (Fig. 5b), whereas the NAAG contents were not changed by Shati/Nat8l overexpression (Figs. 5b). NAAG is metabolized to NAA and glutamate by glutamate carboxypeptidase II (GCPII) [67]. Previous reports have shown that Shati/Nat8l overexpression increases GCPII levels [27]. Therefore, the activation of NAAG metabolism by Shati/Nat8l induced-GCPII overexpression may induce no alteration in the NAAG content, even though Shati/Nat8l is overexpressed in these mice. NAA levels have been reported to decrease in the aging brain [68], which is consistent with the decrease in Shati/Nat8l levels with age. Patients with aging-induced Alzheimer’s disease also showed decreased NAA levels [49]. These reports and our results suggest that NAA regulates cognitive functions. One possible mechanism for the restoration of cognitive impairment by NAA is the enhancement of myelination. Dysfunction of myelination, which is a consequence of aging [69], contributes to cognitive impairment [70]. Indeed, several studies using diffusion tensor imaging indicate a linear decline in myelination from young adulthood to older age [71, 72]. Aged rodents also show decreased myelin basic protein in the hippocampus compared to young individuals, resulting in myelin degeneration [73]. NAA is transported to oligoadenylate and, then converted to aspartate and acetate by aspartoacylase, where acetate is converted to acetyl-coenzyme A, which is then utilized for myelination [74]. We previously reported that juvenile genetic Shati/Nat8l-knockout mice showed dysfunction of myelination, which was normalized by GTA treatment for supplementation with acetate [22]. These results suggest that the Shati/Nat8l-NAA pathway may control myelination in aged mice. Another possible explanation is that Shati/Nat8l affects autophagy. Genome-wide analysis demonstrated a transcriptional decline in autophagy following aging in the human brain [75], suggesting that age-related decreases in autophagic activity result in age-dependent impairment [76]. Furthermore, the downregulation of autophagy in the hippocampus has been observed in aged mice [77], and inducing autophagy reverses age-related memory impairment by controlling neuronal plasticity [78]. Transcription factor EB is known to activate autophagy [79], and its expression is elevated by the activation of the NAA pathways [80]. Increased Shati/Nat8l levels in the dorsal hippocampus, followed by activation of the NAA pathway might be an underlying mechanism of cognitive function. These possibilities should be considered when elucidating the pathogenesis of age-related cognitive dysfunctions.
In conclusion, our results demonstrate that Shati/Nat8l in the dorsal hippocampus determines aging-dependent cognitive function. To the best of our knowledge, this is the first report on the role of Shati/Nat8l in aged mice. Although the detailed mechanisms underlying this regulatory ability of Shati/Nat8l must be clarified in future studies, the present results further indicate that Shati/Nat8l and NAA in the dorsal hippocampus should be considered as potential novel targets for therapy of cognitive dysfunction with aging.
Data Availability
The datasets generated and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
References
Ni Y, Yang X, Zheng L, Wang Z, Wu L, Jiang J, Yang T, Ma L, Fu Z (2019) Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol Nutr Food Res 63:e1900603. https://doi.org/10.1002/mnfr.201900603
Riaz M, Vangberg TR, Vasylenko O, Castro-Chavira S, Gorecka MM, Waterloo K, Rodríguez-Aranda C (2021) What does hand motor function tell us about our aging brain in association with WMH? Aging Clin Exp Res 33:1577–1584. https://doi.org/10.1007/s40520-020-01683-0
Anderson ND, Craik FI (2017) 50 years of cognitive aging theory. J Gerontol B Psychol Sci Soc Sci 72:1–6. https://doi.org/10.1093/geronb/gbw108
Sengoku R (2020) Aging and Alzheimer’s disease pathology. Neuropathology 40:22–29. https://doi.org/10.1111/neup.12626
Chauhan A, Chauhan V (2020) Beneficial effects of walnuts on cognition and brain health. Nutrients 12:550. https://doi.org/10.3390/nu12020550
Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N (2017) Dementia prevention, intervention, and care. Lancet 390:2673–2734. https://doi.org/10.1016/S0140-6736(17)31363-6
Russell JK, Jones CK, Newhouse PA (2019) The role of estrogen in brain and cognitive aging. Neurotherapeutics 16:649–665. https://doi.org/10.1007/s13311-019-00766-9
Zahodne LB, Zajacova A (2020) Education and cognitive aging: an introduction to the special section. J Gerontol B Psychol Sci Soc Sci 75:e78–e80. https://doi.org/10.1093/geronb/gbaa091
Bartsch T, Wulff P (2015) The hippocampus in aging and disease: from plasticity to vulnerability. Neuroscience 309:1–16. https://doi.org/10.1016/j.neuroscience.2015.07.084
Bettio LEB, Rajendran L, Gil-Mohapel J (2017) The effects of aging in the hippocampus and cognitive decline. Neurosci Biobehav Rev 79:66–86. https://doi.org/10.1016/j.neubiorev.2017.04.030
Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ (2018) Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22:589-599.e5. https://doi.org/10.1016/j.stem.2018.03.015
Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR (1991) Cerebral structure on MRI, part I: localization of age-related changes. Biol Psychiatry 29:55–67. https://doi.org/10.1016/0006-3223(91)90210-d
Golomb J, de Leon MJ, Kluger A, George AE, Tarshish C, Ferris SH (1993) Hippocampal atrophy in normal aging. An association with recent memory impairment. Arch Neurol 50:967–973. https://doi.org/10.1001/archneur.1993.00540090066012
Fang EF, Hou Y, Palikaras K, Adriaanse BA, Kerr JS, Yang B, Lautrup S, Hasan-Olive MM, Caponio D, Dan X, Rocktäschel P, Croteau DL, Akbari M, Greig NH, Fladby T, Nilsen H, Cader MZ, Mattson MP, Tavernarakis N, Bohr VA (2019) Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat Neurosci 22:401–412. https://doi.org/10.1038/s41593-018-0332-9
Foster TC (2006) Biological markers of age-related memory deficits: treatment of senescent physiology. CNS Drugs 20:153–166. https://doi.org/10.2165/00023210-200620020-00006
Poulose SM, Miller MG, Scott T, Shukitt-Hale B (2017) Nutritional factors affecting adult neurogenesis and cognitive function. Adv Nutr 8:804–811. https://doi.org/10.3945/an.117.016261
Niwa M, Nitta A, Mizoguchi H, Ito Y, Noda Y, Nagai T, Nabeshima T (2007) A novel molecule “shati” is involved in methamphetamine-induced hyperlocomotion, sensitization, and conditioned place preference. J Neurosci 27:7604–7615. https://doi.org/10.1523/JNEUROSCI.1575-07.2007
Haddar M, Uno K, Azuma K, Muramatsu SI, Nitta A (2020) Inhibitory effects of Shati/Nat8l overexpression in the medial prefrontal cortex on methamphetamine-induced conditioned place preference in mice. Addict Biol 25:e12749. https://doi.org/10.1111/adb.12749
Miyanishi H, Uno K, Iwata M, Kikuchi Y, Yamamori H, Yasuda Y, Ohi K, Hashimoto R, Hattori K, Yoshida S, Goto YI, Sumiyoshi T, Nitta A (2020) Investigating DNA methylation of SHATI/NAT8L promoter sites in blood of unmedicated patients with major depressive disorder. Biol Pharm Bull 43:1067–1072. https://doi.org/10.1248/bpb.b19-01099
Miyanishi H, Nitta A (2021) A role of BDNF in the depression pathogenesis and a potential target as antidepressant: the modulator of stress sensitivity “Shati/Nat8l-BDNF system” in the dorsal striatum. Pharmaceuticals (Basel) 14:889. https://doi.org/10.3390/ph14090889
Nitta A, Noike H, Sumi K, Miyanishi H, Tanaka T, Takaoka K, Nagakura M, Iegaki N, Kaji JI, Miyamoto Y, Muramatsu SI, Uno K (2018) Shati/Nat8l and N-acetylaspartate (NAA) have important roles in regulating nicotinic acetylcholine receptors in neuronal and psychiatric diseases in animal models and humans. In: Akaike A, Shimohama S, Misu Y (eds) Nicotinic acetylcholine receptor signaling in neuroprotection. Springer, Singapore, pp 89–111
Sumi K, Uno K, Noike H, Tomohiro T, Hatanaka Y, Furukawa-Hibi Y, Nabeshima T, Miyamoto Y, Nitta A (2017) Behavioral impairment in SHATI/NAT8L knockout mice via dysfunction of myelination development. Sci Rep 7:16872. https://doi.org/10.1038/s41598-017-17151-1
Iida A, Takino N, Miyauchi H, Shimazaki K, Muramatsu S (2013) Systemic delivery of tyrosine-mutant AAV vectors results in robust transduction of neurons in adult mice. BioMed Res Int 2013:974819. https://doi.org/10.1155/2013/974819
Krzyzosiak A, Szyszka-Niagolov M, Wietrzych M, Gobaille S, Muramatsu S, Krezel W (2010) Retinoid X receptor gamma control of affective behaviors involves dopaminergic signaling in mice. Neuron 66:908–920. https://doi.org/10.1016/j.neuron.2010.05.004
Paxinos G, Franklin KBJ (2008) The mouse brain in stereotaxic coordinates: Compact, 3rd edn. Elsevier, Amsterdam
Uno K, Miyanishi H, Sodeyama K, Fujiwara T, Miyazaki T, Muramatsu SI, Nitta A (2019) Vulnerability to depressive behavior induced by overexpression of striatal Shati/Nat8l via the serotonergic neuronal pathway in mice. Behav Brain Res 376:112227. https://doi.org/10.1016/j.bbr.2019.112227
Miyamoto Y, Iegaki N, Fu K, Ishikawa Y, Sumi K, Azuma S, Uno K, Muramatsu SI, Nitta A (2017) Striatal N-acetylaspartate synthetase Shati/Nat8l regulates depression-like behaviors via mGluR3-mediated serotonergic suppression in mice. Int J Neuropsychopharmacol 20:1027–1035. https://doi.org/10.1093/ijnp/pyx078
Fu K, Miyamoto Y, Sumi K, Saika E, Muramatsu SI, Uno K, Nitta A (2017) Overexpression of transmembrane protein 168 in the mouse nucleus accumbens induces anxiety and sensorimotor gating deficit. PLoS ONE 12:e0189006. https://doi.org/10.1371/journal.pone.0189006
Miyamoto Y, Ishikawa Y, Iegaki N, Sumi K, Fu K, Sato K, Furukawa-Hibi Y, Muramatsu S, Nabeshima T, Uno K, Nitta A (2014) Overexpression of Shati/Nat8l, an N-acetyltransferase, in the nucleus accumbens attenuates the response to methamphetamine via activation of group II mGluRs in mice. Int J Neuropsychopharmacol 17:1283–1294. https://doi.org/10.1017/S146114571400011X
Haddar M, Azuma K, Izuo N, Kyosuke U, Asano T, Muramatsu SI, Nitta A (2021) Impairment of cognitive function induced by Shati/Nat8l overexpression in the prefrontal cortex of mice. Behav Brain Res 397:112938. https://doi.org/10.1016/j.bbr.2020.112938
Miyanishi H, Muramatsu SI, Nitta A (2021) Striatal Shati/Nat8l-BDNF pathways determine the sensitivity to social defeat stress in mice through epigenetic regulation. Neuropsychopharmacology 46:1594–1605. https://doi.org/10.1038/s41386-021-01033-2
Bolognin S, Buffelli M, Puoliväli J, Iqbal K (2014) Rescue of cognitive-aging by administration of a neurogenic and/or neurotrophic compound. Neurobiol Aging 35:2134–2146. https://doi.org/10.1016/j.neurobiolaging.2014.02.017
Budni J, Bellettini-Santos T, Mina F, Garcez ML, Zugno AI (2015) The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis 6:331–341. https://doi.org/10.14336/AD.2015.0825
Molinari C, Morsanuto V, Ruga S, Notte F, Farghali M, Galla R, Uberti F (2020) The role of BDNF on aging-modulation markers. Brain Sci 10:285. https://doi.org/10.3390/brainsci10050285
Patterson SL (2015) Immune dysregulation and cognitive vulnerability in the aging brain: interactions of microglia, IL-1beta, BDNF and synaptic plasticity. Neuropharmacology 96:11–18. https://doi.org/10.1016/j.neuropharm.2014.12.020
Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S (2008) New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Rev 59:201–220. https://doi.org/10.1016/j.brainresrev.2008.07.007
Pietrelli A, Matković L, Vacotto M, Lopez-Costa JJ, Basso N, Brusco A (2018) Aerobic exercise upregulates the BDNF-serotonin systems and improves the cognitive function in rats. Neurobiol Learn Mem 155:528–542. https://doi.org/10.1016/j.nlm.2018.05.007
Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T (2007) Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res 85:525–535. https://doi.org/10.1002/jnr.21139
Palomer E, Martín-Segura A, Baliyan S, Ahmed T, Balschun D, Venero C, Martin MG, Dotti CG (2016) Aging triggers a repressive chromatin state at Bdnf promoters in hippocampal neurons. Cell Rep 16:2889–2900. https://doi.org/10.1016/j.celrep.2016.08.028
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677–736. https://doi.org/10.1146/annurev.neuro.24.1.677
Schliebs R, Arendt T (2011) The cholinergic system in aging and neuronal degeneration. Behav Brain Res 221:555–563. https://doi.org/10.1016/j.bbr.2010.11.058
Fernandez A, Meechan DW, Karpinski BA, Paronett EM, Bryan CA, Rutz HL, Radin EA, Lubin N, Bonner ER, Popratiloff A, Rothblat LA, Maynard TM, LaMantia AS (2019) Mitochondrial dysfunction leads to cortical under-connectivity and cognitive impairment. Neuron 102:1127–1142. https://doi.org/10.1016/j.neuron.2019.04.013
Netto MB, de Oliveira Junior AN, Goldim M, Mathias K, Fileti ME, da Rosa N, Laurentino AO, de Farias BX, Costa AB, Rezin GT, Fortunato JJ, Giustina AD, Barichello T, Dal-Pizzol F, Petronilho F (2018) Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain Behav Immun 73:661–669. https://doi.org/10.1016/j.bbi.2018.07.016
Reutzel M, Grewal R, Dilberger B, Silaidos C, Joppe A, Eckert GP (2020) Cerebral mitochondrial function and cognitive performance during aging: a longitudinal study in NMRI mice. Oxid Med Cell Longev 2020:4060769. https://doi.org/10.1155/2020/4060769
Pessentheiner AR, Pelzmann HJ, Walenta E, Schweiger M, Groschner LN, Graier WF, Kolb D, Uno K, Miyazaki T, Nitta A, Rieder D, Prokesch A, Bogner-Strauss JG (2013) NAT8L (N-acetyltransferase 8-like) accelerates lipid turnover and increases energy expenditure in brown adipocytes. J Biol Chem 288:36040–36051. https://doi.org/10.1074/jbc.M113.491324
Gleyzer N, Vercauteren K, Scarpulla RC (2005) Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol 25:1354–1366. https://doi.org/10.1128/MCB.25.4.1354-1366.2005
Ariyannur PS, Moffett JR, Manickam P, Pattabiraman N, Arun P, Nitta A, Nabeshima T, Madhavarao CN, Namboodiri AM (2010) Methamphetamine-induced neuronal protein NAT8L is the NAA biosynthetic enzyme: implications for specialized acetyl coenzyme A metabolism in the CNS. Brain Res 1335:1–13. https://doi.org/10.1016/j.brainres.2010.04.008
Neale JH, Olszewski RT, Zuo D, Janczura KJ, Profaci CP, Lavin KM, Madore JC, Bzdega T (2011) Advances in understanding the peptide neurotransmitter NAAG and appearance of a new member of the NAAG neuropeptide family. J Neurochem 118:490–498. https://doi.org/10.1111/j.1471-4159.2011.07338.x
Ackl N, Ising M, Schreiber YA, Atiya M, Sonntag A, Auer DP (2005) Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer’s disease. Neurosci Lett 384:23–28. https://doi.org/10.1016/j.neulet.2005.04.035
Scoville WB, Milner B (1957) Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20:11–21. https://doi.org/10.1136/jnnp.20.1.11
Smith TD, Calhoun ME, Rapp PR (1999) Circuit and morphological specificity of synaptic change in the aged hippocampal formation. Neurobiol Aging 20:357–358. https://doi.org/10.1016/s0197-4580(99)00073-1
Dees RL, Kesner RP (2013) The role of the dorsal dentate gyrus in object and object-context recognition. Neurobiol Learn Mem 106:112–117. https://doi.org/10.1016/j.nlm.2013.07.013
Tuscher JJ, Taxier LR, Fortress AM, Frick KM (2018) Chemogenetic inactivation of the dorsal hippocampus and medial prefrontal cortex, individually and concurrently, impairs object recognition and spatial memory consolidation in female mice. Neurobiol Learn Mem 156:103–116. https://doi.org/10.1016/j.nlm.2018.11.002
Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB, Initiative ADN (2014) What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog Neurobiol 117:20–40. https://doi.org/10.1016/j.pneurobio.2014.02.004
Fjell AM, Walhovd KB (2010) Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci 21:187–221. https://doi.org/10.1515/revneuro.2010.21.3.187
Malykhin NV, Bouchard TP, Camicioli R, Coupland NJ (2008) Aging hippocampus and amygdala. NeuroReport 19:543–547. https://doi.org/10.1097/WNR.0b013e3282f8b18c
Reichel JM, Bedenk BT, Czisch M, Wotjak CT (2017) Age-related cognitive decline coincides with accelerated volume loss of the dorsal but not ventral hippocampus in mice. Hippocampus 27:28–35. https://doi.org/10.1002/hipo.22668
Uno K, Miyazaki T, Sodeyama K, Miyamoto Y, Nitta A (2017) Methamphetamine induces Shati/Nat8L expression in the mouse nucleus accumbens via CREB- and dopamine D1 receptor-dependent mechanism. PLoS ONE 12:e0174196. https://doi.org/10.1371/journal.pone.0174196
Brightwell JJ, Gallagher M, Colombo PJ (2004) Hippocampal CREB1 but not CREB2 is decreased in aged rats with spatial memory impairments. Neurobiol Learn Mem 81:19–26. https://doi.org/10.1016/j.nlm.2003.08.001
Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A (2001) Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci 21:4066–4073. https://doi.org/10.1523/JNEUROSCI.21-11-04066.2001
Yu XW, Curlik DM, Oh MM, Yin JC, Disterhoft JF (2017) CREB overexpression in dorsal CA1 ameliorates long-term memory deficits in aged rats. eLife 6:e19358. https://doi.org/10.7554/eLife.19358
Heldt SA, Stanek L, Chhatwal JP, Ressler KJ (2007) Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry 12:656–670. https://doi.org/10.1038/sj.mp.4001957
Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, Paylor RE, Lubin FD (2010) Histone methylation regulates memory formation. J Neurosci 30:3589–3599. https://doi.org/10.1523/JNEUROSCI.3732-09.2010
Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA (2015) DNA methylation of BDNF as a biomarker of early-life adversity. Proc Natl Acad Sci USA 112:6807–6813. https://doi.org/10.1073/pnas.1408355111
Jang JY, Blum A, Liu J, Finkel T (2018) The role of mitochondria in aging. J Clin Invest 128:3662–3670. https://doi.org/10.1172/JCI120842
Sumi K, Uno K, Matsumura S, Miyamoto Y, Furukawa-Hibi Y, Muramatsu S, Nabeshima T, Nitta A (2015) Induction of neuronal axon outgrowth by Shati/Nat8l by energy metabolism in mice cultured neurons. NeuroReport 26:740–746. https://doi.org/10.1097/WNR.0000000000000416
Bzdega T, Turi T, Wroblewska B, She D, Chung HS, Kim H, Neale JH (1997) Molecular cloning of a peptidase against N-acetylaspartylglutamate from a rat hippocampal cDNA library. J Neurochem 69:2270–2277. https://doi.org/10.1046/j.1471-4159.1997.69062270.x
Riederer F, Bittsanský M, Lehner-Baumgartner E, Baumgartner C, Mlynárik V, Gruber S, Moser E, Kaya M, Serles W (2007) Decrease of NAA with aging outside the seizure focus in mesial temporal lobe epilepsy–a proton-MRS study at 3 tesla. Brain Res 1179:131–139. https://doi.org/10.1016/j.brainres.2007.06.063
Chapman TW, Hill RA (2020) Myelin plasticity in adulthood and aging. Neurosci Lett 715:134645. https://doi.org/10.1016/j.neulet.2019.134645
Xin W, Chan JR (2020) Myelin plasticity: sculpting circuits in learning and memory. Nat Rev Neurosci 21:682–694. https://doi.org/10.1038/s41583-020-00379-8
Arshad M, Stanley JA, Raz N (2016) Adult age differences in subcortical myelin content are consistent with protracted myelination and unrelated to diffusion tensor imaging indices. Neuroimage 143:26–39. https://doi.org/10.1016/j.neuroimage.2016.08.047
Sullivan EV, Pfefferbaum A (2006) Diffusion tensor imaging and aging. Neurosci Biobehav Rev 30:749–761. https://doi.org/10.1016/j.neubiorev.2006.06.002
Ahn JH, Lee TK, Park JH, Cho JH, Kim IH, Lee JC, Hong S, Jeon YH, Kang IJ, Lee YJ, Won MH, Lee CH (2017) Age-dependent differences in myelin basic protein expression in the hippocampus of young, adult and aged gerbils. Lab Anim Res 33:237–243. https://doi.org/10.5625/lar.2017.33.3.237
Namboodiri AM, Peethambaran A, Mathew R, Sambhu PA, Hershfield J, Moffett JR, Madhavarao CN (2006) Canavan disease and the role of N-acetylaspartate in myelin synthesis. Mol Cell Endocrinol 252:216–223. https://doi.org/10.1016/j.mce.2006.03.016
Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, Xavier RJ, Li C, Yankner BA, Scherzer CR, Yuan J (2010) Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc Natl Acad Sci USA 107:14164–14169. https://doi.org/10.1073/pnas.1009485107
Li P, Ma Y, Yu C, Wu S, Wang K, Yi H, Liang W (2021) Autophagy and aging: roles in skeletal muscle, eye, brain and hepatic tissue. Front Cell Dev Biol 9:752962. https://doi.org/10.3389/fcell.2021.752962
Yu Y, Feng L, Li J, Lan X, A L, Lv X, Zhang M, Chen L, (2017) The alteration of autophagy and apoptosis in the hippocampus of rats with natural aging-dependent cognitive deficits. Behav Brain Res 334:155–162. https://doi.org/10.1016/j.bbr.2017.07.003
Glatigny M, Moriceau S, Rivagorda M, Ramos-Brossier M, Nascimbeni AC, Lante F, Shanley MR, Boudarene N, Rousseaud A, Friedman AK, Settembre C, Kuperwasser N, Friedlander G, Buisson A, Morel E, Codogno P, Oury F (2019) Autophagy is required for memory formation and reverses age-related memory decline. Curr Biol 29:435–448. https://doi.org/10.1016/j.cub.2018.12.021
Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, Sardiello M, Rubinsztein DC, Ballabio A (2011) TFEB links autophagy to lysosomal biogenesis. Science 332:1429–1433. https://doi.org/10.1126/science.1204592
Huber K, Hofer DC, Trefely S, Pelzmann HJ, Madreiter-Sokolowski C, Duta-Mare M, Schlager S, Trausinger G, Stryeck S, Graier WF, Kolb D, Magnes C, Snyder NW, Prokesch A, Kratky D, Madl T, Wellen KE, Bogner-Strauss JG (2019) N-acetylaspartate pathway is nutrient responsive and coordinates lipid and energy metabolism in brown adipocytes. Biochim Biophys Acta Mol Cell Res 1866:337–348. https://doi.org/10.1016/j.bbamcr.2018.08.017
Acknowledgements
We thank Naomi Takino and Mika Ito for technical assistance in producing the Shati/Nat8l vectors.
Funding
This research was supported by a Grant-in-Aid for Scientific Research (KAKENHI) (B) [JSPS KAKENHI Grant Number, 26293213, JP21H02632] (SM, AN), Kobayashi Foundation (AN), AdAMS (Ac210045, AN) and Smoking Research Foundation Grant for Biomedical Research and Foundation (AN). HM was supported by a Nagai Memorial Research Scholarship from the Pharmaceutical Society of Japan. The funders played no role in the study design, data collection or analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
Hajime Miyanishi, Ayumu Kitazawa and Atsumi Nitta conceived and designed the study. Material preparation, data collection, and analysis were performed by Hajime Miyanishi, Ayumu Kitazawa, Naotaka Izuo, and Atsumi Nitta. AAV vectors were provided by Shin-ichi Muramatsu. The first draft of the manuscript was written by Hajime Miyanishi and it was confirmed by Atsumi Nitta. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
SM has equity in Gene Therapy Research Institution, Co., Ltd., which commercializes the use of AAV vectors for gene therapy applications. To the extent that the work in this manuscript increases the value of these commercial holdings, SM has conflicts of interest. The other authors have no relevant financial or nonfinancial interests to disclose.
Ethical Approval
All experimental procedures followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and they were approved by the Committee for Animal Experiments at the University of Toyama (2021PHA-16, 20).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miyanishi, H., Kitazawa, A., Izuo, N. et al. N-Acetyl Transferase, Shati/Nat8l, in the Dorsal Hippocampus Suppresses Aging-induced Impairment of Cognitive Function in Mice. Neurochem Res 47, 2703–2714 (2022). https://doi.org/10.1007/s11064-022-03594-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03594-0