Abstract
Epilepsy comes after stroke as the most common chronic neurological disorder worldwide. Inflammation enhances neuronal hyperexcitability that could provide a background setting for the development of epilepsy. The aim of this study was to assess the effect of valproate (VAL), diclofenac (DIC), meloxicam (MEL), VAL + MEL and VAL + DIC in pentylenetetrazol (PTZ) kindled mice. Seventy mice were randomly allocated into 7 equal groups; Control, PTZ, VAL, DIC, MEL, VAL + MEL and VAL + DIC groups. Kindling was induced by PTZ (40 mg/kg, i.p.) injection every other day for 17 days. The drugs were administered, 30 min before each PTZ injection till the end of the schedule. Seizure score, latency, duration and mortality rate were recorded in all groups. Tumor necrosis factor- α (TNF-α), interleukin-1β (IL-1β), malondialdehyde (MDA) and prostaglandin E2 (PGE2) levels as well as reduced glutathione (GSH) content were assessed in brain homogenate at the end of the schedule. VAL, DIC, MEL, VAL + MEL and VAL + DIC decreased seizure score and duration. Meanwhile, they increased the latency period. PTZ increased TNF-α, IL-1β, MDA, and PGE2 levels meanwhile, it decreased GSH content. Administration of VAL, DIC, MEL, VAL + MEL and VAL + DIC decreased TNF-α, IL-1β, MDA, and PGE2 levels meanwhile, they increased GSH content in the brain homogenates. Effects of VAL + DIC combination on the studied parameters were significant in relation to VAL. VAL, DIC, MEL, VAL + MEL and VAL + DIC produced anticonvulsant effect and mitigated inflammation and oxidative stress in PTZ-kindled mice. Interestingly, DIC rather than MEL enhanced the anticonvulsant effect VAL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a common neurological disorder that affects people of different ages [1]. Recruitment of inflammatory cells was demonstrated in epileptic brains [2]. Cyclooxygenase (COX)-2 has a role in the brain inflammation [3]. The inflammatory mediators (i.e., cytokines and prostaglandins) were detected in epileptic brain specimens. They promote local inflammation and function directly on cognate receptors to modulate neuronal excitability [4]. The inflammatory processes result in hyperexcitable neuronal networks, and consequently the development of epilepsy [5].
Pentylenetetrazol (PTZ), GABAA receptor antagonist, is used as experimental chemoconvulsant. Repeated injections of subconvulsant doses of PTZ induce kindling model of epilepsy. This model is applicable for investigation of the pathophysiology of epilepsy and screening of anti-epileptic drugs [6].
The available antiepileptic drugs provide a symptomatic relief without cure of the disease. Furthermore, a wide range of adverse effects is associated with their long-term use limiting their compliance [7]. Therefore, the need for new antiepileptic drugs with better compliance is a promising target.
Diclofenac (DIC), non-selective COX inhibitor, has potent anti-inflammatory, analgesic, and antipyretic effects. This drug is widely used for the alleviation of pain, fever, and inflammation associated with arthritis and gout [8]. Meloxicam (MEL) inhibits prostaglandin (PG) biosynthesis during inflammatory conditions with preferential COX-2 inhibitory effect [9].
In previous studies diclofenac decreased the severity of seizures in PTZ kindling model of epilepsy, as well as the levels of interlukin-6 and TNF-α levels in hippocampus [10]. The drug depressed cortical neuronal activity and shifted the voltage dependent potassium channel activation to more hyperpolarization potentials [11]. Meloxicam increased the latency period to convulsions in acute PTZ model of epilepsy [12], and reduced myeloperoxidase and malondialdehyde levels and restored the glutathione content of the brain [13].
The rationale of our study was based on many previous studies of NSAIDs such as celicoxib, asprin, roficoxib, parecoxib and diclofenac performed in different models of epilepsy and showed variations in their antiepileptic effects.
The current work is designed to study and compare the potential anticonvulsant effect of DIC, MEL and their interactions with VAL in PTZ-kindled mice.
Materials and Methods
Animals
Male Swiss albino 8-week-old mice, weighing 15–35 g, purchased from the faculty of veterinary medicine, Zagazig University, Egypt and kept in colony cages with free access to food and tap water, under standardized housing conditions (temperature of 22 ± 1 °C). After 7 days of adaptation to laboratory conditions, the animals were randomly assigned to seven experimental groups. All experimental protocols were approved by the Ethics Committee of Zagazig University.
Drugs
Meloxicam: powder (Adwia, Egypt). Sodium valproate: powder (Sanofi-Aventis, France). Pentylenetetrazol: powder (Sigma-Aldrich, USA). Diclofenac sodium: powder (Novartis, Switzerland).
Kindling Model
PTZ was injected, 40 mg/kg, i.p. on alternate days. After each PTZ injection, seizures were evaluated using a scoring scale: 0, no effect; 1, jerks; 2, Straub’s tail; 3, clonus [14]. The maximum kindling score is approached if the animal shows all the phases of convulsions (i.e. up to full-blown clonus) and equals 6 (sum of 0 + 1 + 2 + 3). On day 17 the maximum seizure score was reached in PTZ-treated group.
Experimental Design
70 mice were randomly allocated into seven equal groups. Saline control group: mice were injected with normal saline (10 ml/kg mice, i.p.) on alternate days for 17 days. PTZ group: mice were received PTZ (40 mg/kg mice, i.p.) on alternate days for 17 days. VAL group: mice were injected with VAL (50 mg/kg/day, i.p.) [15] for 17 days and PTZ (40 mg/kg, i.p.) on alternate days injected 30 min after VAL. MEL group: mice were injected with MEL, 10 mg/kg/day, i.p. [16] for 17 days and PTZ (40 mg/kg, i.p.) on alternate days 30 min after MEL injection. DIC group: mice were injected with DIC, 10 mg/kg/day, i.p. [17] for 17 days, and PTZ (40 mg/kg, i.p.) on alternate days, 30 min after DIC injection. VAL + MEL group: mice were injected with MEL (10 mg/kg/day, i.p.) followed by VAL 50 mg/kg/day, i.p. for 17 days while, 30 min latter PTZ (40 mg/kg, i.p.) was injected on alternate days. VAL + DIC group: mice were injected with DIC (10 mg/kg/day, i.p.) followed by VAL (50 mg/kg, i.p.) for 17 days, while 30 min latter PTZ (40 mg/kg, i.p.) was injected on alternate days.
The selected doses of the drugs were based on the results of previous studies used the same drugs in other models of epilepsy and confirmed by pilot study. DIC or MEL doses utilized in the study were equivalent to human doses in clinical practices. As the human equivalent dose (mg/kg) = mice dose (mg/kg) × mice Km/human Km; while, Km = weight/body surface area [18]. So, the human doses = 10 × 3/37 = 0.8 mg/kg [for 60 kg human about 50 mg].
Mice were kept under direct observation and video recording during the experiment to facilitate the calculation of the results, seizure score and the latency period between PTZ injection and the beginning of convulsions were recorded, the duration of convulsions are calculated from the start of the convulsion movements till their quit.
Biochemical Assays
Mice were euthanized by decapitation 24 h after the last PTZ administration and brains were quickly removed in liquid nitrogen then perfused with phosphate buffered saline solution, pH 7.4, Containing 0.16 mg/ ml heparin to remove any red blood cells and clots. The tissue was homogenized in 5–10 ml cold buffer (i.e., 50 mM potassium phosphate, pH 7.5. 1 mM EDTA) per gram tissue. The homogenate was centrifuged at 100,000×g for 15 min at 4 °C. The supernatant was removed for assessment of TNF-α, IL-1β, MDA, GSH, and PGE2 [19].
Estimation of Reduced Glutathione (GSH) Content and Malondialdehyde (MDA) Level
GSH ELISA kits were utilized for estimation of GSH content according to Crowther [14]. Estimation of MDA levels were performed using MDA ELISA kits according to Jain et al. [20].
Estimation of IL-1β and TNFα
IL-1β and TNFα levels were determined by using their related ELISA kits according to Prostmann and Kiessing [21].
Estimation of PGE2
Prostaglandin E2 level was determined using PGE2 ELISA kits (ab133021) according to manufacturer instructions.
Statistical Analysis
The results obtained were statistically analyzed using the SPSS 15.0 software package for Windows (SPSS Inc., Chicago, IL). Statistical analysis of data was performed with one-way ANOVA followed by the post hoc Tukey–Kramer test for multiple comparisons. Differences among values were considered statistically significant if p < 0.05.
Results
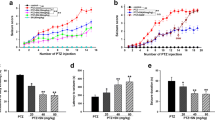
Effects of VAL (50 mg/kg), DIC (10 mg/kg), MEL (10 mg/kg), VAL + MEL and VAL + DIC on Seizure Score and Latency Period in PTZ-Kindled Mice (Figs. 1, 2)
Effects of VAL (50 mg/kg), DIC (10 mg/kg), MEL (10 mg/kg), VAL + MEL and VAL + DIC on seizure score in PTZ-kindled mice. Data represent mean ± Standard deviation. aSignificant in relation to PTZ group. bSignificant in relation to PTZ group, VAL and DIC groups. cSignificant in relation to PTZ and MEL groups. dSignificant in relation to PTZ group, VAL, DIC and MEL groups, p < 0.05. F value between the groups = 18.651
Effects of VAL (50 mg/kg), DIC (10 mg/kg), MEL (10 mg/kg), VAL + MEL and VAL + DIC on latency period (minutes) in PTZ-kindled mice. Data represent mean ± Standard deviation. aSignificant in relation to PTZ group. bSignificant in relation to PTZ group, VAL and DIC groups. cSignificant in relation to PTZ and MEL groups. dSignificant in relation to PTZ group, VAL, DIC and MEL groups, p < 0.05. F value between the groups = 30.555
The seizure score in PTZ control group was 5.4 ± 1.2 (range value: 6–3). This score was significantly decreased to 2.4 ± 1.7 (range value: 6–1), 2.7 ± 1.5 (range. value: 6–1) and 4 ± 1.8 (range value: 6–3) in VAL, DIC and MEL groups respectively. In the MEL group the score was significantly increased in relation to both VAL and DIC groups. Combination of VAL + MEL significantly decreased seizure score to 2 ± 1.05 (range value: 3–1) in relation to PTZ while the decrease was insignificant in relation to VAL, MEL or DIC groups. In VAL + DIC combination group, seizure score was significantly decreased to 1 ± 0.8 (range value:1–0) in relation to PTZ, VAL, DIC and MEL groups. Percentages of mice reached maximal score in the 17th day of experiment in PTZ, VAL, DIC, MEL, VAL + DIC and VAL + MEL groups were 90, 10, 40, 50, 0 and 0 respectively. Percentage of resistant mice to PTZ seizures was 10% in VAL + DIC group and 0% in all other groups.
The latency period of convulsions was 3.7 ± 0.39 (range value: 4.50–3.20) min in PTZ control group. In VAL, DIC and MEL groups, it was significantly increased to 10.3 ± 0.5(range value: 10.30–9.50), 9.8 ± 0.45 (range value: 10.30–8.40) and 7.6 ± 0.8 (range value: 9–7.50) min respectively in relation to PTZ group. The latency period in MEL group was significantly decreased in relation to VAL and DIC groups. MEL + VAL and VAL + DIC significantly (p˂0.05) increased the latency period to 9.9 ± 0.38 (range value: 10.50–9.30) and 11.6 ± 3.77 (range value: 15–11.90) min respectively in relation to MEL and PTZ groups but these values were insignificant in relation to VAL and DIC groups.
Effects of VAL (50 mg/kg), DIC (10 mg/kg), MEL (10 mg/kg), VAL + MEL and VAL + DIC on the Duration of Convulsions (minutes) in PTZ-Kindled mice (Fig. 3)
Effects of VAL (50 mg/kg), DIC (10 mg/kg), MEL (10 mg/kg), VAL + MEL and VAL + DIC on the duration of convulsion (in minutes) in PTZ-kindled mice. Data represent mean ± Standard deviation aSignificant in relation to PTZ group. bSignificant in relation to PTZ and VAL groups. cSignificant in relation to PTZ, DIC and MEL groups. dSignificant in relation to PTZ, VAL, DIC, MEL and VAL + MEL groups, p < 0.05
The mean duration of convulsions in PTZ control group was 6.5 ± 0.57 (range value: 7.20–5.36) min. The latter was significantly decreased in VAL, DIC and MEL groups to 2.3 ± 0.94 (range value: 3.60–2.12), 4.1 ± 1.1 (range value: 4.20–2.16) and 4.6 ± 1.2 (range value: 5.40–3.50) min respectively. The mean duration of convulsions in DIC and MEL groups was significantly increased in relation to VAL group. VAL + MEL significantly (p˂0.05) decreased the duration of convulsions to 2.7 ± 0.49 (range value: 3.80–2.13) min in relation to PTZ, DIC and MEL groups while this decrease was insignificant in relation to VAL group. In VAL + DIC group the duration of convulsions was significantly decreased to 1.4 ± 0.8 (range value: 2.50–1.5) min in relation to PTZ, VAL, DIC, MEL and VAL + MEL groups.
Effects of VAL (50 mg/kg), DIC (10 mg/kg), MEL (10 mg/kg), VAL + MEL and VAL + DIC on MDA Level and GSH Content, IL-1β, TNF-α and PGE2 Levels in the Brain of PTZ Kindled Mice (Table 1)
In saline control group, the MDA level was 1.54 ± 0.12 ng/g brain tissue. This level was significantly elevated in PTZ control group to 17.19 ± 1.18 ng/g. The latter was significantly decreased to 8.27 ± 0.55, 6.39 ± 0.48 and 10.16 ± 0.65 ng/g in VAL, DIC and MEL groups respectively. In DIC group, MDA level was significantly decreased in relation to VAL and MEL groups. In MEL group, MDA level was significantly increased in relation to VAL and DIC groups. In VAL + MEL and VAL + DIC groups, MDA levels were significantly decreased to 4.4 ± 0.49 and 2.83 ± 0.62 ng/g respectively. In VAL + DIC group, the level was significantly decreased in relation to PTZ, VAL, DIC, MEL and VAL + MEL groups.
In saline group, GSH content was 26.85 ± 1.59 pg/g brain tissue and significantly (p˂0.05) decreased in PTZ control group to 2.04 ± 0.42 pg/g. In VAL, DIC and MEL groups GSH contents were significantly increased to 7.16 ± 0.67, 9.97 ± 1.18 and 5.04 ± 0.58 pg/g respectively in relation to PTZ group. In the MEL group, the GSH content was significantly decreased in relation to VAL and DIC groups. GSH contents in VAL + MEL and VAL + DIC groups were significantly increased to 13.71 ± 0.72 and 18.53 ± 1.67 pg/g respectively in relation to PTZ, VAL, DIC and MEL groups. In VAL + DIC group, GSH content was significantly increased in relation to PTZ, VAL, DIC, MEL and VAL + MEL groups.
In saline group IL-1β level was 5.97 ± 0.25 pg/g tissue and significantly increased to 49.98 ± 3.82 pg/g in PTZ group. In VAL, DIC and MEL groups, IL-1β levels were significantly decreased to 24.6 ± 1.48, 19.11 ± 1.21 and 30.6 ± 2.07 pg/g respectively in relation to PTZ group. The IL-1β levels in VAL + MEL and VAL + DIC groups were significantly decreased to 7.65 ± 0.84 and 11.18 ± 0.77 pg/g groups respectively in relation to PTZ, VAL, DIC and MEL groups. The IL-1β level in VAL + DIC group was significantly decreased in relation to VAL + MEL group also.
In saline control group, the level of TNF-α was 4.16 ± 0.21 pg/g brain tissue. The latter was significantly increased to 40.48 ± 4.77 pg/g in PTZ group in relation to saline control group. In VAL, DIC and MEL groups, the levels of TNF-α were significantly decreased to 22.39 ± 1.09, 16 ± 97 ± 1.12 and 27.22 ± 1.43 pg/g respectively in relation to PTZ group. The level in MEL group was significantly increased in relation to DIC group. In VAL + MEL and VAL + DIC groups, the levels of TNF-α were significantly decreased to 9.23 ± 0.58 and 6.12 ± 0.55 pg/g respectively in relation to PTZ, VAL, DIC and MEL groups. The TNF-α level in VAL + DIC group was significantly decreased in relation to VAL + MEL group.
The PGE2 level in saline control group was 4.07 ± 0.12 pg/g brain tissue. The latter level was significantly increased in PTZ control group to 33.38 ± 2.48 pg/g in relation to normal group. In VAL, DIC and MEL groups, PGE2 levels were significantly decreased to 19.94 ± 1.02, 14.1 ± 0.84 and 20.64 ± 1.79 pg/g respectively in relation to PTZ group. In VAL + MEL and VAL + DIC groups, the levels of PGE2 were significantly decreased to 9.78 ± 0.83 and 6.01 ± 0.63 pg/g respectively in relation to PTZ, VAL, DIC and MEL groups. The PGE2 level in VAL + DIC group was significantly decreased in relation to VAL + MEL group.
Discussion
Drug interactions with antiepileptic drugs (AEDs) are common which may enhance their antiepileptic effect [7]. In the present work, the effects of VAL, MEL, DIC, VAL + MEL and VAL + DIC were examined in PTZ-kindled mice.
PTZ induced generalized convulsions and oxidative stress. The latter was represented by reduction in GSH content and elevation of MDA level in mice brain. Oxidative stress was found to be implicated in the initiation and progression of epilepsy [22]. Previous study reported decrement in GSH content in PTZ-kindled mice brain [23]. Epilepsy is associated with NMDA-mediated increase in neuronal calcium and cysteine deprivation due to binding of glutamate to cysteine transporter with subsequent GSH overwhelming [24]. In the same context, antioxidants ameliorated seizure activity in epileptic patients [25].
In the current study, IL1β and TNF-α levels were elevated in kindled mice brain. Increased cytokines levels in the cerebrospinal fluid of epileptic rats were previously reported [26]. In addition, toll-like receptor/interleukin-1 receptor signaling was enhanced in neurons of kindled mice [27]. Moreover, inhibition of IL-1β biosynthesis ameliorated PTZ-kindling development in rats [28]. Indeed, reduction of inflammation displayed anticonvulsant effect in drug-resistant epilepsy [29]. Inflammatory mediators were reported to decrease seizure threshold by inducing the expression of genes involved in neuronal cell death and synaptic plasticity [30]. IL-1β and TNF-α could induce glutamate release from astrocytes and inhibit GABA-mediated chloride influx [31]. On the contrary, IL-1β neuroprotective effect was reported [32]. Indeed, IL-1β and TNF-α can either reduce or exacerbate glutamate receptor mediated excitotoxicity depending on their extracellular concentrations and the length of time the tissue is exposed to these cytokines [33].
The present findings showed that VAL decreased seizure score, reduced the duration of convulsions and increased the latency period between PTZ injection and the occurrence of convulsions with increasing in brain GSH level content and reduction of MDA, IL1β, and TNF α, MDA and PGE2 levels. In previous study, oxidative damage of cortical neurons after ischemia/reperfusion (I/R) injury was reduced by VAL in rats [34]. The drug suppressed lipopolysaccharide-induced production of TNF-α, IL-1β, IL-2, IL-5, and IL-6 in human T cells and monocytes. The inhibitory effect on cytokine production could be a complementary mechanism for valproate antiepileptic action [35]. Furthermore, blockage of Na+ channels by AEDs inhibited the production of the pro-inflammatory cytokines induced by lipopolysaccharides [36].
In the present work, DIC exerted anticonvulsant effect, decreased seizure score, the duration of convulsions, and increased the latency period between PTZ injection and the occurrence of convulsions.Also, DIC reduced TNFα, IL-1β, MDA, PGE2 level while elevated of GSH content. In accordance with our results, DIC has been reported to increase the latency period in maximal electroshock seizure model [37]. In addition to blockade of PGs synthesis due to non-selective COX inhibition, the drug exhibited potassium channel opening effect that depressed cortical neuron in rats and enhanced its anticonvulsant effect in previous study [38].
In line with our results, selective inhibition of COX2 was reported to prevent neuronal damage and epileptic activity through inhibition of MAPK and ERK signalling pathway [39]. Nimuslide, another NSAID, decreased the effect of PTZ and decreased the neuronal oxidative stress in mice in another study [40]. In the current study, MEL produced anticonvulsant effect and decreased TNFα, IL-1β, PGE2, MDA meanwhile, it increased GSH content. Indeed, COX-2 inhibition was found to up-regulate GABAA receptors expression yielding reduction in neuronal excitability [41]. Moreover, celecoxib, another selective COX2 inhibitor was found to reduce the severity of PTZ-induced seizures [42].
In the present findings, combination of MEL or DIC with VAL decreased seizure score as well as levels of MDA, TNFα, IL-1β, PGE2 and increased GSH content in the brain tissue. DIC rather than MEL enhanced the anticonvulsant effect of VAL with better anti-inflammatory and antioxidant effects than VAL alone or with MEL. The beneficial effect of DIC could be attributed to the previously reported K channel opening effect as well as non-selectivity for COX subtypes. In this context, it was reported that selective COX-2 inhibitor (SC58236) did not modify microglia activation in the hippocampus [43].
The pharmacokinetic drug interactions could share in the enhancement of the anti-convulsant effects concluded in the present study. Valproate inhibits CYP2C9 enzyme that metabolises diclofenac [44]. Whereas, diclofenac can displace valproate from plasma protein binding sites. Meloxicam is metabolized by CYP2C9 and CYP3A4 which are inhibited by valproate [45].
In Conclusion: VAL, MEL, DIC and their combinations exhibited anticonvulsant effects and reduced inflammation as well as oxidative stress in PTZ kindled mice. DIC enhanced the anticonvulsant effect of VAL; an effect could be partially attributed to its better anti-inflammatory and antioxidant effects than MEL. Further experimental and clinical studies are needed for confirmation of these results.
References
England MJ, Liverman CT, Schultz AM, Strawbridge LM (2012) A reprint from epilepsy across the spectrum: promoting health and understanding. Epilepsy Curr 12(6):245–253
AlyuDikmen FM (2017) Inflammatory aspects of epileptogenesis: contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr 29(1):1–16
Vezzani A, Friedman A, Dingledine RJ (2013) The role of inflammation in epileptogenesis. Neuropharmacol 69:16–24
Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T (2011) IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun 25(7):1281–1289
Wilcox KS, Vezzani A (2014) Does brain inflammation mediate pathological outcomes in epilepsy? Adv Exp Med Biol 813:169–183
Shimada T, Yamagata K. (2018). Pentylenetetrazole-induced kindling mouse model. J Vis Exp, 136.
Wahab A (2010) Difficulties in treatment and management of epilepsy and challenges in new drug development. Pharmaceuticals 3(7):2090–2110
Altman R, Bosch B, Brune K, Patrignani P, Young C (2015) Advances in NSAID development: evolution of diclofenac products using pharmaceutical technology. Drugs 75(8):859–877
Tegeder I, Neupert W, Guhring H, Geisslinger G (2000) Effects of selective and unselective cyclooxygenase inhibitors on prostanoid release from various rat organs. J pharmacol Exp Ther 292(3):1161–1168
Vieiraa V, Glassmannb D, Marafonb P, Pereirac P, Gomezc R, Coitinho AS (2016) Effect of diclofenac sodium on seizures and inflammatory profile induced by kindling seizure model. Epilepsy Res 127:107–113
Peretz A, Degani N, Nachman R (2005) Meclofenamic acid and diclofenac, novel templates of KCNQ2/Q3 potassium channel openers, depress cortical neuron activity and exhibit anticonvulsant properties. Mol Pharmacol 67:1053–1066
Darvishi H, Rezaei M, Khodayar MJ, Zargar HR, Dehghani MA, Vardanjani HR, Ghanbari S (2016) Differential effects of meloxicam on pentylenetetrazole- and maximal electroshock-induced convulsions in mice. Jundishapur J Nat Pharm Prod 12(2):e36412
Hakan T, Toklu HZ, Biber N, Ozevren H, Solakoglu S, Demirturk P, Aker FV (2010) Effect of COX-2 inhibitor meloxicam against traumatic brain. Neurol Res 32(6):629–635
Erkec OE, Arihan O (2015) Pentylenetetrazole kindling epilepsy model. Epilepsy 21(1):6–12
Abdel-kader ZY, Khorshid NEA, Abd El motteleb D, Elwany NE (2015) Protective effect of montelukast against pentylenetetrazole induced acute seizures and kindling in mice. ZUMJ 21:6
Almaghour HG, Zawawi NM, Sherif FM (2014) Effect of non-steroidal anti-inflammatory drugs on anticonvulsant activity of diazepam in mice. Pharm pharmacol Int J 1(1):4
Akula KK, Dhir A, Kulkarni SK (2008) Rofecoxib, a selective cyclooxygenase-2 (COX- 2) inhibitor increase pentylenetetrazole seizure threshold in mice: possible involvement of adenosoinergic mechanism. Epilepsy Res 78(1):60–70
Reagan-Shaw NM, Ahmad N (2008) Dose translation from animal to human study should be revisited. FASEB J 22:659–666
Crowther J R. (2009). Stages in ELISA. Methods Mol Bio, 516–78.
Jain S, Bharal N, Khurana S, Mediratta PK, Sharma KK (2011) Anticonvulsant and antioxidant actions of trimetazidine in pentylenetetrazole-induced kindling model in mice. Naunyn Schmiedebergs Arch Pharmacol 383(4):385–392
Prostmann T, Kiessing ST (1992) Enzyme immunoassay techniques. An overview. J Immunol Methods 150:5–21
Shin EJ, Jeong JH, Chung YH, Kim WK, Ko KH, Bach JH (2011) Role of oxidative stress in epileptic seizures. Neurochem Int 59(2):122–137
Reddy AJ, Dubey AK, Handu SS, Sharma P, Mediratta PK, Ahmed QM, Jain S (2018) Anticonvulsant and antioxidant effects of musa sapientum stem extract on acute and chronic experimental models of epilepsy. Pharmacognosy Res 10(1):49–54
Van Den Pol AN, Obrientan K, Belousov A (1996) Glutamate hyperexitability and seizure-like activity throughout the brain and spinal cord upon relief from chronic glutamate receptor blockade in culture. Neuroscience 74(3):653–674
Martinc B, Grabnar I, Vovk T (2014) Antioxidants as a preventive treatment for epileptic process: a review of the current status. Curr Neuropharmacol 12(6):527–550
Gómez CD, Buijs RM, Sitges M (2014) The anti-seizure drugs vinpocetine and carbamazepine, but not valproic acid, reduce inflammatory IL-1β and TNF-α expression in rat hippocampus. J Neurochem 130(6):770–779
Lenz QF, Arroyo DS, Temp FR, Poersch AB, Masson CJ, Jesse AC (2014) Cysteinyl leukotriene receptor (CysLT) antagonists decrease pentylenetetrazol-induced seizures and blood–brain barrier dysfunction. Neuroscience 277:859–871
Maroso M, Balosso S, Ravizza T, Iori V, Wright CI, French J (2011) Interleukin-1beta biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics 8:304–315
Vezzani A, Granata T (2005) Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 46(11):1724–1743
Vezzani A, Aronica E, Mazarati A, Pittman QJ (2013) Epilepsy and brain inflammation. Exp Neurol 244:11–21
Balosso S, Ravizza T, Aronica E, Vezzani A (2013) The dual role of TNF-α and its receptors in seizures. Exp Neurol 247:267–271
He Y, Jackman NA, Thorn TL, Vought VE, Hewett SJ (2015) Interleukin-1β protects astrocytes against oxidant-induced injury via an NF-ĸB-development up regulation of glutathione synthesis. Glia 63:1568–1580
Vezzani A, Balosso S, Ravizza T (2008) The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun 22:797–803
Suda S, Katsura K, Kanamaru T, Saito M, Katayama Y (2013) Valproic acid attenuates ischemia-reperfusion injury in the rat brain through inhibition of oxidative stress and inflammation. Eur J Pharmacol 707(1–3):26–31
Ximenes JC, Verde EC, Naffah-Mazzacoratti M, Viana GS. (2012). Valproic acid, a drug with multiple molecular targets related to its potential neuroprotective action. Neurosci & Med, 3 (1): Article ID:17746, 14 pages.
Himmerich H, Bartsch S, Hamer H, Mergl R, Schönherr J, Petersein C. (2014). Modulation of cytokine production by drugs with antiepileptic or mood stabilizer properties in anti-CD3- and anti-d40-stimulated blood in vitro. Oxid Med Cell Longev, 806162.
Black JA, Liu S, Waxman SG (2009) Sodium channel activity modulates multiple functions in microglia. Glia 57(10):1072–1081
Khattab MI, Kamel EM, Abbas NAT, Kaoud A (2018) Diclofenac influence on the anticonvulsant effect of retigabine: the potential role of KCNQ channels. Egyptian J Basic Clin Pharmacol 8:1–17
Zhang HJ, Sun RP, Lei GF, Yang L, Liu CX (2008) Cyclooxygenase-2 inhibitor inhibits hippocampal synaptic reorganization in pilocarpine-induced status epilepticus rats. J Zhejiang Univ Sci B 9(11):903–915
Dhir A, Akula KK, Kulkarni SK (2008) Rofecoxib potentiates the anticonvulsant effect of topiramate. Inflammopharmacology 16(2):83–86
Haiju Z, Ruopeng S, Gefei L, Lu Y, Chunxi L (2008) Cyclooxygenase-2 inhibitor inhibits the hippocampal synaptic reorganization by inhibiting MAPK/ERK activity and modulating GABAergic transmission in pilocarpine-induced status epilepticus rats. Med Chem Res 18(2):71–90
Oliveira MS, Furian AF, Royes LF, Fighera MR, Fiorenza NG, Castelli M, Machado P, Bohrer D, Veiga M, Ferreira J, Cavalheiro EA, Mello CF (2008) Cyclooxygenase-2/PGE2 pathway facilitates pentylenetetrazol-induced seizures. Epilepsy Res 79(1):14–21
Holtman L, van Vliet EA, van Schaik R, Queiroz CM, Aronica E, Gorter JA (2009) Effects of SC58236, a selective COX-2 inhibitor, on epileptogenesis and spontaneous seizures in a rat model for temporal lobe epilepsy. Epilepsy Res 84(1):56–66
Patsalos PN. (2010). Drug to Drug Interactions of Antiepileptic Drugs: Mechanisms of Interaction and Management Strategies. In: Panayiotopoulos C.P. (Eds) Atlas of Epilepsies. Springer, London.
Chesné C, Guyomard C, Guillouzo A, Schmid J, Ludwig E, Sauter T (1998) Metabolism of Meloxicam in human liver involves cytochromes P4502C9 and 3A4. Xenobiotica 128(1):1–13
Funding
self-funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
No conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elgarhi, R., Shehata, M.M., Abdelsameea, A.A. et al. Effects of Diclofenac Versus Meloxicam in Pentylenetetrazol-Kindled Mice. Neurochem Res 45, 1913–1919 (2020). https://doi.org/10.1007/s11064-020-03054-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03054-7