Abstract
Astrocytes make up 20–40% of glial cells within the central nervous system (CNS) and provide several crucial functions, ranging from metabolic and structural support to regulation of synaptogenesis and synaptic transmission. Although these cells are morphologically and functionally complex, astrocytes have been historically regarded as homogenous cell populations and studied as one population of cells. Fortunately, recent evidence in RNA profiling and imaging data has begun to refute this view. These studies suggest heterogeneity of astrocytes across brain regions, differing in many aspects such as morphology, function, physiological properties, developmental origins, and response to disease. Increased understanding of astrocyte heterogeneity is critical for investigations into the function of astrocytes in the brain and neuro–glia interactions. Furthermore, insights into astrocyte heterogeneity can help better understand their role in neurological disorders and potentially produce novel approaches to treating these diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astrocytes are the most functionally diverse glial cells in the CNS and whose role in the nervous system has begun to be appreciated more over the past few decades. Astrocytes serve a multitude of versatile roles in providing metabolic and trophic support, regulating synaptogenesis, ion homeostasis, neurotransmitter buffering, maintaining blood-brain barrier integrity, and contribute to patterns of neuronal network activity [1,2,3]. In neural development, they function as neural stem cells, stimulate neurite outgrowth, guide axon projections, and promote synapse formation [4, 5]. They also have essential roles in responding to local neuropathology and in the mediation of innate immune responses, contributing to both inflammation and its resolution [6]. It is not possible to address all the studies on astrocyte function and pathology here, and we refer readers to these thorough reviews [1,2,3,4,5,6,7,8]. With all of these advancements in comprehending astrocytes and their role in the nervous system, what elements are still lagging in our understanding of astrocytes? One area of astrocyte science that remains relatively unexplored, and the topic of this review, is astrocyte heterogeneity.
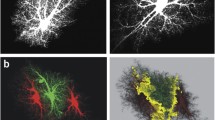
Cellular heterogeneity is a defining feature of all organ systems, where diverse cell populations operate together to ensure homeostatic function and proper physiological activities. While the same is true for the nervous system, studies in CNS cellular heterogeneity has been primarily investigated in neurons. Neuronal diversity was a certainty with early morphological and regional differences recorded by Camillo Golgi and Ramon y Cajal (Fig. 1a, [9]), and further verified with physiological and functional differences observed between differing neuronal subtypes. However, even with early morphological differences detected between astroglia (Fig. 1b, [10]), astrocytes were grouped as a homogenous population. For the better part of the century, astrocytes were treated as two separate populations: protoplasmic astroglia of the gray matter and fibrous astroglia of the white matter [11,12,13,14]. While there have been special cases such as Müller glia in the retina and Bergmann glia in the cerebellum, protoplasmic and fibrous astroglia were considered the major subpopulations of astrocytes [15]. Fortunately, this view has been challenged in the past few years with advancements in experimental techniques and functional understanding of astrocytes. This review aims to briefly explore some aspects of astrocyte heterogeneity: developmental diversity, subpopulations that have been determined, functional differences, and differences in the response to neuropathology. We suggest further reading into this subject highlighted in other reviews [6, 10, 16,17,18,19,20].
Early morphological discoveries in neurons and astrocyte demonstrate morphological diversity in both cell types. a Diversity of neuronal cells. a, Purkinjie neuron (human); b, pyramidal neuron (rabbit); c, motor neuron (cat); d,e horizontal neuron (cat); f, pre-motor interneuron (locust); g, visual amacrinal neuron (fly); h, multipolar neuron (fly); i, visual monopolar neuron (fly); j, visual interneuron (locust); k, pre-motor interneuron (crayfish); l, mechanical sensory neuron (cray fish); (from Cajal, Fisher and Boycott, Burrows, Strausfeld, O’Shea, Rowell and Reichert). Illustration taken from H. Reichert; Neurobiolgie, page 23 [9]. b Golgi staining of astrocytes in human cerebellum by Ramon y Cajal showing diversity of astrocytes. b, Bergmann glia; s, smooth protoplasmic astrocytes; v, velate astrocytes; f, fibrous astrocytes. Reproduced with permission from Springer Science & Business media: H. Kimelberg; Astrocytes in (patho)physiology of the nervous system, astrocyte heterogeneity or homogeneity? page 3 [10].
Development Heterogeneity
Cellular diversification of organ systems is determined by developmental patterning of embryonic tissue. For example, in the developing spinal cord, Sonic Hedgehog (Shh) and BMP/Wnt morphogen gradients along the neural tube participate in the control of diverse neuronal identities that emerge [21]. Initial evidence that astrocyte diversity is the result of patterning surprisingly came from the understanding of how oligodendrocytes are derived in the spinal cord. A majority of oligodendrocytes are derived from the pMN domain, a domain located near the ventral neural tube that specifically expresses the bHLH transcription factor Olig2 [22, 23]. Meanwhile, the surrounding domains (termed p1–p3) generate astrocytes in the ventral area. Analysis in an Olig2-null mouse model demonstrated that, in the absence of Olig2, the pMN domain is converted to a p2 domain and cells which once became oligodendrocytes were converted to astrocytes [24, 25]. Subsequent studies showed that Olig2 interacts with the factor Scl between the pMN and p2 domains, repressing one another to control astrocyte verse oligodendrocyte fates [26].
These initial studies provided evidence of developmental patterning in organizing glia diversity but did not explain the formation of distinct subpopulations of astrocytes within the p1–p3 domains. To address this, subsequent studies analyzed the gene expression profiles of astrocytes generated in these domains in the absence of Olig2 using the Olig2-null mouse model [27]. These studies demonstrated that the transcription factors Pax6 and Nkx6.1 selectively pattern these subsets of astrocytes. Additionally, the subpopulations are further demarcated by expression of Reelin and Slit. These subpopulations were designated as VA1, VA2, and VA3 (related to the p1–3 domains respectively). Pax6 is required for VA1 and VA2 identity, while Nkx6.1 is required for VA3. VA1 expresses Reelin, VA2 expresses both Reelin and Slit, and VA3 expresses Slit.
These developmental astrocyte patternings were further validated by lineage-tracing approaches with Cre-expressing mouse lines active in the specific spinal cord domains, demonstrating positional identity along the dorsal/ventral axis within the distinct subpopulations of astrocytes [28]. These approaches were also extended to the developing forebrain, showing similar results of local embryonic regions being linked to regional location of astrocytes. Interestingly, this study also showed that each domain generates both fibrous and protoplasmic astrocytes, suggesting that morphologically diverse astrocyte populations develop from common origins [28]. While these studies begin to suggest that astrocyte heterogeneity within the CNS is specified early by developmental patterning, future studies will need elucidate region-specific patterning mechanisms in other regions of the CNS.
Subpopulations and Heterogeneity in the Adult Brain
Due to a lack of available tools in isolating populations of astrocytes, our understanding of astrocyte heterogeneity in the adult brain was limited to early morphological techniques. The first of which came in differences between the protoplasmic and fibrous astrocytes. Protoplasmic astrocytes were shown to have long unbranched processes and generally express S100β, while fibrous astrocytes have short and highly branched extensions and express GFAP [1, 29, 30]. This analysis was expanded in a recent study where nine morphologically distinct GFAP or S100β expressing astrocytes were identified, distributed in varying proportions across different brain regions [15]. Interestingly, changes in expression of GFAP and other astrocyte intermediate filaments, such as vimentin, synemin, and nestin, provided some of the earliest evidence of the heterogeneous response astrocytes have to disease and injury, a topic highlighted in a future section [31,32,33]. While these studies gave an early indication of astrocyte heterogeneity, a considerable amount of evidence on astrocyte heterogeneity would arise in the past decade with two scientific advancements.
The first being a novel translational profiling approach called TRAP, translating ribosome affinity purification, in which translated mRNAs could be compared between astrocyte populations. Initial studies using the approach compared cortical astrocytes, cerebellar astrocytes, and cerebellar Bergman glia, and reported extensive differences in gene expression between astrocytes of different brain regions [34]. This study, combined with microarray analysis and genome-wide gene expression studies, found a large number of genes that are differentially expressed by subsets of astrocytes including genes that encode for proteins such as neuropeptides, sodium and potassium channels, various glutamate receptors and transporters, and surface glycoproteins [18]. Recently, this approach was improved upon, isolating and developing molecular profiles of astrocytes from six different brain regions utilizing BAC-TRAP (using BAC-transgenic mouse lines generated for TRAP methodology) [35]. Profiling astrocyte mRNAs in major cortical and subcortical brain regions (cortex, hippocampus, caudate-putamen, nucleus accumbens, thalamus, and hypothalamus), this study showed extensive molecular heterogeneity across these regions. Interestingly, this study used multiple coculture systems to show that the astrocytes preferentially promote neurite growth and synapse formation of neurons from the same brain regions over neurons from different brain regions.
The second advancement came in better understanding of molecular markers for astrocytes (outside of GFAP and S100β) and developing mouse models around these markers. One such marker that emerged as a broad marker for astrocytes in the adult brain is the folate enzyme Aldh1l1 (aldehyde dehydrogenase 1 family member L1) [36]. A BAC transgenic mouse line was generated expressing GFP under the control of the Aldh1l1 promoter, allowing for fluorescence-activated cell sorting (FACS) isolation of astrocyte subpopulations (as well as the BAC-TRAP analysis highlighted above) [37]. A recent study utilizing FACS and immunological approaches in this mouse model detailed five different populations (termed A–E) of astrocytes within the mouse CNS [38]. These astrocyte populations were characterized by their affinity and expression to several antibodies such as CD51, CD63, and CD71. Further, each astrocyte population was shown to differentially regulated synaptogenesis of neurons. Interestingly, this study also showed correlative astrocyte populations in human glioma and found specific subpopulations during tumor progression which corresponded with the onset of seizures and tumor invasion. A parallel study utilizing the Aldh1l1-GFP mouse performed an extensive comparison of hippocampal and striatal astrocytes [39]. This study utilized multiple transcriptomic, proteomic, morphological, and functional assays to thoroughly characterize and compare astrocytes from these two regions, demonstrating highly specific functional and morphological differences between these two populations (suggest reading [39] for all the detailed differences).
Recently, another marker used to determine astrocyte subpopulations in mice came from an old astrocyte protein expressed in most astrocytes, EAAT2 (excitatory amino acid transporter 2). Various promoter lengths of EAAT2 were used to drive tdTomato expression in mouse models, which showed neuronal expression of tdTomato of a promoter length up to 7.9 kb [40]. However, when the promoter length was increased to 8.3 kb, a subpopulation of astrocytes were highlighted within the cortical layers, showing higher populations in layers II/III and V [40, 41]. Microarray and functional analysis showed higher expression in genes such as Kcnj10 (encoding Kir4.1), LGR6, and Norrin and that the proteins LGR6 and Norrin were important for maintaining neuronal dendrites and spines. Subsequent studies were performed comparing cortical astrocytes with low and high expression of tdTomato to cortical astrocytes absent of tdTomato signal [42]. RNA-seq comparisons in this approach showed cortical astrocytes absent of tdTomato signal displayed elevated levels of several non-astrocyte genes such as Iba1 and mog.
Taken together, these studies have provided a multitude of new evidence elucidating astrocyte heterogeneity and subpopulations within the adult brain. However, one caveat with these approaches is relying heavily on gene expression changes to support astrocyte heterogeneity. Future studies utilizing the approach highlighted in [39], while costly, will help not only circumvent this issue but generate a highly detailed map characterizing astrocyte subpopulations throughout the CNS. In this approach, not only are transcriptomic differences highlighted, but proteomic, functional, and morphological differences are detailed.
Functional Heterogeneity
Given that astrocytes provide support to a diverse group of neurons, it is rational to believe that there is functional heterogeneity between astrocytes in different niches. Such functional differences were highlighted in the previous section, specifically that populations of astrocytes preferentially promote neurite growth and synapse formation of neurons in the same brain regions [35, 38]. These findings, as well as the others highlighted above, suggest astrocyte heterogeneity has a strong connection to surrounding neuron population. Potentially, astrocyte subpopulations may be induced by interactions with specific neuron populations to produce astrocytes which function in a precise manner to fit the needs of the surrounding neurons.
Another functional difference in astrocyte populations which may support this concept is in ion buffering. Kir4.1 (Kcnj10) is an inward-rectified potassium channel expressed in astrocytes that is critical for homeostatic regulation of potassium ions in the extracellular space [43]. Kir4.1 protein levels vary highly in gray matter astroglia, and the neocortex and hippocampus exhibit high heterogeneity in astroglia Kir4.1 levels [44, 45]. However, Kir4.1 is enriched in ventral horn astrocytes in the spinal cord and in cortical layers II/III and layers V in the cortex [16, 40, 45]. These astrocytes support lower motor neurons in the spinal cord and upper motor neurons in the motor cortex, suggesting enrichment in Kir4.1 expression of astrocytes to regulated the potassium ion needs of motor neurons. However, this notion is based on immunohistochemical observations and need to be further supported with functional measurements. Unfortunately, this brings up an issue when exploring functional diversity of astrocytes and that is the lack of available metrics for astrocyte physiology.
Electrophysiology, which has been a valuable tool for neuronal diversity, is difficult and does not provide much information in astrocytes. Astrocytes rarely diverge from the potassium equilibrium potential, have low membrane resistance making voltage clamping problematic, and are extensively coupled intracellularly to several other astrocytes [16]. The only study which extensively studied electrophysiological heterogeneity in astrocytes was only able to find very subtle differences between hippocampal and striatal astrocytes [39]. Another physiological readout of astrocytes which has gained increased attention is intracellular calcium signaling, which has been suggested to represent a method of excitability that astrocytes use for communication [46, 47]. Tools known as genetically encoded calcium indicators (GECIs) have been used to view calcium signaling in astrocytes, however they have diverse properties and can only be visualized on a single imaging plane. Interestingly, the only study to measure calcium signaling differences between astrocyte populations using GECIs comes again from the extensive study done between hippocampal and striatal astrocytes [39]. These studies showed few differences between the calcium signaling. Hippocampal astrocytes displayed higher spontaneous calcium signal frequency while striatal astrocytes significantly relied on entry for basal calcium levels more than hippocampal astrocytes.
Although there is some evidence in functional heterogeneity in astrocytes, it is clear that future studies will require novel tools to assess astrocyte physiology. Transcriptomics, proteomics, and immunohistochemical analysis give an early indicator of potential functional differences, but these are incomplete without proper metrics to connect these findings with function.
Astrocyte Heterogeneity in Disease
Astrocytes respond to a variety of injuries, infections, and diseases and astrocyte pathology is a major hallmark in a wide range of neurological disorders such as epilepsy, brain tumors, and neurodegeneration [38, 48,49,50]. Generally referred to as astrogliosis, reactive astrocytes undergo remarkable cellular, molecular, and functional alterations serving to support neurons, regulated the blood–brain barrier, remodel the extracellular space, control immune cells, and control synapse formation in response to disease and injury [51,52,53,54]. These alterations include increased astrocyte proliferation and number, increased expression and rearrangement of GFAP and intermediate filaments, and varying expression levels of cytokines and signaling molecules [1, 31,32,33, 51,52,53,54]. However, the responses and changes in reactive astrocytes are very heterogeneous and can have both detrimental and beneficial effects. In response to CNS injuries, astrocytes have been shown to inhibit axon regeneration and can produce pro-inflammatory cytokines that worsen spinal cord injuries [55,56,57]. On the other hand, ablation of reactive astrocytes in models of injury and ischemia showed reactive astrocytes are crucial for withstanding insult and improving recovery [58,59,60]. In an epileptic brain, reactive astrocytes produce a variety of mechanisms which can both promote and oppose seizure development [18]. These heterogeneous responses raised the question of whether there might be different subtypes of reactive astrocyte which elicit different responses.
To begin to understand the profiles of reactive astrocytes, transcriptome analysis was performed on quiescent and reactive astrocytes isolated from healthy and injured brains, through lipopolysaccaride (LPS) injections or middle cerebral artery occlusions to induce ischemia [54]. Through these studies, two different types of reactive astroglia were characterized: A1 and A2. A1 astrocytes lose the ability to promote neuronal survival and synaptogenesis and induce the death of neurons and oligodendrocytes through toxic soluble factors [54, 61]. A2 astrocytes upregulate many neurotrophic factors and promote neuronal survivability [54, 61]. A1 astrocytes were also shown to be induced by activated microglial inflammatory signaling molecules, which when inhibited prevented A1 astrocyte development [61]. Furthermore, A1 astrocytes were shown to be present in aged mouse CNS tissue and around areas of disease pathology in multiple neurodegenerative diseases in mouse and human [61,62,63,64]. However, while these populations show heterogeneity in the molecular phenotype of reactive astrocytes, it is unclear if these astrocytes are separate populations or the same astrocytes undergoing a continuum of progressive changes.
Regional heterogeneity of astrocyte populations in neurological disorders is an interesting topic given the regional specificity of neurological disorders with astrocyte pathology. Astrocytomas, one of the most common types of brain tumors, primarily occur in specific brain regions suggesting regional differences in the ability for astrocytes to proliferate and form tumors [18]. In one study, gene expression profiles from different brain regions showed heterogeneity in the express of the tumor-suppressor gene neurofibromatosis type 1 (NF1). As previously highlighted, a recent studied demonstrated that gene expression profiles of identified astrocyte subpopulations correlated with the profiles of different subtypes of human glioblastomas [38]. Additionally, specific subpopulations were found during tumor progression which corresponded with the onset of seizures and tumor invasion. Another neurological disorder with strong astroglia pathology is epilepsy. Astrocytes in epilepsy were shown to have downregulated Kir4.1 and Glutamate transporter-1 (Glt1), potentially allowing accumulation of glutamate and potassium ions in the extracellular space and exacerbating disease pathology [49, 65]. While downregulation of those proteins is more related to global astrocyte changes versus astrocyte heterogeneity, point mutations in Kir4.1 and the astroglial water channel Aquaporin-4 (AQP4) are present in patients with temporal lobe epilepsy, suggesting some astrocyte heterogeneity in epilepsy.
Astrocyte heterogeneity becomes an even more enticing subject when looking at neurodegenerative diseases. Most neurodegenerative diseases present as loss of specific populations of neurons, so most studies focus on what disease factors are present in specific neuronal pools that make them susceptible to death over other neuron types. However, similar pathology can be present in susceptible neurons of different diseases, such as TDP43, Tau, and FUS pathologies in both amyotrophic lateral sclerosis (ALS) and dementia [66]. Therefore, regional differences between astrocyte functionality or pathology may provide a key to understanding why specific neuronal pools die in neurodegenerative disease.
In Parkinson’s disease (PD), there is selective degeneration of dopamine neurons in the substantia nigra pars compacta (SN), while the neighboring dopamine neurons in the ventral tegmental area (VTA) are spared. The SN has been shown to have the lowest density of astrocytes and the levels of GFAP expression are reduced in PD post-mortem tissue, suggesting suppressed astrocyte response and decreased neuroprotection [67,68,69]. Recently, one study has demonstrated that there are vast transcriptional differences between VTA and SN astrocytes [70]. Of these differences, GDF15, a member of the TGFβ superfamily, was expressed ~ 200 times higher in VTA astrocytes and provided a neuroprotective effect to SN neurons when applied in vitro. PD astroglia have also been shown to exhibit senescence and release pro-inflammatory molecules that exacerbate dopaminergic neuron degeneration [71, 72]. In Huntington’s disease (HD), astrocytes from the striatum, where neuronal degeneration occurs, were shown to have significant downregulation of Kir4.1 [73]. Increased expression of Kir4.1 in striatal astrocytes through adeno-associated viruses (AAVs) showed increased striatal neuron survival and improved motor deficits in an HD mouse model [73].
Alzheimer's disease (AD) is characterized by a progressive atrophy of cortical and subcortical structures. Astrocytes have been shown to undergo both degeneration and reactivity in a time and regional specific manner in AD [68, 74]. In the entorhinal and prefrontal cortices, astrocytes become atrophic and fail to mount a response, suggesting that these regions have increase vulnerability to AD pathology [68, 75, 76]. Reactive astrocytes, such as the A1 astrocytes highlighted above, have been shown to localize to the regions of degeneration in human AD patient post-mortem tissue and in mouse models of tauopathy [61, 77]. Interestingly, astrocytes derived from AD patient iPSCs demostrate lower EAAT1 and glutamine synthetase levels, reduced morphological heterogeneity, increased atrophy, and altered release of soluble inflammatory mediators [78]. Given the complex degeneration and reactivity of astrocytes in AD, it has been suggested that astrocytes should be a key criteria for development of effective AD therapeutics [79].
ALS has long been connected to astrocytes, exhibiting astroglia pathology even before neuronal death has occurred [65, 80]. In the SOD1 (G93A) transgenic mouse model, prominent astrocyte degeneration and atrophy occurs, which precedes both neuronal death and the appearance of clinical symptoms [68]. In ALS, degeneration occurs in upper cortical motor neurons in layer V and lower motor neurons in the ventral horn of the spinal cord. Astrocyte subpopulations within these areas demonstrated downregulation of both Kir4.1 and Glt1, providing excess glutamate and potassium ions within the extracellular space which could serve to initiate or exacerbate the hyperexcitable deficits seen in ALS motor neurons [81,82,83]. Additionally, in vitro models of human and rodent ALS astrocytes demonstrate an increased release of neurotoxic factors to motor neurons that increase their susceptibility to death [84].
Taken together, these studies provide not only provide evidence for the role of astrocyte heterogeneity in neurological disorders but that astrocytes may provide potential therapeutic targets. Future studies into astrocyte heterogeneity in disease would benefit from the newer approaches taken to isolate and screen astrocyte subpopulations, combining mouse models used to analyze astrocyte subpopulations with mouse models of neurodegenerative diseases.
Conclusion
Astrocyte heterogeneity is a topic that has become more appreciated in the past few decades. Insights into astrocyte heterogeneity are crucial for our understanding of astrocyte biology in the developing and adult brain. Additionally, as astrocytes are crucial for neuronal function, maintenance, and neural development, understanding astrocyte heterogeneity is important to further understand neuronal function and development. Furthermore, the role astrocyte subpopulations may serve in neurological disorders provides an interesting target for future therapeutic targets. However, this field is still in the early stages and requires advancement in tools as early insights rely on transcriptional changes between astrocyte populations. Future studies would benefit greatly from tools for functional assessments as well as additional molecular tools for labeling and manipulating astrocyte pools. Astrocyte heterogeneity remains a fascinating topic that offers a promising addition to our understanding of the biology of the nervous system.
References
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35
Verkhratsky A, Nedergaard M (2018) Physiology of astroglia. Physiol Rev 98(1):239–389
Khakh BS, Sofroniew MV (2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18(7):942–952
Clarke LE, Barres BA (2013) Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci 14(5):311–321
Allen NJ, Barres BA (2009) Neuroscience: glia—more than just brain glue. Nature 457(7230):675–677
Cunningham C, Dunne A, Lopez-Rodriguez AB (2018) Astrocytes: heterogeneous and dynamic phenotypes in neurodegeneration and innate immunity. Neuroscientist 25:455–474
Guttenplan KA, Liddelow SA (2019) Astrocytes and microglia: models and tools. J Exp Med 216(1):71–83
Allen NJ, Eroglu C (2017) Cell biology of astrocyte-synapse interactions. Neuron 96(3):697–708
Reichert H (1990) Neurobiologie. Georg Thieme Verlag, Stuttgart
Kimelberg HK (2009) Astrocyte heterogeneity or homogeneity. Astrocytes in (Patho)Physiology of the Nervous System. Springer, Boston
Tabata H (2015) Diverse subtypes of astrocytes and their development during corticogenesis. Front Neurosci 9:114
Lundgaard I et al (2014) White matter astrocytes in health and disease. Neuroscience 276:161–173
Gallo V, Deneen B (2014) Glial development: the crossroads of regeneration and repair in the CNS. Neuron 83(2):283–308
Rodnight RB, Gottfried C (2013) Morphological plasticity of rodent astroglia. J Neurochem 124(3):263–275
Emsley JG, Macklis JD (2006) Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol 2(3):175–186
Khakh BS, Deneen B (2019) The emerging nature of astrocyte diversity. Annu Rev Neurosci 42:187–207
Oberheim NA, Goldman SA, Nedergaard M (2012) Heterogeneity of astrocytic form and function. Methods Mol Biol 814:23–45
Zhang Y, Barres BA (2010) Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol 20(5):588–594
Chaboub LS, Deneen B (2012) Developmental origins of astrocyte heterogeneity: the final frontier of CNS development. Dev Neurosci 34(5):379–388
Ben Haim L, Rowitch DH (2017) Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18(1):31–41
Ulloa F, Briscoe J (2007) Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle 6(21):2640–2649
Zhou Q, Wang S, Anderson DJ (2000) Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron 25(2):331–343
Lu QR et al (2000) Sonic hedgehog–regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron 25(2):317–329
Lu QR et al (2002) Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109(1):75–86
Zhou Q, Anderson DJ (2002) The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109(1):61–73
Muroyama Y et al (2005) Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature 438(7066):360–363
Hochstim C et al (2008) Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 133(3):510–522
Tsai HH et al (2012) Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337(6092):358–362
Miller RH, Raff MC (1984) Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci 4(2):585–592
Bignami A et al (1972) Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res 43(2):429–435
Hol EM, Pekny M (2015) Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol 32:121–130
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46(6):957–967
Pekny M et al (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol 131(3):323–345
Doyle JP et al (2008) Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell 135(4):749–762
Morel L et al (2017) Molecular and functional properties of regional astrocytes in the adult brain. J Neurosci 37(36):8706–8717
Anthony TE, Heintz N (2007) The folate metabolic enzyme ALDH1L1 is restricted to the midline of the early CNS, suggesting a role in human neural tube defects. J Comp Neurol 500(2):368–383
Cahoy JD et al (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28(1):264–278
John Lin CC et al (2017) Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 20(3):396–405
Chai H et al (2017) Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron 95(3):531-549 e9
Miller SJ et al (2019) Molecularly defined cortical astroglia subpopulation modulates neurons via secretion of Norrin. Nat Neurosci 22(5):741–752
Yang Y et al (2011) Molecular comparison of GLT1 + and ALDH1L1 + astrocytes in vivo in astroglial reporter mice. Glia 59(2):200–207
Morel L et al (2019) Intracortical astrocyte subpopulations defined by astrocyte reporter Mice in the adult brain. Glia 67(1):171–181
Farmer WT, Murai K (2017) Resolving astrocyte heterogeneity in the CNS. Front Cell Neurosci 11:300
Olsen ML, Campbell SL, Sontheimer H (2007) Differential distribution of Kir4.1 in spinal cord astrocytes suggests regional differences in K + homeostasis. J Neurophysiol 98(2):786–793
Kelley KW et al (2018) Kir4.1-dependent astrocyte-fast motor neuron interactions are required for peak strength. Neuron 98(2):306–319e7
Charles AC et al (1991) Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron 6(6):983–992
Cornell-Bell AH et al (1990) Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247(4941):470–473
Molofsky AV et al (2012) Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 26(9):891–907
Ohno Y (2018) Astrocytic Kir4.1 potassium channels as a novel therapeutic target for epilepsy and mood disorders. Neural Regen Res 13(4):651–652
Cabezas R et al (2014) Astrocytic modulation of blood brain barrier: perspectives on Parkinson's disease. Front Cell Neurosci 8:211
Sofroniew MV (2014) Astrogliosis. Cold Spring Harb Perspect Biol 7(2):a020420
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32(12):638–647
Pekny M, Nilsson M (2005) Astrocyte activation and reactive gliosis. Glia 50(4):427–434
Zamanian JL et al (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32(18):6391–6410
Fitch MT, Silver J (2008) CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol 209(2):294–301
Alilain WJ et al (2011) Functional regeneration of respiratory pathways after spinal cord injury. Nature 475(7355):196–200
Brambilla R et al (2005) Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med 202(1):145–156
Bush TG et al (1999) Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23(2):297–308
Faulkner JR et al (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24(9):2143–2155
Voskuhl RR et al (2009) Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29(37):11511–11522
Liddelow SA et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487
Clarke LE et al (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci USA 115(8):E1896-E
Rothhammer V et al (2018) Microglial control of astrocytes in response to microbial metabolites. Nature 557(7707):724–728
Yun SP et al (2018) Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat Med 24(7):931–938
Dossi E, Vasile F, Rouach N (2018) Human astrocytes in the diseased brain. Brain Res Bull 136:139–156
Ferrari R et al (2011) FTD and ALS: a tale of two diseases. Curr Alzheimer Res 8(3):273–294
Mena MA, Garcia de Yebenes J (2008) Glial cells as players in parkinsonism: the "good," the "bad," and the "mysterious" glia. Neuroscientist 14(6):544–560
Verkhratsky A et al (2017) Neuroglia: functional paralysis and reactivity in alzheimer's disease and other neurodegenerative pathologies. Adv Neurobiol 15:427–449
Tong J et al (2015) Low levels of astroglial markers in Parkinson's disease: relationship to alpha-synuclein accumulation. Neurobiol Dis 82:243–253
Kostuk EW, Cai J, Iacovitti L (2019) Subregional differences in astrocytes underlie selective neurodegeneration or protection in Parkinson's disease models in culture. Glia 67(8):1542–1557
Booth HDE, Hirst WD, Wade-Martins R (2017) The role of astrocyte dysfunction in Parkinson's disease pathogenesis. Trends Neurosci 40(6):358–370
Chinta SJ et al (2018) Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson's disease. Cell Rep 22(4):930–940
Tong X et al (2014) Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat Neurosci 17(5):694–703
Olabarria M et al (2010) Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer's disease. Glia 58(7):831–838
Rodriguez-Arellano JJ et al (2016) Astrocytes in physiological aging and Alzheimer's disease. Neuroscience 323:170–182
Verkhratsky A et al (2015) Glial asthenia and functional paralysis: a new perspective on neurodegeneration and Alzheimer's disease. Neuroscientist 21(5):552–568
Shi Y et al (2017) ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549(7673):523–527
Jones VC et al (2017) Aberrant iPSC-derived human astrocytes in Alzheimer's disease. Cell Death Dis 8(3):e2696
Sadick JS, Liddelow SA (2019) Don't forget astrocytes when targeting Alzheimer's disease. Br J Pharmacol 176(18):3585–3598
Haidet-Phillips AM, Maragakis NJ (2015) Neural and glial progenitor transplantation as a neuroprotective strategy for Amyotrophic Lateral Sclerosis (ALS). Brain Res 1628(Pt B):343–350
Yang Y et al (2009) Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron 61(6):880–894
Rosenblum LT, Trotti D (2017) EAAT2 and the molecular signature of amyotrophic lateral sclerosis. Adv Neurobiol 16:117–136
Kaiser M et al (2006) Progressive loss of a glial potassium channel (KCNJ10) in the spinal cord of the SOD1 (G93A) transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem 99(3):900–912
Phatnani HP et al (2013) Intricate interplay between astrocytes and motor neurons in ALS. Proc Natl Acad Sci USA 110(8):E756–E765
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue: In Honor of Professor Michael Robinson.
Rights and permissions
About this article
Cite this article
Westergard, T., Rothstein, J.D. Astrocyte Diversity: Current Insights and Future Directions. Neurochem Res 45, 1298–1305 (2020). https://doi.org/10.1007/s11064-020-02959-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-02959-7