Abstract
Depressive state adversely affects the memory functions, especially in the geriatric population. The initial stage of memory deficits associated with depression is particularly called as pseudodementia. It is the starting point of memory disturbance before dementia. The purpose of this research was to study depression and its consequent pseudodementia. For this purpose 24 male albino Wistar rats were divided into four groups. Depression was induced by 14 days of chronic restraint stress (CRS) daily for 4 h. After developing a depression model, pattern separation test was conducted to monitor pseudodementia in rats. Morris water maze test (MWM) was also performed to observe spatial memory. It was observed that model animals displayed impaired pattern separation and spatial memory. Treatment was started after the development of pseudodementia in rats. Curcumin at a dose of 200 mg/kg was given to model rats for one week along with the stress procedure. Following the treatment with curcumin, rats were again subjected to the aforementioned behavioral tests before decapitation. Corticosterone levels, brain derived neurotrophic factor (BDNF) and neurochemical analysis were conducted. Model rats showed depressogenic behavior and impaired memory performance. In addition to this, high corticosterone levels and decreased hippocampal BDNF, 5-HT, dopamine (DA), and acetylcholine (ACh) levels were also observed in depressed animals. These behavioral biochemical and neurochemical changes were effectively restored following treatment with curcumin. Hence, it is suggested from this study that pseudodementia can be reversed unlike true dementia by controlling the factors such as depression which induce memory impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is the most prevalent and debilitating psychiatric disorder worldwide that alters a person’s thoughts, feelings, and behavior. Prevalence of the major depressive disorder is about 9–23% among the general population [1]. Depression and other psychiatric illnesses are brutally affecting the health and quality of life. Daily life stressors, genetic, and environmental factors may be involved in the etiology of depression [2]. Chronic stress increases corticosterone level that leads to depression-like illness causing hippocampal damage that can be a risk factor for the onset of prodromal stage of dementia [3,4,5]. Depression induces mood disturbances which also alters the mental capability of an affected person leading to reduced performance in daily routine life. Depression is a major risk factor for inducing dementia [6]. However, the symptoms of dementia due to depression are different from ‘true’ dementia, therefore, dementia secondary to depression is called ‘pseudodementia’ [7]. The difference between true dementia and pseudodementia is reversibility, as the symptoms of pseudodementia can be reversed unlike true dementia [8]. Moreover, memory deficits in depression are noticeably mild in comparison to dementia [9]. Evidence shows that pseudodementia is the precursor of true dementia and if the underlying psychiatric illness is treated then pseudodementia can be treated [1, 10]. This emphasizes the significance of prevention and early treatment of mood disorders.
Depressive patients show less improvement in psychomotor, verbal, and visual memory as compared to schizophrenic patients [11]. Several studies have described that serotonergic and dopaminergic neurotransmission decreases in depressive disorder [12,13,14]. The brain regions mainly affected by depression are hippocampus, amygdala, and prefrontal cortex [15]. It has been shown that individuals with major depression exhibit decreased neurogenesis in the hippocampus [16]. Depression is involved in hippocampal atrophy leading to impaired memory functions [17]. Depression has also been associated with impaired pattern separation which can be described as misperceived stimuli and impaired ability to distinguish information at a basic sensory processing level [18]. Pattern separation is the ability to discriminate among similar experiences. It is hypothesized that pattern separation is associated with hippocampal functioning [19]. BDNF helps to regulate hippocampal functions and its plasticity [20]. Decreased BDNF expression has been reported in depression which ultimately halts neuronal regeneration [21].
For the management of depression and mood disorders mostly drugs are used that regulate serotonergic transmission [22]. Curcumin is a component of turmeric possessing anti-inflammatory, antistress, and neurotropic effects. Traditionally curcumin is used for the treatment of depression as it increases serotonergic and dopaminergic neurotransmission and BDNF protein expression in the brain [23, 24]. Curcumin decreases acetylcholinesterase (AChE) activity and hence increases acetylcholine (ACh) function in hippocampus and cerebral cortex [25]. Oral administration of curcumin was found to enhance memory functions by having a positive effect on cholinergic and serotonergic neurotransmission [26, 27].
The reversibility of pseudodementia increases the possibility to treat it by eliminating the underlying psychiatric illness. By this strategy, it is possible to halt the progress of pseudodementia into true dementia. Previously, curcumin has been consistently reported for its antidepressant effects. The present study was, therefore, particularly aimed to investigate the biochemical and neurochemical changes in memory deficits associated with depression following the treatment with curcumin. Curcumin has been used as an antidepressant and memory-enhancing compound but in the present study, the role of curcumin was investigated as a therapeutic agent to overcome depression-induced pseudodementia in rats.
Experimental Procedures
Animals
Twenty four locally bred, male albino Wistar rats (body weight: 180–200 g) were purchased from Dow University of Health Sciences, OJHA campus Karachi. Animals were kept individually in their cages in a quiet room for at least a week so that the rats adapt to the laboratory environment. The study was reviewed and approved by the ethical clearance committee, University of Karachi (Advance Studies and Research Board) and obtained ethical clearance under ASRB no: 03344/Sc. The study was also performed in accordance with National Institute of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 2011). All experimental procedures were performed in a balanced design to avoid the effect of time and order.
Drug
Curcumin used in the experiment was purchased from Sigma Aldrich. The drug was prepared in sunflower oil and administered orally to the test animals at a dose of 200 mg/kg/ml using a feeding tube. Untreated rats received an equal volume of sunflower oil. Effects of curcumin were monitored after seven days of treatment.
Experimental Protocol
Animals (n = 24) were randomly divided into two experimental groups unstressed (n = 12) and stressed (n = 12). Stressed rats were subjected to chronic restraint stress in restrainer tube. The stress procedure was continued for 14 days for 4 h daily. Depressive-like behaviors were then assessed in forced swim test (FST). After observing depression-like symptoms, pseudodementia in rats was checked by conducting pattern separation test for similar new object (PST-SNO) and pattern separation test for different location of object (PST-DLO). Spatial memory was also monitored by using Morris water maze (MWM) paradigm. After the initial behavioral phase, both groups were further divided for vehicle and curcumin treatment resulting in four groups (n = 6 per group) including control, depression (DEP), curcumin (CUR), and depression + curcumin (DEP + CUR). The control and DEP groups were treated with vehicle whereas CUR and DEP + CUR groups were treated with curcumin at a dose of 200 mg/kg for seven days. The stress procedure was continued during the supplementation of curcumin for DEP and DEP + CUR groups. After 7 days of curcumin treatment, rats were again subjected to behavioral analyses including FST, PST-SNO, PST-DLO and MWM. Behaviors were performed between 0800 and 1600 h. Following the behavioral analysis, rats were subjected to decapitation to dissect the brain and the hippocampal region was separated for neurochemical analysis. Plasma samples were also collected for the estimation of corticosterone. All samples were immediately stored at − 20 °C till further analysis (Fig. 1).
Schematic diagram showing experimental protocol of the current study. After chronic restraint stress (CRS) the rats for 14 days, rats were subjected to behavioral tests using forced swim test (FST), pattern separation test for similar new object (PST-SNO), pattern separation test for different location of objects (PST-DLO), and Morris water maze (MWM). There was a gap of 3 h between the tests which were performed on the same day. Curcumin supplementation was continued for 7 days followed by same sequence of behavioral analysis and decapitation to collect the samples
Restraint Stress Procedure
The stress groups were subjected to stress procedure by restraining them in a ventilated closed plastic tube that only allowed mild lateral movement. The stress procedure was continued for 4 h daily for 14 days [28].
Assessment of Depression-Like Symptoms
The expression for depression was monitored by FST paradigm. The apparatus and procedure was essentially the same as described previously [29]. It was one day protocol of FST. Rats were allowed to swim for 5 min followed by a pre-test of 2 min. Immobility time was observed during testing duration of the experiment. The increase in immobility time showed depression-like behavior of rats.
Pattern Separation for Similar New Object (PST-SNO)
This test was conducted to evaluate the ability of rats to differentiate between similar but new objects. This test has been suggested to determine the tendency of pattern separation to identify the minute differences between two similar objects. The apparatus consisted of an open field 40 × 40 × 40 cm. The test comprised of three sessions; habituation, familiarization, and test sessions. During habituation, each rat was placed in the box and allowed to explore the area for 10 min in order to habituate with the box. After 24 h of habituation, two similar objects were placed in the box and rat was allowed to explore both objects for 5 min so that rat got familiarized with both objects. The test session was carried out after 20 min of familiarization phase during which one of the objects was replaced by a new but similar object. The old object which was not replaced was designated as ‘a’ whereas the new object was assigned as ‘b’. During the test session exploring time for each object was recorded. Discrimination index (DI) was calculated by using the formula (b − a/a + b), where ‘a’ is the time to sniff the old object and ‘b’ is the time to sniff the new object placed during the test session. This test is based on the recognizing ability of rodent using olfactory cues. The discrimination index ranges from − 1 to + 1. The lower values of discrimination index represent inability of the rat to differentiate between similar but new objects [1].
Pattern Separation Test for Different Location of Object (PST-DLO)
This test was also used to analyze the ability of pattern separation in rats. This test was based on the ability to discriminate similar events [30]. In this test an apparatus having the dimension of 76 × 76 × 42 cm was used as an open field area. This test also comprised of three sessions; habituation, familiarization and test sessions. The rat was allowed to habituate with the open field arena during habituation for 10 min which was followed by familiarization phase after 24 h. During familiarization two massive and identical (12 × 12 × 12 cm) wooden blocks were placed in the middle area on a same horizontal line. Rat was introduced into the open field area facing towards the wall and was allowed to explore both objects for 5 min. After 20 min of second phase, test session was conducted during which one of the objects was displaced from its position to a definite point to change its location so that the objects aligned diagonally. Time to sniff both objects was recorded during testing phase. The object which was not displaced from its location was designated as ‘a’ whereas the object with new location was named as ‘b’. Discrimination index was calculated by the same formula as mentioned above. Discrimination index value ranges from − 1 to + 1. The impaired pattern separation was determined by lower discrimination index values indicating that rat was unable to identify the new location of displaced object.
Assessment of Spatial Memory by Morris Water Maze (MWM)
Spatial memory was observed by MWM apparatus. The dimensions of the apparatus were same as described earlier [31]. The MWM tank was divided into four quadrants as North (N), West (W), East (E), and South (S). The test consisted of two sessions; training and testing. During training session rats were allowed to swim in a circular tank filled with opaque water (having milk in it). Rats were given 120 s to find hidden platform; if the rat found the platform it was allowed to stay there for 10 s to familiarize and find cues related to the platform. In case rat failed to find platform then it was dragged gently to the platform. After initial training for 120 s, rats were trained from four different directions NE, SE, NW, and SW (target quadrant) to find the platform. There was 15 min gap between each trial. The cumulative escape latency was calculated for each rat by the summation of escape latencies observed during four training trials to find out the learning ability. After one hour of last trial, the test session was conducted during which platform was removed from the target quadrant. During test session time spent in target quadrant was noted. Cut off time was 120 s during testing. More time spent in target quadrant showed improved memory function.
Corticosterone Estimation
Blood samples were collected in heparinized tubes following decapitation of rats. These samples were centrifuged at 4 °C and plasma was separated for the estimation of corticosterone. Procedure for extraction of corticosterone was exactly same as described in [32]. It is a fluorimetric method in which 0.2 ml plasma was extracted with 1.5 ml of dichloromethane. It was shaken well and centrifuged. The upper layer was aspirated and 1 ml dichloromethane layer was transferred to another tube and shaken with 1 ml of sulphuric acid-ethanol reagent (7:3, v/v). The solvent was removed and sulphuric acid-ethanol reagent layer was transferred to a cuvette. It was read in fluorimeter at 470 nm excitation and 570 nm emission. The stock solution was prepared in ethanol and diluted with distilled water. The concentration of corticosterone was expressed in µg/dl.
Estimation of Hippocampal BDNF Levels
Hippocampal BDNF levels were analyzed by ELISA kit method. Commercially available BDNF kit (Rat BDNF Elisa Kit, Cat. No. E0476Ra, Bioassay Technology Laboratory, Shanghai, China) was used for the estimation. Homogenization and centrifugation of hippocampal tissue in PBS buffer (pH = 7.4) was done to separate the supernatant which was then used for the estimation of BDNF levels by using the manufacture‘s protocol. The BDNF levels were expressed as ng/g of hippocampal tissue.
Monoamine Estimation
For the estimation of 5-HT, dopamine (DA), and dihydroxyphenylacetic acid (DOPAC), the same method was used as described previously [33]. Frozen hippocampal tissues were homogenized in an extraction medium by using an electrical homogenizer (Polytron; Kinematica). The biogenic amines were estimated by reversed-phase HPLC with fluorescence detector (RF-10\({A}_{XL}\)). The stationary phase used for separation is a 5-µ Shim-pack ODS column having an internal diameter of 4.0 mm and a length of 250 mm. The mobile phase (pH = 3.5) that passes through the column consisted of 0.0025% 1-octanesulfonic acid sodium salt in acetate buffer having 12 mM acetic acid, 0.26 mM Na2EDTA, and 14% methanol. The fluorescence was observed at 279 nm excitation and 320 nm emission wavelengths.
Acetylcholine (ACh) Estimation
ACh content was estimated by the method of Hestrin [34] as described previously [35]. Hippocampal tissue was boiled to set free the bounded ACh and inactivate the enzyme. Free ACh bound with ferric chloride and brown color was obtained. Samples were read at 540 nm against blank.
Statistical Analysis
The behavioral data obtained before curcumin treatment was analyzed by independent t-test as the data comprised of two groups (CONTROL and DEP). The data obtained after curcumin treatment comprised of four groups (CONTROL, CUR, DEP, DEP + CUR) was analyzed by one-way ANOVA followed by Bonferroni test. Two-way repeated measure was applied to assess data for MWM training trials. Data is presented as mean ± SEM, p values < 0.05 were taken as significant.
Results
Effects of Stress and Curcumin on Forced Swim Activity
In this study, depression-like behavior was monitored using FST paradigm. Test was conducted after 14 days of stress exposure. Independent t-test showed a significant increase in immobility time [t(22) = 4.83, p < 0.01] in depressed group as compared to control animals (Fig. 2a). After curcumin treatment statistical analysis showed significant effect of treatment [F(3, 20) = 8.69, p < 0.01] on immobility time (Fig. 2b). Post-hoc analysis by Bonferroni test revealed significantly elevated immobility time in DEP group (p < 0.05) as compared to control animals. However, curcumin treatment in DEP + CUR group normalized the depressive symptoms and became comparable to controls.
Effects of stress on depression-like symptoms were observed before (a) and after (b) curcumin treatment by forced swim test in terms of immobility time. Data is represented as mean ± SEM (n = 6). Significant difference were obtained by Bonferroni post-hoc test; *p < 0.05, **p < 0.01 as compared to control group
Effects of Stress and Curcumin on Pattern Separation Test for Similar New Objects
Effects of depression on pattern separation were observed by PST-SNO in which discrimination index between similar new objects was monitored (Fig. 3). Before curcumin treatment, depression significantly induced impaired pattern separation as evident by decreased discrimination index [t(22) = 5.28, p < 0.01] in DEP group as compared to controls (Fig. 3a). After curcumin treatment statistical analysis showed significant effect of treatment [F(3, 20) = 14.01, p < 0.01]. DEP group showed impaired ability of pattern separation (p < 0.05) as compared to controls whereas, treatment with curcumin improved the discrimination of similar new objects from old object when compared with DEP group (p < 0.01) (Fig. 3b).
Effects of stress on total object exploration time and discrimination index in pattern separation test for similar new object was assessed before (a) and after (b) curcumin treatment. Data is represented as mean ± SEM (n = 6). Significant difference was obtained by Bonferroni post-hoc test; *p < 0.05, **p < 0.01 as compared to control group; ++p < 0.01 as compared to DEP group
Effects of Stress and Curcumin on Pattern Separation Test for Different Location Object Locations
Pattern separation was also observed by PST-DLO paradigm in which discrimination index for different location of object was monitored (Fig. 4). Depression significantly [t(22) = 5.50, p < 0.01] induced impairment in the ability of pattern separation of DEP group as compared to control animals (Fig. 4a). Statistical analysis after curcumin treatment showed significant effects of treatment [F(3, 20) = 19.87, p < 0.01] on discrimination index. Group treated with curcumin alone exhibited significantly (p < 0.05) improved discrimination index as compared to controls (Fig. 4b). DEP group showed impaired discrimination index (p < 0.01) when compared with control animals whereas, treatment with curcumin in DEP + CUR reduced the depression-induced impaired pattern separation as evident by significantly increased discrimination index when compared with DEP group.
Effects of stress on total object exploration time and discrimination index in pattern separation test for different location of object was assessed before (a) and after (b) curcumin treatment. Data is represented as mean ± SEM (n = 6). Significant difference was obtained by Bonferroni post-hoc test; *p < 0.05, **p < 0.01 as compared to control group; ++p < 0.01 as compared to DEP group
Effects of Stress and Curcumin on Morris Water Maze Test
Spatial memory of rats was evaluated by MWM test. One-way ANOVA with repeated measure showed significant effect of treatment [F(1, 22) = 5.227, p < 0.05] and trials [F(3, 66) = 38.77, p < 0.01] on escape latency during trials. Bonferroni test revealed impaired learning ability in DEP group as evident by significantly increased escape latency during training trials as compared to controls (p < 0.05; p < 0.01) (Fig. 5a). In addition to this, DEP group also showed impaired memory function (p < 0.01) as these rats spent less time in target quadrant during probe trial as compared to control animals (Fig. 5b). After curcumin administration statistical analysis showed significant effects of treatment [F(3, 20) = 55.63, p < 0.01] and trials [F(3, 60) = 36.83, p < 0.01] on learning ability. In comparison to control animals, DEP group showed significantly increased escape latency during 2nd (p < 0.05), 3rd (p < 0.01) and 4th (p < 0.05) training trial whereas, curcumin treatment in DEP + CUR group significantly reduced the escape latency during 3rd (p < 0.05) and 4th (p < 0.01) training trial as compared to DEP group, indicating improved learning ability following the treatment with curcumin (Fig. 5c). Statistical analysis for the time spent in target quadrant also showed significant effects of treatment [F(3, 20) = 17.13, p < 0.01]. The group treated with curcumin alone showed significantly increased (p < 0.05) time spent in target quadrant as compared to control animals (Fig. 5d). DEP group on the other hand spent significantly (p < 0.01) reduced time in target quadrant when compared with controls. However, DEP + CUR group exhibited a significant (p < 0.01) increase in time spent in target quadrant as compared to DEP group, demonstrating improved memory function by curcumin treatment.
Effects of stress on escape latency during 4 training trials and time spent in target quadrant observed in Morris water maze test before (a, b) and after (c, d) curcumin treatment. Data is represented as mean ± SEM (n = 6). Significant difference were obtained by Bonferroni post-hoc test; *p < 0.05, **p < 0.01 as compared to control group; +p < 0.05, ++p < 0.01 as compared to DEP group
Effects of Stress and Curcumin on Plasma Corticosterone Levels
Analysis of data by one-way ANOVA showed significant effects of treatment [F(3, 20) = 18.80, p < 0.01] on plasma corticosterone levels. The levels of corticosterone were significantly increased in DEP group as compared to that of control group (p < 0.01). Elevated levels of corticosterone confirmed depression-like condition in DEP rats (Fig. 6). CUR treatment, however, significantly reduced depression-induced increase in corticosterone levels in DEP + CUR group as compared to DEP (p < 0.01).
Effects of Stress and Curcumin on Hippocampal BDNF Levels
It was observed that there was also a significant effect of treatment [F(3, 20) = 38.70, p < 0.01] on hippocampal BDNF levels. CUR group exhibited significantly increased levels of BDNF as compared to that of controls (p < 0.01). DEP group showed significantly reduced levels of BDNF as compared to control rats (p < 0.01). DEP + CUR showed significantly increased levels of BDNF as compared to DEP and control groups (p < 0.01) which showed improvement in hippocampal functions by curcumin treatment (Fig. 7).
Effects of Stress and Curcumin on Hippocampal 5-HT Levels
Statistical analysis showed significant effects of treatment [F(3, 20) = 82.78, p < 0.01] on 5-HT levels in hippocampus. Post-hoc analysis revealed significant reduction in 5-HT levels in DEP group as compared to that of control group (p < 0.01). This alteration in hippocampal 5-HT levels were not observed in DEP + CUR group which showed significantly increased levels of 5-HT as compared to DEP group (p < 0.01) (Fig. 8).
Effect of stress on hippocampal 5-HT neurotransmission was determined by reversed-phase HPLC with fluorescence detector after curcumin treatment. Data is represented as mean ± SEM (n = 6). Significant difference were obtained by Bonferroni post-hoc test; *p < 0.05, **p < 0.01 as compared to control group; ++p < 0.01 as compared to DEP group
Effects of Stress and Curcumin on Hippocampal DA Levels
Hippocampal DA was also significantly affected by the treatment [F(3, 20) = 2005.8, p < 0.01] Bonferroni post-hoc test showed increased levels of hippocampal DA in CUR group as compared to control animals (p < 0.01) whereas, DA levels were significantly reduced in DEP group as compared to that of control group (p < 0.01). Curcumin treatment inhibited the reduction in DA levels in DEP + CUR group as compared to DEP group (p < 0.01) showing beneficial effects of curcumin on monoamine neurotransmission (Fig. 9).
Effect of stress on hippocampal dopamine neurotransmission was estimated by reversed-phase HPLC with fluorescence detector after curcumin treatment. Data is represented as mean ± SEM (n = 6). Significant difference were obtained by Bonferroni post-hoc test; **p < 0.01 as compared to control group; ++p < 0.01 as compared to DEP group
Effects of Stress and Curcumin on Hippocampal DOPAC Levels
One-way ANOVA showed significant effects of stress [F(3, 20) = 1772.4, p < 0.01] on hippocampal DOPAC levels. DEP group exhibited significantly increased levels of DA degradative product as compared to controls (p < 0.01). Whereas curcumin treatment significantly protected the degradation of DA as evident by decreased levels of DOPAC in DEP + CUR as compared to DEP group (p < 0.01) (Fig. 10).
Effect of stress on hippocampal DOPAC neurotransmission was estimated by reversed-phase HPLC with fluorescence detector after curcumin treatment. Data is represented as mean ± SEM (n = 6). Significant difference were obtained by Bonferroni post-hoc test; **p < 0.01 as compared to control group; ++p < 0.01 as compared to DEP group
Effects of Stress and Curcumin on Hippocampal ACh Levels
Analysis of hippocampal ACh levels by one-way ANOVA showed significant effects of treatment [F(3, 20) = 26.41, p < 0.01]. CUR group exhibited significantly increased levels of ACh as compared to controls (p < 0.01). DEP group showed a significant reduction in ACh levels when compared with control group (p < 0.05). Curcumin treatment in DEP + CUR group, however, significantly increased the levels of hippocampal ACh as compared to DEP and control groups (p < 0.01) (Fig. 11).
Effect of stress on hippocampal acetylcholine levels (µmol/g of brain tissue) was determined after curcumin treatment. Data is represented as mean ± SEM (n = 6). Significant difference were obtained by Bonferroni post-hoc test; *p < 0.05, **p < 0.01 as compared to control group; ++p < 0.01 as compared to DEP group
Discussion
In the present study depressive-like symptoms were observed in FST paradigm after subjecting rats to 14 days of chronic restraint stress for 4 h. Immobility time was longer in stressed group and impairment in pattern separation and spatial memory function was observed in the depressed group. It has been reported that 2 h stress for 4–8 weeks induces depression-like behavior which can be observed by FST [36]. Another experiment showed that 2 h CRS stress for 14 days was valid for animal model of depression [37]. It has been shown previously that depressive patients also suffer from pseudodementia [38]. Depressive patients perform abruptly in effort demanding situations. This led the scientists to suggest that motivation is linked with effort demanding activities in depressed patients which also affects learning ability [39]. In depression, it is difficult to recognize minor differences between two objects, which make the basis of diagnosis of dementia associated with depression [8]. In the present study, pseudodementia was monitored by using pattern separation test. The depressed rats showed impaired recognition to discriminate between similar objects and different location of objects. This indicates that depressed rats were unable to focus on the minute differences thus resulting in impairment of learning and memory and showed deterioration in pattern separation ability. Hence, the present findings are consistent with the previous research that showed impaired memory function following depression [40].
Activation of hypothalamic-pituitary-adrenal (HPA) axis following stress has been reported earlier [41]. This leads to the elevation of glucocorticoids which results in hippocampal atrophy and cognitive abnormalities. Increased cortisol levels were found in depressive patients [5]. Another research showed that elevated levels of glucocorticoids and reduced hippocampal BDNF mRNA expression are involved in the pathophysiology of depression [42]. The current findings also showed increased corticosterone levels in depressive animals. Moreover, hippocampal BDNF levels were also decreased in present research which is in accordance with the previous findings [43]. This could be the cause of impaired learning and memory observed in depressed rats. Evidence showed that animal model of depression has decreased levels of BDNF in hippocampus and amygdala resulting in impaired hippocampal dependent memory processing (pattern separation ability) [44]. Convergent evidence from studies also shows that decreased BDNF levels cause hippocampal atrophy [45, 46]. Moreover, human postmortem studies of depressive and suicidal cases also indicated a reduction in BDNF [20, 47]. Impaired memory and pattern separation ability observed in the present study in depressed rats may be attributed to the decreased hippocampal BDNF levels.
The role of 5-HT and DA in depression and memory is extensively studied. Decreased hippocampal and cortical 5-HT and DA have been observed in depressive illness [48]. The present study also exhibited decreased levels of 5-HT and DA in the hippocampus of depressed rats. DOPAC levels were significantly higher in the depressed group as compared to control rats. Increased DOPAC levels in the present study suggest increased turnover of DA resulting in the low levels of DA in the hippocampus. In depressive illness degradation of DA is increased and its neurotransmission is decreased [49]. Previously alteration in DA and DOPAC levels has been shown in different areas of the brain in depressed rats [50]. It has been studied that in depressive illness DA neurons are disrupted resulting in impaired memory, mainly affecting pattern separation ability. Present findings are consistent with the previous findings of Dillon and Pizzagalli [44] that decreased DA in the hippocampus is involved in impaired pattern separation. Hippocampal ACh neurotransmission is also reported to decline in depression which further worsens the pattern separation in rats [51]. A study showed that initially, a chronic elevation in ACh in depression leads towards down-regulation of ACh receptors, hence, ACh levels ultimately decrease in depression [52]. Depressed rats in the present study also exhibited decreased levels of ACh in hippocampus. Impaired memory and cognition in the present study in depressed rats may indeed be attributed to decreased ACh levels. Increased levels of ACh in the hippocampal formation are well studied and a decline in this neurotransmitter is related to impaired memory and cognitive processes [53].
It has been shown previously that curcumin treatment reversed depression and memory impairment [54]. Curcumin in this study was used as an antidepressant and nootropic drug. It was previously described that it has profound antidepressant activity. Its antidepressant effects have been reported in Wistar Kyoto rat model of depression [55]. This putative model of depression was administered with curcumin at the doses of 50, 100, and 200 mg/kg for 1–10 days and dose-dependent antidepressant effects were observed in FST and open field test [55]. Consistent with this study, present findings also showed that one-week curcumin treatment significantly restored the depressive symptoms in rats exposed to CRS. Curcumin can help to alleviate stress-induced dysfunction of HPA-axis and decreases the level of stress hormone such as corticosterone. Xu et al. examined the amelioration of depression and dysfunction of HPA-axis following the administration of curcumin at the doses of 2.5, 5, and 10 mg/kg in rats subjected to 20 days of stress protocol [56]. Reduced level of stress hormone is involved in the alleviation of depressogenic behavior which was also observed in this study following the treatment with curcumin. Neurotrophic factor expressions are also modulated by curcumin treatment [56]. Curcumin treatment in control and depressed rats improved learning and memory via regulating BDNF in our study. It was described earlier that curcumin reversed memory impairment in stressed rats by regulating BDNF in the hippocampus [54].
Curcumin has a marked effect on neurotransmission in mental illness. In an animal study, curcumin administration increased 5-HT and DA levels [27]. It inhibits monoamine oxidase activity and hence is involved in increasing monoamine neurotransmission [23]. Increased hippocampal DA and 5-HT levels were observed in DEP rats following treatment with curcumin whereas the control animals treated with curcumin alone also showed increased DA levels in hippocampus. Moreover, DEP rats treated with curcumin also displayed reduced depressive symptoms and improved learning and memory. Previous research showed that curcumin increased 5-HT receptor expression and improved cognitive functions in depressed animals [57]. Curcumin is an allosteric modulator of cholinergic receptors and acts in positive way and hence increases the level of ACh [58]. Consistent with these results we also observed increased hippocampal ACh levels following the administration of curcumin. It was shown that polyphenolic compounds like curcumin can decrease the activity of AChE and combat memory decline due to reduced levels of ACh [59]. Increased ACh in curcumin treated rats observed in this study may be attributed to inhibitory activity of curcumin on AChE as reported earlier [60]. Here it is suggested that curcumin can ameliorate pseudodementia via regulating corticosterone and hippocampal BDNF. Further, improvement in hippocampal neurochemical profile may also be involved in overcoming pseudodementia.
Conclusions
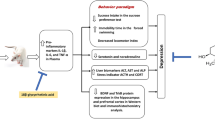
The present findings showed that stress-induced depression results in various behavioral, and biochemical changes (Fig. 12). Increased levels of corticosterone and reduced neurotrophic factor may lead to impaired memory function resulting in pseudodementia. Administration of curcumin effectively attenuated the depressive symptoms and related memory impairments. Hence, it is suggested that curcumin can be a potent therapeutic agent in combating depression and associated pseudodementia.
Schematic representation of findings of current study. Chronic restraint stress (CRS) induced activation of HPA-axis through the activation of corticotrophin releasing factor (CRF) and adrenocorticotropic hormone (ACTH) leading to increased levels of corticosterone. CRS also reduced the levels of serotonin, dopamine, acetylcholine and BNDF in hippocampus causing depressogenic behavior and associated pseudodementia. On the other hand, treatment with curcumin for a week helped in the reversal of depression-like symptoms by normalizing HPA-axis, neurotransmitters and BDNF levels that ultimately attenuated depression-induced pseudodementia
References
Afzal A, Ahmad S, Agha F, Batool Z, Tabassum S, Liaquat L et al (2018) Administration of 5-HT-1B agonist ameliorates pseudodementia induced by depression in rats. Pak J Pharm Sci 31:2179–2184
Uchida S, Yamagata H, Seki T, Watanabe Y (2018) Epigenetic mechanisms of major depression: targeting neuronal plasticity. Psychiatry Clin Neurosci 72(4):212–227
Veenit V, Cordero MI, Tzanoulinou S, Sandi C (2013) Increased corticosterone in peripubertal rats leads to long-lasting alterations in social exploration and aggression. Front Behav Neurosci 7:26
Zhao Y, Ma R, Shen J, Su H, Xing D, Du L (2008) A mouse model of depression induced by repeated corticosterone injections. Eur J Pharmacol 581(1–2):113–120
Byers AL, Yaffe K (2011) Depression and risk of developing dementia. Nat Rev Neurol 7(6):323–331
Brommelhoff JA, Gatz M, Johansson B, McArdle JJ, Fratiglioni L, Pedersen NL (2009) Depression as a risk factor or prodromal feature for dementia? Findings in a population-based sample of Swedish twins. Psychol Aging 24(2):373–384
Pozzoli S, De Carlo V, Madonna D (2019) Depression, dementia, and pseudodementia. In: Altamura AC, Brambilla P (eds) Clinical cases in psychiatry: integrating translational neuroscience approaches. Springer, Cham, pp 171–188
Kang H, Zhao F, You L, Giorgetta CDV, Sarkhel S, Prakash R (2014) Pseudo-dementia: a neuropsychological review. Ann Indian Acad Neurol 17(2):147–154
Morimoto SS, Kanellopoulos D, Manning KJ, Alexopoulos GS (2015) Diagnosis and treatment of depression and cognitive impairment in late life. Ann NY Acad Sci 1345(1):36–46
Kennedy J (2015) Depressive pseudodementia how “pseudo” is it really? Old Age Psychiatrist 62:30–37
Neu P, Gooren T, Niebuhr U, Schlattmann P (2019) Cognitive impairment in schizophrenia and depression: a comparison of stability and course. Appl Neuropsychol Adult 26(3):215–228
Belujon P, Grace AA (2017) Dopamine system dysregulation in major depressive disorders. Int J Neuropsychopharmacol 20(12):1036–1046
Nautiyal KM, Hen R (2017) Serotonin receptors in depression: from A to B. F1000Res 6:123
Liu Y, Zhao J, Guo W (2018) Emotional roles of mono-aminergic neurotransmitters in major depressive disorder and anxiety disorders. Front Psychol 9:2201
Kircanski K, Joormann J, Gotlib IH (2012) Cognitive aspects of depression. Wiley Interdiscip Rev Cogn Sci 3(3):301–313
Eisch AJ, Petrik D (2012) Depression and hippocampal neurogenesis: a road to remission? Science 338(6103):72–75
Kosteniuk JG, Morgan DG, O’Connell ME, Crossley M, Kirk A, Stewart NJ et al (2014) Prevalence and covariates of elevated depressive symptoms in rural memory clinic patients with mild cognitive impairment or dementia. Dement Geriatr Cogn Dis Extra 4(2):209–220
Gandy K, Kim S, Sharp C, Dindo L, Maletic-Savatic M, Calarge C (2017) Pattern separation: a potential marker of impaired hippocampal adult neurogenesis in major depressive disorder. Front Neurosci 11:571
Yassa MA, Stark CE (2011) Pattern separation in the hippocampus. Trends Neurosci 34(10):515–525
Phillips C (2017) Brain-derived neurotrophic factor, depression, and physical activity: making the neuroplastic connection. Neural Plast 2017:7260130
Jin Y, Sun LH, Yang W, Cui RJ, Xu SB (2019) The role of BDNF in the neuroimmune axis regulation of mood disorders. Front Neurol 10:515
Stiedl O, Pappa E, Konradsson-Geuken Å, Ögren SO (2015) The role of the serotonin receptor subtypes 5-HT1A and 5-HT7 and its interaction in emotional learning and memory. Front Pharmacol 6:162
Kulkarni SK, Bhutani MK, Bishnoi M (2008) Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacology 201(3):435–442
Choi GY, Kim HB, Hwang ES, Lee S, Kim MJ, Choi JY et al (2017) Curcumin alters neural plasticity and viability of intact hippocampal circuits and attenuates behavioral despair and COX-2 expression in chronically stressed rats. Mediators Inflamm 2017:6280925
Rajasekar N, Dwivedi S, Tota SK, Kamat PK, Hanif K, Nath C et al (2013) Neuroprotective effect of curcumin on okadaic acid induced memory impairment in mice. Eur J Pharmacol 715(1–3):381–394
Sarlak Z, Oryan S, Moghaddasi M (2015) Interaction between the antioxidant activity of curcumin and cholinergic system on memory retention in adult male Wistar rats. Iran J Basic Med Sci 18(4):398–403
Chimakurthy J, Murthy TE (2010) Effect of curcumin on quinpirole induced compulsive checking: An approach to determine the predictive and construct validity of the model. Am J Med Sci 2(2):81–86
Nawaz A, Batool Z, Ahmed S, Khaliq S, Sajid I, Anis L et al (2017) Attenuation of restraint stress-induced behavioral deficits by environmental enrichment in male rats. Pak Vet J 37:435–439
Haider S, Nawaz A, Batool Z, Tabassum S, Perveen T (2019) Alleviation of diazepam-induced conditioned place preference and its withdrawal-associated neurobehavioral deficits following pre-exposure to enriched environment in rats. Physiol Behav 208:112564
van Hagen BT, van Goethem NP, Lagatta DC, Prickaerts J (2015) The object pattern separation (OPS) task: a behavioral paradigm derived from the object recognition task. Behav Brain Res 285:44–52
Haider S, Tabassum S, Perveen T (2016) Scopolamine-induced greater alterations in neurochemical profile and increased oxidative stress demonstrated a better model of dementia: a comparative study. Brain Res Bull 127:234–247
Haider S, Saleem S, Tabassum S, Khaliq S, Shamim S, Batool Z et al (2013) Alteration in plasma corticosterone levels following long term oral administration of lead produces depression like symptoms in rats. Metab Brain Dis 28(1):85–92
De Benedetto GE, Fico D, Pennetta A, Malitesta C, Nicolardi G, Lofrumento DD et al (2014) A rapid and simple method for the determination of 3,4-dihydroxyphenylacetic acid, norepinephrine, dopamine, and serotonin in mouse brain homogenate by HPLC with fluorimetric detection. J Pharm Biomed Anal 98:266–270
Hestrin S (1949) The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J Biol Chem 180(1):249–261
Batool Z, Sadir S, Liaquat L, Tabassum S, Madiha S, Rafiq S et al (2016) Repeated administration of almonds increases brain acetylcholine levels and enhances memory function in healthy rats while attenuates memory deficits in animal model of amnesia. Brain Res Bull 120:63–74
Lapmanee S, Charoenphandhu J, Teerapornpuntakit J, Krishnamra N, Charoenphandhu N (2017) Agomelatine, venlafaxine, and running exercise effectively prevent anxiety- and depression-like behaviors and memory impairment in restraint stressed rats. PloS one 12(11):e0187671
Kim MH, Leem YH (2014) Chronic exercise improves repeated restraint stress-induced anxiety and depression through 5HT1A receptor and cAMP signaling in hippocampus. J Exerc Nutrition Biochem 18(1):97–104
Fischer P, Bailer U, Hilger E, Leitner I (2002) Depressive pseudodemenzen (Depressive pseudodementia). Wien Med Wochenschr 152(3–4):62–65
Crowe SF, Hoogenraad K (2000) Differentiation of dementia of the Alzheimer’s type from depression with cognitive impairment on the basis of a cortical versus subcortical pattern of cognitive deficit. Arch Clin Neuropsychol 15(1):9–19
Shelton DJ, Kirwan CB (2013) A possible negative influence of depression on the ability to overcome memory interference. Behav Brain Res 256:20–26
Stephens MA, Wand G (2012) Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res 34(4):468–483
Vollmayr B, Faust H, Lewicka S, Henn FA (2001) Brain-derived-neurotrophic-factor (BDNF) stress response in rats bred for learned helplessness. Mol Psychiatry 6(4):471–474
Lee BH, Kim YK (2010) The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig 7(4):231–235
Dillon DG, Pizzagalli DA (2018) Mechanisms of memory disruption in depression. Trends Neurosci 41(3):137–149
Miranda M, Morici JF, Zanoni MB, Bekinschtein P (2019) Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci 13:363
Mizoguchi Y, Yao H, Imamura Y, Hashimoto M, Monji A (2020) Lower brain-derived neurotrophic factor levels are associated with age-related memory impairment in community-dwelling older adults: the Sefuri study. Sci Rep 10(1):1–9
Erickson KI, Miller DL, Roecklein KA (2012) The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist 18(1):82–97
Hei M, Chen P, Wang S, Li X, Xu M, Zhu X et al (2019) Effects of chronic mild stress induced depression on synaptic plasticity in mouse hippocampus. Behav Brain Res 365:26–35
Ruiz N, Del Ángel DS, Olguín HJ, Silva ML (2018) Neuroprogression: the hidden mechanism of depression. Neuropsychiatr Dis Treat 14:2837–2845
Scholl JL, Renner KJ, Forster GL, Tejani-Butt S (2010) Central monoamine levels differ between rat strains used in studies of depressive behavior. Brain Res 1355:41–51
Fink KB, Göthert M (2007) 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev 59(4):360–417
Fernandes SS, Koth AP, Parfitt GM, Cordeiro MF, Peixoto CS, Soubhia A et al (2018) Enhanced cholinergic-tone during the stress induce a depressive-like state in mice. Behav Brain Res 347:17–25
Barry C, Heys JG, Hasselmo ME (2012) Possible role of acetylcholine in regulating spatial novelty effects on theta rhythm and grid cells. Front Neural Circuits 6:5
Liu D, Wang Z, Gao Z, Xie K, Zhang Q, Jiang H et al (2014) Effects of curcumin on learning and memory deficits, BDNF, and ERK protein expression in rats exposed to chronic unpredictable stress. Behav Brain Res 271:116–121
Hurley LL, Akinfiresoye L, Nwulia E, Kamiya A, Kulkarni AA, Tizabi Y (2013) Antidepressant-like effects of curcumin in WKY rat model of depression is associated with an increase in hippocampal BDNF. Behav Brain Res 239:27–30
Xu Y, Ku B, Tie L, Yao H, Jiang W, Ma X et al (2006) Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res 1122(1):56–64
Lian L, Xu Y, Zhang J, Yu Y, Zhu N, Guan X et al (2018) Antidepressant-like effects of a novel curcumin derivative J147: involvement of 5-HT1A receptor. Neuropharmacology 135:506–513
El Nebrisi EG, Bagdas D, Toma W, Al Samri H, Brodzik A, Alkhlaif Y et al (2018) Curcumin acts as a positive allosteric modulator of α7-nicotinic acetylcholine receptors and reverses nociception in mouse models of inflammatory pain. J Pharmacol Exp Ther 365(1):190–200
Desai A (2016) Dietary polyphenols as potential remedy for dementia. Adv Neurobiol 12:41–56
Akinyemi AJ, Oboh G, Fadaka AO, Olatunji BP, Akomolafe S (2017) Curcumin administration suppress acetylcholinesterase gene expression in cadmium treated rats. Neurotoxicol 62:75–79
Acknowledgements
Authors kindly acknowledge the Higher Education Commission (HEC), Pakistan, for Funding the project NRPU-3766.
Funding
This study was funded by Higher Education Commission (HEC), Pakistan (project NRPU-3766) received by S. Haider. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Afzal, A., Batool, Z., Sadir, S. et al. Therapeutic Potential of Curcumin in Reversing the Depression and Associated Pseudodementia via Modulating Stress Hormone, Hippocampal Neurotransmitters, and BDNF Levels in Rats. Neurochem Res 46, 3273–3285 (2021). https://doi.org/10.1007/s11064-021-03430-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03430-x