Abstract
The hippocampus is critical for memory and emotion and both N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl- 4-isoxazolepropionic acid (AMPA) receptors are known to contribute for those processes. However, the underlying molecular mechanisms remain poorly understood. We have previously found that mice undergo memory decline upon dcf1 deletion through ES gene knockout. In the present study, a nervous system-specific dcf1 knockout (NKO) mouse was constructed, which was found to present severely damaged neuronal morphology. The damaged neurons caused structural abnormalities in dendritic spines and decreased synaptic density. Decreases in hippocampal NMDA and AMPA receptors of NKO mice lead to abnormal long term potentiation (LTP) at DG, with significantly decreased performance in the water maze, elevated- plus maze, open field and light and dark test. Investigation into the underlying molecular mechanisms revealed that dendritic cell factor 1 (Dcf1) contributes for memory and emotion by regulating NMDA and AMPA receptors. Our results broaden the understanding of synaptic plasticity’s role in cognitive function, thereby expanding its known list of functions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The brain mechanisms of learning and memory are among the most attractive issues for neuroscientists. Although progress has been made in the past few decades, the understanding of such mechanisms still poses a huge challenge. The hippocampus is a key brain region for learning and memory and LTP is often used to study learning and memory associated behavior [1,2,3]. In animal models, LTP formation enhances learning, whereas its blockage decreases it. Therefore, LTP reflects an increase in hippocampal synaptic transmission [4]. During LTP induction, postsynaptic NMDA receptors are activated, resulting in increased calcium concentration, which triggers a series of intracellular signaling cascades [1, 5,6,7,8], This in turn, leads to LTP expression through AMPAR phosphorylation and insertion into the postsynaptic membrane.
In the central nervous system, N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl- 4-isoxazolepropionic acid (AMPA) receptors are important excitatory amino acid receptors [9]. The NMDA receptor is regulated by AMPAR PAMs, acetylcholine receptors, and GABA receptors, among others [10,11,12], and plays an important role in nervous system development, including neuronal survival, development of neuronal dendrites and axonal structure, and formation of synaptic plasticity, as well as in learning and memory [13]. The AMPA receptor mediates rapid excitatory synaptic transmission, neuronal death, and synaptic plasticity [14]. However, the molecular mechanisms underlying the regulatory roles of NMDA and AMPA receptors remain unclear.
Dcf1 (dendritic cell factor 1), also known as TMEM59, is a membrane protein that we previously found to be involved in neural stem cell differentiation (NSCs) [15]. Upon overexpression of the dcf1 gene, NSCs remained undifferentiated, whereas silencing dcf1 induced their differentiation into neurons and astrocytes [16]. We further discovered that microRNA-351 (miR-351) could target dcf1 and negatively regulate its expression in the mouse NSC cell line C17.2 [17]. Moreover, dcf1 defines a novel ATG16L1‐binding motif and promotes LC3 activation in response to Staphylococcus aureus infection [18]. Our recent mouse study indicates that Dcf1 knockout (KO) leads to decreased dendritic complexity and dendritic spine density. Dcf1 KO mice exhibit spatial memory impairment and anxiety-like behavior [19]. However, the mouse model used in that study was a dcf1 systemic rather than a neurospecific knockout and the mechanism underlying the observed behaviors remains unclear. Therefore, in the present study, we knocked out dcf1 specifically in the nervous system to reveal the mechanism underlying the effects of dcf1 on memory and emotion, via the regulation of NMDA and AMPA receptors expression. The results will extend and deepen our understanding of dcf1′s involvement in brain function.
Material and Methods
Animals
All animal experimental procedures were approved by the Shanghai University Ethics Committee and all animal husbandry procedures were in compliance with the institutional and NIH guidelines. Experimental animals were male C57BL/6 J mice, aged 3–6 months. The animals were housed under a 12-h light–dark cycle (08:00–20:00) at a controlled temperature (22 ± 1 °C) and provided food and water.
Construction of NKO Mice
All mice used in the hybridization experiment were 3–6 months old. Mice carrying loxP on both sides of the Dcf1 exon 1 were hybridized with and Nestin-cre mice to produce nervous system-specific Dcf1 knockout mice (NKO). NKO was confirmed by polymerase chain reaction (PCR)-amplifying a 435 bp band on agarose gel electrophoresis, unlike WT, in which a 400 bp band is amplified.
Morris Water Maze
The Morris water maze test was performed as previously described with slight modifications [20]. A circular water tank (122 cm in diameter) was filled with water (22 °C ± 1 °C) made opaque through the addition of milk. The tank walls were marked with graphics placed approximately 1 m from the bottom of the sink. After a 60 s free swimming trial, the mice were trained in four trials (visual training phase) to find a visible platform (10 cm in diameter), placed 1 cm above the water surface. The starting locations were varied across tests. On the day after space training completion, mice were evaluated in a 60 s exploration trial in which the platform was removed. On the final day, mice underwent four trials in which the hidden platform was placed in the quadrant opposite to the original position (reversal learning). All sessions ended once the mouse touched or went up the platform or once 60 s had elapsed.
Open Field Test
The open field test assesses the mice tendency to explore novel objects and the environment. The open field arena (70 cm × 70 cm) was placed in the video acquisition area. The video camera and room light were adjusted as needed. A behavior analysis software was set up. Each mouse was placed near the wall of the open field and allowed to explore the new environment for a total of 10 min. The mouse’s movements were recorded through the video camera and the time spent in the center (35 × 35 cm) was analyzed (EthoVision XT 5.0). Data acquisition was performed automatically using the Image software.
Elevated Plus Maze
The EPM apparatus was an elevated two-arm plus maze, placed 40 cm above the floor. It consisted of two enclosed arms of the same size, each with two 15 cm high transparent walls, opposed to two open arms (5 × 30 cm) and an open platform. The EPM apparatus was placed in the video acquisition area, the camera image and room brightness were adjusted as needed and a software was set up. Each mouse was placed in the maze’s central square (5 × 5 cm) facing an enclosed arm and allowed to move freely for 5 min. The mouse’s movement was recorded through the camera and the time spent in the open arms was analyzed (EthoVision XT 5.0). Data acquisition was performed automatically using the Image software.
Dendritic Spine Morphology
For Golgi staining, the hippocampal DG regions of 3–6-month-old wild-type and dcf1NKO mice were immersed in Golgi-Kirks solution according to the Golgi program (Hito Golgi-Cox Optim StainTM Kit). Sections of 100 µm thickness were cut and mounted in bright-field z-series. The number of dendritic spines in each section was counted in at least 30 µm segments. All pictures and analyses were performed by an investigator who was blinded to the genotype.
Electron Microscopy
Adult mice were anesthetized with 2% pentobarbital sodium. The hippocampal DG region was quickly isolated in phosphate buffer, 1 mm3 of tissue was extracted with micro-tweezers and tissue blocks were fixed in 2.5% glutaraldehyde for 2 h, then washed with phosphate buffer and put in phosphate buffer containing 1% osmium tetroxide for 2 h at 4 °C. Subsequently, the brains were dehydrated and embedded in 618# resin. Ultrathin sections were cut with a Reichert ultramicrotome, then contrasted with uranyl acetate and lead citrate, and finally subjected to 1100 × examination under a FE1 5018/11 electron microscope. In electron micrographs, excitatory synapses were identified by the presence of dendritic spines containing a clear electron dense postsynaptic density, opposed to an asymmetric presynaptic compartment containing at least three synaptic vesicles. All measurements were performed by an investigator who was blinded to the genotype.
Electrophysiology
A MEA 2100 system (Multi Channel Systems, Reutlingen, Germany) was used to examine fEPSPs in hippocampal DG regions. Electrophysiological recordings were performed according to Chong et al. [21] and Balemans et al. [22]. For dissection and recording, 400 ml of ice-cold, artificial cerebrospinal fluid (ACSF; ice water mixture) and 1L of room temperature ACSF were prepared and saturated with 95% O2/5% CO2. Mice were anesthetized and decapitated. The brain was quickly removed in frozen ACSF and then placed in a vibratome chamber filled with ice-cold ACSF saturated with 95% O2/5% CO2. Coronal slices (250 µm) were cut at a 20 °C inclination and allowed to recover in oxygenated ACSF, at room temperature, for at least 2 h. After recovery, slices were transferred to a 200/30 MEA and continuously perfused with oxygenated ACSF (2 ml/min) at 31 °C. Stimulation electrodes were placed in hippocampal DG and evoked fEPSPs were monitored. Stimulus intensity was adjusted to produce fEPSPs at 40% of the maximal response. Paired-pulses were delivered three times with a 10 s interval to obtain average values. Test stimuli were delivered every 60 s with biphasic voltage pulses lasting 200 μs. After 30 min of recording to obtain a stable baseline, short-term potentiation was induced by two trains of high-frequency stimulation (HFS; 100 Hz) separated by a 20 s interval. Test stimuli were then delivered every 60 s with biphasic voltage pulses lasting 200 μs.
Western Blot
For the western blot assay, the hippocampal tissues of 3-month-old mice brains were isolated and homogenized in ice-cold radioimmune precipitation assay lysis buffer. The homogenates were centrifuged at 12,000×g revolutions per minute for 15 min at 4 °C, and the supernatants were collected for protein concentration using the BCA-100 protein assay kit. An equal concentration of protein (20 μg) from each sample was boiled for 10 min in 5 × sodium dodecyl sulfate buffer, then loaded onto 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. The membranes were blocked with 5% nonfat milk powder for 60 min at 37 °C, then incubated overnight at 4 °C with primary antibodies, including anti-β-actin (1:800, Santa Cruz), anti-GluR1 (1:800, ABclonal), anti-GluR2 (1:800,ABclonal), and anti-NR2A (1:800,ABclonal), anti-NR2B (1:800,ABclonal), anti-Gsr (1:800, ABclonal), anti-Glo1 (1:800, Santa Cruz).
Statistical Analysis
All data were analyzed using the GraphPad Prism software. Protein and mRNA expressions were analyzed by one-way Analysis of Variance (ANOVA). A level of P < 0.05 was considered statistically significant.
Results
Abnormal Hippocampal System in NKO Mice
Our previous study revealed an important role of Dcf1 in dendritic spine formation and memory [19]. Considering the specificity of the nervous system, we created an NKO mouse in which dcf1 was only deleted in the nervous system (Fig. 1a). RT-PCR was used to confirm the WT and NKO genotypes (Fig. 1b). First, we investigated whether the absence of Dcf1 in the nervous system would lead to neuronal changes. Immunofluorescence analysis revealed a significantly decreased number of astrocytes (GFAP) in the hippocampus, after Dcf1 deletion in the nervous system (Fig. 1c). Further, we investigated the expression level of the neurogenetic marker (DCX), to understand whether Dcf1 deletion in the nervous system leads to hippocampal neurogenesis defects. DCX immunofluorescence showed that new hippocampal neurons were mainly produced in the DG area, and that NKO mice showed a significantly decreased number of newborn neurons (Fig. 1d). We measured the distribution of mature neurons in the hippocampus using the mature neuron marker NeuN, and found that NKO mice presented a significantly increased number of neurons in hippocampal DG area 1 (Fig. 1e). Nissl bodies are chromophilic granules found in neurons’ dendritic cytoplasm. Upon tissue pretreatment with DNase, PI stained the cytoplasmic granules of neurons but not of glial cells. Neurons’ occurrence, distribution and pathological changes can be identified through Nissl body morphology [23, 24]. We found that, after Dcf1 deletion, hippocampal DG area 1 presented a decreased number of Nissl bodies and that their cell morphology was altered. This indicates the partial inhibition of neuronal protein synthesis (Fig. 1f). Although, in general, the number of GFAP and NeuN in the hippocampus was increased in NKO mice, neuronal morphology was severely damaged, suggesting that Dcf1 may affect neuron development.
Abnormal hippocampal neural system in NKO mice. a Strategy for construction of nervous system knockout (NKO) mice. Mice carrying LoxP on either side of the dcf1 gene Exon 1 were crossed with Nestin-Cre mice to generate nervous system-specific dcf1 Knockout mice (bottom). b RT-PCR analysis of WT and NKO mice, NKO mice are represented by the 435 bp band and WT mice are represented by the 400 bp band. c The number of GFAP-positive neurons was significantly decreased in NKO mice’ DG region. Scale bar 100 μm. d The number of DCX-positive neurons was increased in NKO mice’ DG region. Scale bar 100 μm. e The number of NeuN-positive neurons was significantly increased NKO mice’ DG region. Scale bar 100 μm. f Expression of the Nissl bodies, chromophilic granules found in the cytoplasm of neuron dendrites, in the hippocampus. F1, F2 Scale bar 100 μm. F3, F4, F5, F6 Scale bar 10 μm. 3 mice per group
Decreased Density of Dendritic Spines and Synapses in the Hippocampus of NKO Mice
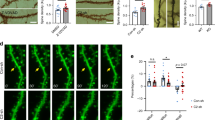
Do damaged neurons affect the development of dendritic spines and synapses? As a storage site of synaptic strength, dendritic spines help to convey electrical signals to neuronal bodies [25, 26]. The number and shape of dendritic spines affect synaptic plasticity and suggest a link to memory formation. In mice, dendritic spines play an important role not only in learning and memory, but also in the regulation of emotional behaviors, such as anxiety. The changes in dendritic spines and synapses observed in NKO mice were analyzed by Golgi staining and transmission electron microscopy (TEM). In both NKO and WT mice, dendritic spine changes were observed using a motorized microscope (Fig. 2a). Results showed that NKO mice presented significantly reduced dendritic spines in the hippocampal DG region (Fig. 2b, c). This was further confirmed through TEM observations (Fig. 2d, e). These findings indicate that Dcf1 deletion leads to changes in neuronal dendritic spines and synapses.
NKO mice presented decreased hippocampal dendritic spine and synapse density. a A sketch of Golgi staining of neurons in the Coronal hippocampal area. The red square signs the location of B. b, c Golgi staining of DG neurons (b) indicates a significant reduction in spine density (c). Scale bar 1 μm. (d and e) TEM ultrastructural analysis of hippocampal DG synapses. Scale bar 1 μm. Two-tailed t tests, *P < 0.05, **P < 0.01, ***P < 0.001; all data are presented as mean ± SEM; 4 mice per group (Color figure online)
Changes in Hippocampal AMPA and NMDA Receptors in NKO Mice
Do changes in dendritic spines and synapses influence AMPA and NMDA receptors? AMPA and NMDA receptors play an important role in synaptic plasticity [13, 14] and four NMDA and AMPA receptor subunits were detected in mice hippocampus: GluR1, GluR2, NR2A, NR2B. Our results revealed that all four proteins presented down-regulated expression, and that GluR1, and NR2B were significant reduced in the hippocampus of NKO mice compared to WT mice (Fig. 3a, b). Postsynaptic NMDA receptors are activated increasing calcium concentration and thereby triggering a series of intracellular signaling cascades, which leads to LTP expression through AMPAR phosphorylation and insertion into the postsynaptic membrane. Therefore, we further verified whether NMDA and AMPA receptors were physiologically changed. We looked into PPF (paired pulse facilitation) and found no significant changes in NKO mice compared to WT mice (Fig. 3c). We also performed a short-term potentiation test, which revealed that, following high-frequency stimulation, the amplitude was significantly higher in WT than NKO mice during the first 30 min compared with the basal level. The increased amplitude gradually recovered to the same level of NKO mice (Fig. 3d). These results suggest that Dcf1 deletion in the nervous system affects the AMPA and NMDA receptors, thereby influencing synaptic plasticity.
NKO mice presented changes in hippocampal synaptic plasticity. a, b Changes in APPA and NMDA receptor subunit proteins expression level. c Differences in DG PPT between NKO and WT mice. d Differences in DG LTP between NKO and WT mice. Two-tailed t tests, *P < 0.05, **P < 0.01, ***P < 0.001; all data are presented as mean ± SEM; 4 mice per group
NKO Mice Exhibit Impaired Memory and Anxious Behavior
The hippocampus is a key brain region for learning and memory, in which both NMDA and AMPA receptors play an important role. Does decreased NMDA and AMPA receptor expression cause behavioral changes? NKO mice took longer to locate the hidden platform in the Morris water maze, both in the first and third days of spatial training. Although this difference was not significant (Fig. 4a), in the reversal learning trial, NKO mice showed significantly lower memory than WT mice (Fig. 4b), suggesting that NKO mice have impaired memory. In the open field test, NKO mice remained mostly at the periphery, spending less time in the central area than WT mice (Fig. 4c). Similarly, in the elevated-plus maze, NKO mice spent less time in the open arms (Fig. 4d), which indicates a preference for less exposed environments. Consistently, in the light and dark test NKO mice spent longer in the dark area than WT mice (Fig. 4e). Taken together, these results indicate that NKO mice exhibit anxious behavior. Moreover, since Glyoxalase (Glo1) and Glutathione reductase (GSR) are important anxiety markers, we investigated their expression in the hippocampus and found that both Glo1 and GSR were significantly upregulated in the hippocampus of NKO mice (Fig. 4f, g). Our results show that changes in synaptic plasticity affect learning and anxiety in Dcf1 KO mice.
NKO mice exhibited memory deficit and anxiety behavior. a In the Morris water maze test, NKO mice took longer to locate the hidden platform on the first and third days of spatial training. b NKO mice showed significantly reduced reversal learning compared to WT mice. c In the open field, NKO mice spent less time in the center area than WT mice, although the difference was not significant. d In the elevated-plus maze, NKO mice spent significant less time in the open arms than WT mice. e In the light and dark test, NKO mice spent more time in the dark area than WT mice. f, g Changes in Glo1 and Gsr proteins expression level. Two-tailed t tests, #P < 0.1, *P < 0.05, **P < 0.01; all data are presented as mean ± SEM; n = 7 to 10 mice per group
Discussion
The present study aimed to fully understand the function of Dcf1, by investigating the anatomy, physiology and behavior of NKO mice. The results demonstrated changes in hippocampal dendritic spines and synaptic density and synaptic plasticity, which were associated with memory deficits. Our findings indicated for the first time that Dcf1 in the nervous system is critical for learning and memory. It also indicated that Dcf1 is an important regulatory factor for AMPA and NMDA in synaptic plasticity. Taken together, the results from the present study broadened our understanding of synaptic plasticity and expanded the known roles of synaptic plasticity in cognitive function.
In the present study, a Dcf1 neurological knockout mouse was constructed, to understand the molecular mechanism in detail. Results indicated that dcf1 affects memory and anxiety via NMDA and AMPA receptors regulation. The four behavioral experiments used in this study indicated that Dcf1 knockdown in the CNS influenced memory and anxiety, although the underlying mechanism was not clear. Dcf1 knockout decreased the number of Nissl bodies and changed cell morphology, which reflected the partial inhibition of neuronal protein synthesis. We hypothesized that such changes exerted an effect on dendritic spines and synapses. The number and shape of dendritic spines influence synaptic plasticity, which is related to memory formation [27]. In the present study, NKO mice presented significantly decreased dendritic spines and synapse density, as observed by Golgi staining and electron microscopy. Since dendritic spines and synapses are important for the formation of AMPA and NMDA receptors, these changes were likely to affect NKO mice’ AMPA and NMDA receptors. We therefore evaluated the expression of four NMDA and AMPA receptor family proteins GluR1, GluR2, NR2A and NR2B in the hippocampus of KNO mice and found that all four proteins were down-regulated compared with WT mice. Therefore, we further verified whether NMDA and AMPA receptors were physiologically changed. Electrophysiological experiments revealed decreased short-term potentiation in NKO mice, suggesting that Dcf1 neuronal knockout affects the AMPA and NMDA receptors, thereby influencing memory and anxiety. Our findings provide new insights into Dcf1-mediated neural behavior and information relevant for treating memory loss.
References
Malenka RC, Nicoll RA (1999) Long-term potentiation—a decade of progress? Science 285(5435):1870–1874
Muller D et al (2002) LTP, memory and structural plasticity. Curr Mol Med 2(7):605–611
Cooke SF, Bliss TV (2006) Plasticity in the human central nervous system. Brain 129(Pt 7):1659–1673
Matsuzaki M et al (2004) Structural basis of long-term potentiation in single dendritic spines. Nature 429(6993):761–766
Kirkwood A, Bear MF (1994) Hebbian synapses in visual cortex. J Neurosci 14(3 Pt 2):1634–1645
Myhrer T (2003) Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Res Brain Res Rev 41(2–3):268–287
Cormier RJ, Greenwood AC, Connor JA (2001) Bidirectional synaptic plasticity correlated with the magnitude of dendritic calcium transients above a threshold. J Neurophysiol 85(1):399–406
Baudry M et al (2015) Multiple cellular cascades participate in long-term potentiation and in hippocampus-dependent learning. Brain Res 1621:73–81
Traynelis SF et al (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62(3):405–496
Demidchik V et al (2018) Calcium transport across plant membranes: mechanisms and functions. New Phytol 220(1):49–69
Collingridge GL et al (2013) The NMDA receptor as a target for cognitive enhancement. Neuropharmacology 64:13–26
Zhu S, Paoletti P (2015) Allosteric modulators of NMDA receptors: multiple sites and mechanisms. Curr Opin Pharmacol 20:14–23
Nowak L et al (1984) Magnesium gates glutamate-activated channels in mouse central neurones. Nature 307(5950):462–465
Henley JM, Barker EA, Glebov OO (2011) Routes, destinations and delays: recent advances in AMPA receptor trafficking. Trends Neurosci 34(5):258–268
Wen T, Gu P, Chen F (2002) Discovery of two novel functional genes from differentiation of neural stem cells in the striatum of the fetal rat. Neurosci Lett 329(1):101–105
Wang L et al (2008) A novel function of dcf1 during the differentiation of neural stem cells in vitro. Cell Mol Neurobiol 28(6):887–894
Li X et al (2012) MicroRNA-351 regulates TMEM 59 (DCF1) expression and mediates neural stem cell morphogenesis. RNA Biol 9(3):292–301
Boada-Romero E et al (2013) TMEM59 defines a novel ATG16L1-binding motif that promotes local activation of LC3. EMBO J 32(4):566–582
Liu Q et al (2018) Dcf1 triggers dendritic spine formation and facilitates memory acquisition. Mol Neurobiol 55(1):763–775
Wang XD et al (2011) Forebrain CRF(1) modulates early-life stress-programmed cognitive deficits. J Neurosci 31(38):13625–13634
Chong SA et al (2011) Synaptic dysfunction in hippocampus of transgenic mouse models of Alzheimer's disease: a multi-electrode array study. Neurobiol Dis 44(3):284–291
Balemans MC et al (2013) Hippocampal dysfunction in the Euchromatin histone methyltransferase 1 heterozygous knockout mouse model for Kleefstra syndrome. Hum Mol Genet 22(5):852–866
Pullen AH (1990) Morphometric evidence from C-synapses for phased Nissl body response in alpha-motoneurones retrogradely intoxicated with diphtheria toxin. Brain Res 509(1):8–16
Niu J et al (2015) Propidium iodide (PI) stains Nissl bodies and may serve as a quick marker for total neuronal cell count. Acta Histochem 117(2):182–187
Banerjee J, Fischer CC, Wedegaertner PB (2009) The amino acid motif L/IIxxFE defines a novel actin-binding sequence in PDZ-RhoGEF. Biochemistry 48(33):8032–8043
Tschida KA, Mooney R (2012) Deafening drives cell-type-specific changes to dendritic spines in a sensorimotor nucleus important to learned vocalizations. Neuron 73(5):1028–1039
Gipson CD, Olive MF (2017) Structural and functional plasticity of dendritic spines - root or result of behavior? Genes Brain Behav 16(1):101–117
Acknowledgements
This work was supported by the National Science Foundation of China (Grant Nos. 81271253, and 81471162), the Science and Technology Commission of Shanghai (Grant No. 14JC1402400), and the Key Innovation Project of Shanghai Municipal Education Commission (Grant No. 14ZZ090).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Liu, Q., Xie, J. et al. Dcf1 Affects Memory and Anxiety by Regulating NMDA and AMPA Receptors. Neurochem Res 44, 2499–2505 (2019). https://doi.org/10.1007/s11064-019-02866-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02866-6