Abstract
CX3CL1 (fractalkine), the sole member of chemokine CX3C family, is implicated in inflammatory and neuropathic pain via activating its receptor CX3CR1 on neural cells in spinal cord. However, it has not been fully elucidated whether CX3CL1 or CX3CR1 contributes to the development of morphine tolerance. In this study, we found that chronic morphine exposure did not alter the expressions of CX3CL1 and CX3CR1 in spinal cord. And neither exogenous CX3CL1 nor CX3CR1 inhibitor could affect the development of morphine tolerance. The cellular localizations of spinal CX3CL1 and CX3CR1 changed from neuron and microglia, respectively, to all the neural cells during the development of morphine tolerance. A microarray profiling revealed that 15 members of chemokine family excluding CX3CL1 and CX3CR1 were up-regulated in morphine-treated rats. Our study provides evidence that spinal CX3CL1 and CX3CR1 may not be involved in the development of morphine tolerance directly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morphine is the most important and frequently used opioid for acute and chronic pain in clinical practice. However, repeated usage of morphine can induce drug tolerance, a consequence requiring higher doses of morphine to maintain the same analgesic effect. Dose escalation of morphine could potentially cause serious side effects including respiratory depression [1], hypotension, nausea, constipation, dizziness and addiction [2]. Morphine tolerance could hinder the clinical utilization of morphine and impair the quality of life in patients. Thereby, understanding the mechanism of morphine tolerance is critical for improving pain management.
Chemokines play the pivotal roles in neuroinflammation, nerve injury-induced pain [3,4,5] and morphine analgesia [6,7,8]. CX3CL1 (fractalkine) is the only member of chemokine CX3C family [9] and activates its sole receptor CX3CR1. Previous studies have demonstrated that intrathecal injection of CX3CR1 neutralizing antibody can effectively delay the development of mechanical allodynia and thermal hyperalgesia in neuropathic pain, inflammatory pain and cancer pain [3, 10, 11]. In addition, CX3CL1 has been reported to be involved in diminishing the analgesic effect of opioids in periaqueductal grey [12]. Thus, CX3CL1 plays an important role in the mechanisms of chronic pain.
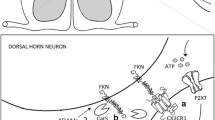
In central nervous system, microglia are generally regarded as the source and target of chemokines [13]. Microglia can regulate and receive chemokine signal between astrocyte and neuron through autocrine and paracrine communications, thus contributing to the development of neuropathic pain [14] and traumatic brain injury [15]. Although it is clear that microglia activation is involved in morphine tolerance [16, 17], the potential signals that causing glial activation have not been well understood. Morphine tolerance and chronic pain may share the similar cellular mechanisms. Previous study reported that CX3CL1 is mainly released by neurons and its receptor CX3CR1 is primarily expressed on microglia [18]. As CX3CL1 plays an important role in chronic pain, these results indicate a potential involvement of CX3CL1/CX3CR1 signaling axis in the activation of microglia, and thus the mechanism of morphine tolerance. Thereby, the present study was designed to investigate the possible roles of spinal CX3CL1/CX3CR1 in the development of morphine tolerance in rats.
Materials and Methods
Animals
Specific pathogen free adult male Sprague–Dawley rats, weighing 220–240 g, were purchased from Laboratory Animal Center, Tongji Medical College, Huazhong University of Science & Technology. Animals were housed under controlled conditions (22 ± 0.5 °C, relative humidity 40–60%, alternate light–dark cycles, food and water ad libitum). To keep the integrity of catheter, rats were housed individually after surgery. All experimental procedures and protocols were reviewed and approved by Experimental Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science & Technology, and carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Intrathecal Catheterization
Lumbosacral indwelling catheters were constructed and planted using a lumbar approach, as described previously [19]. Briefly, rats were deeply anesthetized with 1% pentobarbital sodium [60 mg/kg, intraperitoneal injection (i.p.)]. The lumbar region of rat was shaved, and intrathecal catheterization was performed by implanting a sterile PE-0503 catheter (outer diameter 0.5 mm, inner diameter 0.3 mm. Anilab Software & Instruments, Ningbo, China) into subarachnoid cavity between L4 and L5 vertebrae. The catheter was subcutaneously tunneled, externalized and fixed to the back of neck. Wounds were sutured after disinfection with 75% (v/v) ethanol. Proper location of catheter was confirmed by a temporary motor block of both hind limbs after intrathecal injection of 10 μL of 2% lidocaine. The rats were allowed a 7-day recovery period before the following experiments. Rats with hind limb paralysis or paresis after surgery were excluded and euthanized with overdose of pentobarbital sodium.
Drugs Administration
The drugs used in this study were prepared as follows. Morphine hydrochloride was diluted by saline (North-East Pharmaceutical Group, China). Recombinant rat CX3CL1 protein (rrCX3CL1, R&D, Minneapolis, MN, USA) was dissolved in saline and injected intrathecally (100 or 500 ng). Rabbit anti-rat CX3CR1 (Torrey Pine Biolabs, East Orange, NJ, USA) and placebo rabbit IgG (Sigma, St. Louis, MO, USA) were diluted by saline and administered intrathecally (5 or 10 μg). All drugs or vehicle solutions were injected 30 min before morphine administration in a volume of 5 μL followed by 10 μL of saline to flush the catheter. All the doses of each drug used in this study were determined according to the previous experiments [3, 12, 20].
Morphine Tolerance
To induce chronic tolerance to morphine, rats were intrathecally injected with morphine (10 μg) twice daily for 7 days. Rats in the control group received an equivalent volume of saline at the same time points. The development of morphine tolerance was assessed by behavioral tests on day 1, 3, 5 and 7 [21].
Behavioral Assessment
Thermal pain thresholds in rats were measured by a tail-flick latency test [22] before drug administration and at 30 min after morphine administration on day 1, 3, 5, and 7 [21]. Briefly, rats were placed in plastic containers to hold the body without restraining the head and tail, and one-third to the tip of tail was immersed into water. The temperature of water was adjusted to 50 ± 0.2 °C because this was the proper temperature to record an average tail-flick latency of 2–4 s in naïve rats. A cutoff time of 15 s was determined to prevent tail damage. The test was repeated three times and the mean of three trials was considered as the final latency. The percentage of maximal possible antinociceptive effect (%MPE) was calculated by comparing the test latency before (baseline, BL) and after drug injection (TL) using following equation: %MPE = [(TL − BL)/(cutoff time − BL)] × 100. All behavioral tests were carried out under blind conditions.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Under deep anesthesia with 1% pentobarbital sodium, L1–L5 spinal cord segments of rats were quickly removed on day 7. Total RNA was extracted from tissue sample and the reverse transcription procedure was performed by using RNAiso Plus (Takara, Shiga, Japan) according to the manufacturer’s instructions. One microgram of total RNA from each sample was added into 20 μL reactive solution of reverse transcription, respectively. Specific primers for rat CX3CL1, CX3CR1, mu opioid receptor (MOR) and endogenous control rat GAPDH were obtained from GeneCopoeia Company (USA). The catalogs of each primer were as following: CX3CL1 (RQP052632), CX3CR1 (RQP052471), MOR (RQP048316), GAPDH (RQP049537). StepOne Real-Time PCR System (Applied Biosystems, USA) was used to conduct the qRT-PCR. Relative quantification of mRNA was performed by 2−∆∆Ct method.
Western Blots
L1–L5 spinal cord segments of all animals were quickly removed and dissected. Total protein of spinal cord tissue from each group was extracted by using radio immunoprecipitation assay lysis buffer according to the manufacturer’s instructions (Beyotime, Wuhan, China). The protein concentration of supernatants was measured by using bicinchoninic acid assay. 50 μg protein from each sample was loaded on 10% SDS-PAGE gel after boiling in the sample buffer. Electrophoresis was conducted at 60 V constant voltage for stacking gel and 100 V for separating gel. The proteins were subsequently electro-transferred (200 mA, 60–90 min) to a PVDF membrane (Millipore, Billerica, MA, USA). The membrane was blocked with 5% BSA (Bovine Serum Albumin) for 1 h at room temperature followed by incubating with the following primary antibodies: mouse anti-ionized calcium-binding adapter molecule 1 (Iba1) antibody (1:200, Santa Cruz, Dallas, TX, USA), goat anti-CX3CL1 antibody (1:50, R&D, Minneapolis, MN, USA), rabbit anti-CX3CR1 antibody (1:1000, Abcam, Cambridge, MA, USA), mouse anti-MOR antibody (1:500, R&D, Minneapolis, MN, USA), or rabbit anti-GAPDH (1:2500, Aspen, Wuhan, China) overnight at 4 °C. After being thoroughly washed, the membrane was incubated with HRP-conjugated rabbit anti-goat IgG (1:5000, EarthOx, Millbrae, CA, USA), HRP-conjugated goat anti-rabbit IgG (1:4000, Aspen, Wuhan, China), or HRP-conjugated goat anti-mouse IgG (1:1000, Bioyeartech, Wuhan, China) for 2 h at room temperature. Finally, proteins were detected by ECL reagents (Beyotime, Wuhan, China) and visualized by exposing to X-ray film. The ImageJ analysis system (NIH, Bethesda, MD) was used for the quantification of specific bands. The levels of Iba-1, CX3CL1, CX3CR1 and MOR were exhibited as density relative to the density of GAPDH.
Double Immunofluorescent Staining
After being treated with morphine or saline for 7 days, rats were perfused with saline, followed by 4% ice-cold paraformaldehyde (PFA) in 0.1 M phosphate buffer saline (PBS) under deep anesthesia with pentobarbital sodium. The L1–L5 spinal cord segments were removed and post-fixed for 24 h at 4 °C, then dehydrated in 30% sucrose solution. After being treated with 0.3% Triton X-100 and blocked with 10% donkey serum for 40 min at room temperature, 25 µm-thick sections were incubated overnight at 4 °C with mixtures of the following primary antibodies: goat anti-CX3CL1 antibody (1:25, R&D, Minneapolis, MN, USA) or rabbit anti-CX3CR1 antibody (1:200, Abcam, Cambridge, MA, USA) and mouse anti-NeuN (1:200, Millipore, Billerica, MA, USA), mouse anti-GFAP (1:200, CST, Beverly, MA, USA), goat anti-Iba1 (1:100, Abcam, Cambridge, MA, USA) or rabbit anti-Iba1 (1:200, Wako, Osaka, Japan). Then sections were incubated with mixtures of the following secondary antibodies: Cy3-conjugated donkey anti-goat IgG (1:300, Proteintech, Wuhan, China) and FITC-conjugated donkey anti-mouse IgG (1:100, Proteintech, Wuhan, China) or FITC-conjugated donkey anti-rabbit IgG (1:100, Proteintech, Wuhan, China), or IFKine Red labeled donkey anti-rabbit IgG (1:500, Abbkine, Redlands, CA, USA) and FITC-conjugated donkey anti-mouse IgG (1:100, Proteintech, Wuhan, China) or FITC-conjugated donkey anti-goat IgG (1:100, Proteintech, Wuhan, China) for 2 h at room temperature and stained with 4,6-diamidino-2-phenylindole (DAPI, Boster, Wuhan, China) for 10 min. The stained sections were examined by using Fluorescence Microscope (DM2500, Leica, German) to capture the fluorescent images. Five spinal sections were selected randomly for each rat and the immunoreactivities of CX3CL1 and CX3CR1 were counted in a blinded fashion [23]. The stained sections were analyzed by Image Pro Plus 4 software (Media Cybernetics, Maryland, MD, USA).
Microarray mRNA Profiling
Gene expression profile of spinal cord tissues was established by using Affymetrix Rat Genome 230 2.0 Arrays. L1–L5 spinal cord segments of morphine-treated or saline-treated rats were isolated on day 7 and RNAlater RNA Stabilization Reagent (Qiagen, Germany) was used for stabilization of RNA in tissue samples. Total RNA isolation was performed with TRIzol reagent (Invitrogen, USA) and NucleoSpin® RNA Clean-up (MACHEREY-NAGEL, Germany). The cRNA was generated and labeled by one-cycle target labeling method. Affymetrix Rat Genome 230 2.0 microarray (CapitalBio Corporation, Beijing, China) which contains 31,000 probe sets including 65 probe sets of chemokine family was used to screen the differential expressions of chemokines. The acceptance criteria for RNA quality were 260/280 ratio ≥1.80 and RNA integrity number ≥8.0. The cRNA generated from each sample was hybridized to a single array according to standard Affymetrix protocols. Initial image analysis of microarray chips was performed using the Genechip® Command Console® Software. Data were exported to Significance Analysis of Microarrays software for screening differentially expressed genes. The screening criterion was set as fold change ≥2 or fold change ≤0.5 with false discovery rate (FDR) q-value ≤0.05.
Statistical Analysis
Animal sample size for behavioral experiment was decided by power analysis using SSize2021 software (National University of Singapore, Singapore) (version 2). With anticipated population proportion P 1 = 0.95, P 2 = 0.05, significance level 0.05 and power of test 0.09, the sample size was estimated to be four per group. All data were presented as mean ± SEM. Behavioral test was analyzed by two-way repeated measure ANOVA (treatment group × time) to detect overall differences among treatment groups followed by Bonferroni’s test to detect the changes to %MPE after drug injection over time. The results of qRT-PCR and western blots were analyzed by one-way ANOVA. Individual comparisons were conducted with unpaired t test. Statistical analyses were performed with GraphPad Prism 5 (GraphPad Software Inc.) with statistical significance set at P < 0.05.
Results
Chronic Morphine Treatment Induced Drug Tolerance and Activated Microglia
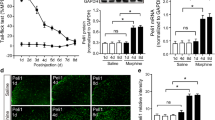
Rats were intrathecally administered with morphine (10 μg/5 μL) or saline (5 μL) twice daily for consecutive 7 days. Behavioral tests were conducted before drug administration and at 30 min after the last drug administration on day 1, 3, 5, and 7. As shown in Fig. 1a, rats received morphine exhibited significantly higher %MPE compared with saline-treated rats on day 1 (P < 0.001) and day 3 (P < 0.01). On day 5, there was no significant difference of %MPE levels between morphine-treated and saline-treated rats (P > 0.05), suggesting that chronic morphine tolerance had been successfully established. Iba-1 is the marker of activated microglia. On day 7, the increased expression of Iba-1 was detected in spinal cord (Fig. 1b), indicating the activation of microglia induced by chronic morphine exposure. Previous study has demonstrated that chronic morphine treatment significantly decreases the expression of MOR in hypothalamus but not in locus ceruleus and nucleus accumbens [24], suggesting that the cellular adaptation for morphine is tissue-specific. In this study, we did not detect any changes in MOR expression in spinal cord of morphine-treated rats compared to that in saline-treated rats on day 7 (Fig. 1c, d).
Expressions of MOR and Iba-1 in lumbar spinal cord of rats. a Thermal pain threshold of rats was assessed using the percentage of maximal possible antinociceptive effect (%MPE) according to the tail-flick latency of rats. The %MPE in rats received morphine (10 μg, twice daily, intrathecally) on day 5 and 7 were dramatically decreased compared with the baseline on day 1. Values represent mean ± SEM; two-way ANOVA, **P < 0.01, ***P < 0.001, vs. naïve and sham, n = 6 in each group. b The expression of Iba-1 protein was significantly increased in morphine-treated rats measured by Western blots. Values represent mean ± SEM; ANOVA, *P < 0.05 vs. naïve and sham, n = 6 in each group. c, d The expressions of MOR mRNA (c) and protein (d) were not affected by morphine treatment measured by real-time PCR and Western blots, respectively. Values represent mean ± SEM; ANOVA, *P < 0.05 vs. naïve and sham, n = 6 in each group
Chronic Morphine Treatment Did Not Affect the Expressions of CX3CL1 and CX3CR1
Previous studies have demonstrated that CX3CL1 and its receptor could play the important roles in antinociceptive effects of opioid agonists in periaqueductal grey [12, 25]. To determinate whether CX3CL1 and CX3CR1 participate in the development of morphine tolerance, the expressions of CX3CL1 and CX3CR1 in spinal cord of rats were examined on day 7 of morphine administration. As shown in Fig. 2, neither the levels of CX3CL1 mRNA (F 2,15 = 0.901, P = 0.427) and CX3CR1 mRNA (F 2,15 = 1.314, P = 0.298), nor expressions of CX3CL1 protein (F 2,12 = 0.999, P = 0.397) and CX3CR1 protein (F 2,21 = 0.833, P = 0.449) was affected by intrathecal administration of morphine when compared to saline-treated rats.
Expressions of CX3CL1 and CX3CR1 in lumbar spinal cord of rats. a The expressions of CX3CL1 and CX3CR1 mRNA were not affected by morphine treatment measured by real-time PCR. Values represent mean ± SEM; ANOVA, *P < 0.05 vs. naïve and sham, n = 6 in each group. b, c The expressions of CX3CR1 (b) and CX3CL1 (c) protein were not affected by morphine treatment measured by Western blots. Values represent mean ± SEM; ANOVA, *P < 0.05 vs. naïve and sham, n = 8 in each group for CX3CR1, n = 5 in each group for CX3CL1
Exogenous CX3CL1 or CX3CR1 Inhibitor Had No Effect on Behavioral Responses During the Development of Morphine Tolerance
There was no significant difference in baseline levels of tail-flick latency measured prior to drug administration among all groups, indicating that intrathecal catheterization did not affect behavioral responses of rats (Fig. 3a). To further investigate the roles of CX3CL1 and CX3CR1 in the development of morphine tolerance, recombinant rat CX3CL1 (100 or 500 ng), anti-CX3CR1 neutralizing antibody (5 or 10 μg) or control IgG (100 or 5 μg) was intrathecally injected 30 min before morphine administration, respectively. As shown in Fig. 3b, neither 100 nor 500 ng rrCX3CL1 exhibited statistically significant effect on %MPE in rats treated with morphine compared with that in IgG-morphine treated rats (F 2,45 = 1.498, P = 0.255 for 100 ng rrCX3CL1; F 2,45 = 0.903, P = 0.426 for 500 ng rrCX3CL1). Moreover, interaction between rrCX3CL1 treatment and time was not considered significantly (F 9,60 = 0.527, P = 0.849). As shown in Fig. 3c, there was no significant effect of 5 or 10 μg anti-CX3CR1 neutralizing antibody on %MPE in rats treated with morphine when compared with that in IgG-morphine treated rats (F 2,45 = 0.905, P = 0.426 for 5 μg; F 2,45 = 1.107, P = 0.356 for 10 μg). There was no significant interaction between anti-CX3CR1 neutralizing antibody treatment and time (F 9,60 = 1.770, P = 0.093). These results suggest that both exogenous CX3CL1 stimulation and CX3CR1 inhibition could not markedly affect the development of morphine tolerance that assessed by tail flick test.
Effects of recombinant rat CX3CL1 and anti-CX3CR1 neutralizing antibody on the development of morphine tolerance. a Tail flick latency of rats in each group did not change after intrathecal catheterization. b, c. Recombinant rat CX3CL1 (100 or 500 ng) (b), anti-CX3CR1 neutralizing antibody (5 or 10 μg) (c) or normal IgG (100 ng for rrCX3CL1 group, 5 μg for anti-CX3CR1 neutralizing antibody group as control dose.) was intrathecally administered 30 min before morphine treatment for 7 days. None of them significantly affected morphine antinociception or alleviated the development of morphine tolerance. Values represent mean ± SEM; two-way ANOVA, ***P < 0.001, *P < 0.05 vs. naïve and sham, n = 6 in each group
Exogenous CX3CL1 or CX3CR1 Inhibitor Had No Influence on the Activation of Microglia Induced by Morphine
In order to further clarify the roles of CX3CL1 and CX3CR1, exogenous CX3CL1 (100 or 500 ng) or CX3CR1 inhibitor (5 or 10 μg) was intrathecally administered respectively and their effects on the activation of spinal microglia in morphine tolerant rats were assessed. As shown in Fig. 4, the expressions of Iba-1 were significantly increased in all morphine-treated rats. Neither rrCX3CL1 nor anti-CX3CR1 neutralizing antibody had statistically significant effect on morphine-induced expressions of Iba-1 (F 5,12 = 0.138, P = 0.980).
Effects of exogenous CX3CL1 and CX3CR1 inhibitor on the microglia activation with repeated morphine administration for 7 consecutive days. Exogenous CX3CL1 (100 or 500 ng), CX3CR1 inhibitor (5 or 10 μg) and IgG (5 μg) were administered 30 min before morphine treatment for 7 day, respectively. Microglia activity was increased significantly in morphine involved groups. However, both exogenous CX3CL1 and CX3CR1 inhibitor showed no influence in quantification of Iba-1 levels from Western blots compared to morphine treated rats. Values represent mean ± SEM; one-way ANOVA, *P < 0.05 vs. sham, n = 3 in each group
Cellular Localizations of Spinal CX3CL1 and CX3CR1 in Morphine Tolerant Rats
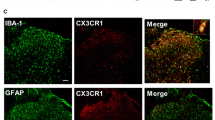
The communication between neurons and glia mediated by CX3CL1 and CX3CR1 contributes to the mechanisms of inflammatory and neuropathic pain [3, 26, 27]. The changes of cellular distribution of CX3CL1 and CX3CR1 in spinal cord have also been reported to be associated with pain conditions [18]. In this study, we examined the cellular localizations of CX3CL1 and CX3CR1 in rat spinal dorsal horn. The results showed that CX3CL1 expression was extensively distributed to all layers of spinal dorsal horn, and CX3CR1 was mainly expressed in lamina I to lamina III of spinal cord (Fig. 5a). There was no significant difference in the expression of CX3CL1 or CX3CR1 between morphine-treated rats and saline-treated rats (Fig. 5b). In saline-treated rats, the immunoreactivity of CX3CL1 was co-localized with neuronal marker NeuN, while CX3CR1 was co-localized with microglia marker Iba-1 (Fig. 5c). However, both CX3CL1 and CX3CR1 were found to be co-localized with NeuN, GFAP and Iba-1 in morphine-treated rats (Fig. 5d, e). These indicate the shift of CX3CL1/CX3CR1 expressions occurred during the development of morphine tolerance.
Changes in the cellular localizations of CX3CL1 and CX3CR1 during the development of morphine tolerance. a, b Expressions of CX3CL1 and CX3CR1 in the spinal cord. There were no changes in the number of CX3CL1 (a, b) and CX3CR1 (c, d) immunoreactive cells in spinal dorsal horn of morphine-tolerant rats compared with those in sham rats (n = 3, scale bar 200 μm). c Double immunostaining of CX3CL1 (a, b, c) or CX3CR1 (d, e, f) and cell-specific markers in the spinal cord in saline-treated rats. CX3CL1 was co-localized with NeuN and CX3CR1 was co-localized with Iba-1. a CX3CL1 and NeuN; b CX3CL1 and GFAP; c CX3CL1 and Iba-1; d CX3CR1 and NeuN; e CX3CR1 and GFAP; f CX3CR1 and Iba-1. Scale bar 200 μm. d Double immunostaining of CX3CL1 and cell-specific markers in morphine-treated rats. CX3CL1 was co-localized with NeuN, GFAP and Iba-1 (indicated by arrows). a, e and i CX3CL1; b NeuN; f GFAP; j Iba-1; c, d CX3CL1 merged with NeuN; g, h CX3CL1 merged with GFAP; k, l CX3CL1 merged with Iba-1. Scale bars 200 μm (a, b, c, e, f, g, i, j and k); scale bar 100 μm (d, h and l). e Double immunostaining of CX3CR1 and cell-specific markers in morphine-treated rats. CX3CR1 was co-localized with NeuN, GFAP and Iba-1 (indicated by arrows). a, e and i CX3CR1; b NeuN; f GFAP; j Iba-1; c, d CX3CR1 merged with NeuN; g, h CX3CR1 merged with GFAP; k, l CX3CR1 merged with Iba-1. Scale bar 200 μm (a, b, c, e, f, g, i, j and k); scale bar 100 μm (d, h and l)
The mRNA Expression Profiling Screened Spinal Chemokines Related to Morphine Tolerance
To screen the possible chemokines which might be involved in the development of morphine tolerance in spinal cord, mRNA of L1–L5 lumbar spinal cord of respective animal was analyzed using microarrays which contains 65 probe sets of chemokines. As shown in Fig. 6, expressions of 15 chemokines were identified to be upregulated in morphine-treated rats when compared with saline-treated rats. All the upregulated genes of chemokine were listed in Table 1. However, the changes of CX3CL1 and CX3CR1 expressions were not detected by microarray analysis.
Heatmap of expression ratios of chemokines family mRNAs. The probe sets that expressions were changed in morphine tolerance rats were identified by microarray analysis. Probe sets with similar expression profiles were clustered together using a Pearson’s correlation-based method with Cluster 3.0 and TreeView software. The expression level of each chemokine probe set was displayed as a log2 ratio of their expression values divided by their expression values in sham rats
Discussion
The results of our study showed that chronic morphine treatment can induce antinociceptive tolerance, but did not affect the expressions of CX3CL1 and its receptor CX3CR1 in spinal cord. Neither intrathecal administration of exogenous CX3CL1 nor CX3CR1 inhibitor affected the development of morphine tolerance. However, morphine treatment could influence the cellular localization of CX3CL1 and CX3CR1 in spinal dorsal horn in rats.
Various signaling pathways have been found to be involved in the mechanism of opioid tolerance. Opioid tolerance could be prevented, attenuated or reversed by inhibiting proinflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α) [28, 29]; blocking the activations of extracellular regulated protein kinases (ERK) and p38 protein [30,31,32]; increasing glial glutamate transporter-1 (GLT-1) and glutamate-aspartate transporters (GLAST) [33, 34]. Recently, toll-like receptor 4 (TLR4) has been reported to participate in the development of opioid tolerance via increasing tumor necrosis factor and IL-1β expressions and downregulating the expressions of GLT-1 and GLAST [35, 36]. Opioid tolerance could be considered as a drug-specific side effect. The mechanisms of drug tolerance induced by different opioids may be distinct. The internalization of MOR, which is the typical feature of opioid tolerance, highly depends on the type of agonist [37]. Endogenous opioids as well as synthetic peptide DAMGO ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin) promote the rapid endocytosis of MOR, but the highly addictive opioid, such as morphine, fails to induce detectable endocytosis [38, 39]. Changes in MOR expression in response to chronic opioid treatment have long been speculated to directly contribute to the development of opioid tolerance. Following chronic treatment with various agonists, the expression of opioid receptor in brain tissue is either increased, decreased or unchanged, indicating that the regulation of opioid receptor expression depends on the type of opioid [40], agonist [41, 42], and the region of brain [26, 43]. In addition, opioid tolerance is not only due to the rapid decrease of receptor activity but also the compensatory mechanism counteracting the function of opioid receptor [44,45,46]. Therefore, it is reasonable to comprehend the expression of MOR in spinal cord was unchanged in our study during the development of morphine tolerance.
Previous studies have shown that CX3CL1/CX3CR1 signaling axis participate in numerous physiological and pathological processes, including neuropathic pain [26], maturation of synaptic connection [47, 48], neuronal survival [49], insulin secretion [50] and atherosclerosis [51]. However, our results revealed the unchanged expressions of CX3CL1 and CX3CR1 in spinal cord of morphine tolerant rats. Recently study in opioid tolerant patients also showed that the concentration of CX3CL1 in cerebrospinal fluid is not significantly different from that in naïve-control patients [6]. Although previous study reported that intrathecal administration of 30 ng exogenous CX3CL1 could induce the behavioral effects such as mechanical allodynia and thermal hyperalgesia [4], the development of morphine tolerance was not affected by much higher doses of exogenous CX3CL1 in our study. Previous study showed that intrathecal administration of 3 μg anti-CX3CR1 neutralizing antibody could effectively inhibit monoarthritis-induced mechanical allodynia and thermal hyperalgesia [3], which illustrates that the doses of anti-CX3CR1 neutralizing antibody used in our study (5 and 10 μg) should be sufficient to block the function of CX3CR1 in spinal cord. However, in our study, intrathecal injection of CX3CR1 inhibitor did not affect either the antinociceptive effect of morphine or the development of morphine tolerance. In contrast, Johnston and colleagues reported that intrathecal injection of anti-CX3CR1 neutralizing antibody could attenuate the development of morphine tolerance [20]. This discrepancy might be due to the different experimental protocols including the evaluation of pain threshold. In Johnston’s study, tail flick latencies were recorded every 20 min for 2 h after morphine infusions to calculate the average response over this time on day 1 and day 5. Their behavioral assessment protocol is quite different from ours which has been most commonly used in the previous studies [21, 22, 37]. The analgesic effect of morphine occurs at 5 min after intrathecal injection, lasts for about 60 min, and dissipates by 100 min [29]. Choosing the average value of tail flick latencies over 2 h as the pain threshold of morphine-treated rats may fail to represent the maximum analgesic potency of morphine. Taken together, CX3CL1 and its receptor CX3CR1 in spinal cord may not participate in the mechanism of morphine tolerance directly. Although sufficient dosages of exogenous CX3CL1 and anti-CX3CR1 neutralizing antibody were used in our study, we could not definitively exclude the possibility that CX3CL1/CX3CR1 signaling play a positive role in the mechanism of morphine tolerance yet. CX3CL1 or CX3CR1 knockout animals might be the ideal option to verify the role of CX3CL1/CX3CR1 in morphine tolerance.
Under physiological conditions, expression of CX3CL1 in spinal cord appears to be restricted to neurons, whereas CX3CR1 in microglia [18]. However, CX3CL1 could be detected not only in neurons but also in astrocytes after spinal nerve ligation [10], suggesting that the distribution of CX3CL1 and CX3CR1 may depend on the diverse pathological processes. Both CX3CR1 and opioid receptors are members of G protein coupled receptor family. The formation of heterodimer among G protein coupled receptors are common. Previous study has found the MOR-CX3CR1 co-localization on neurons in several brain regions, including nucleus accumbens, ventral tegmental area and periaqueductal gray and MOR-CX3CR1 heterologous desensitization has been proved in periaqueductal gray [25]. We found that the cellular localizations of spinal CX3CL1 and CX3CR1 changed from neuron and microglia, respectively, to all the neural cells after chronic morphine administration. These findings support the possibility that morphine treatment may stimulate the cleavage of CX3CL1 in neurons [26] and promote the combination of CX3CL1 with its receptor on glia. It has been reported that simultaneous activations of delta opioid receptor (DOR) and CXCR4 on human peripheral blood mononuclear cells could promote the formation of non-functional DOR-CXCR4 heterodimers which are unable to respond to the agonists [52]. Therefore, we speculate that the newly expressed CX3CR1 on neuron may bind to MOR to form into the heterodimer, which at least partly contribute to morphine analgesia or tolerance. Further studies are still needed to explore the potential interaction between CX3CR1 and opioid receptor in the mechanism of morphine tolerance.
Chemokines and opioids are effective regulators of immune, inflammatory and neuronal responses in pain mechanism in central nervous system. Several chemokines could increase the neuronal excitability and subsequently decrease opioid analgesic efficacy, which may act as the key neuromodulators of pain pathways [53]. Administration of CCL5 or CXCL12 (binding to CCR1 or CXCR4, respectively) into periaqueductal gray could attenuate acute opioid analgesia via heterologous desensitization of opioid receptors [54, 55]. Spinal glial CXCL12 has also been reported to be associated with pain hypersensitivity process induced by bone cancer [56]. Other evidences indicate that CXCL10 could serve as a negative regulator of morphine analgesia [7]. Intrathecal administration of CCL2 neutralizing antibody could attenuate the development of morphine tolerance [21]. Our microarrays analysis revealed the expressions of 15 chemokines, which mainly belong to CXC and CC subfamilies, were significantly increased due to chronic morphine exposure. These results further excluded the direct involvement of CX3CL1 and CX3CR1 in the mechanism of morphine tolerance and also indicated the potential contribution of chemokines to the development of morphine tolerance.
In conclusion, our study reveals that chronic morphine exposure did not alter the expressions of CX3CL1 and CX3CR1 in spinal cord and inhibiting CX3CL1 or CX3CR1 could not affect the morphine analgesia and the development of drug tolerance. But morphine could change the cellular localizations of spinal CX3CL1 and CX3CR1 which indicates the complex interaction between neuron and glia during morphine tolerance. We also found that 15 chemokines were upregulated significantly during the development of morphine tolerant. These might provide the potential research targets for the further studies in morphine tolerance in the future.
Abbreviations
- CCL2:
-
C-C motif ligand 2
- CCL5:
-
C-C motif ligand 5
- CCR1:
-
C-C motif chemokine receptor 1
- CXCL12:
-
C-X-C motif ligand 12
- CX3CL1:
-
C-X3-C motif chemokine 1
- CX3CR1:
-
C-X3-C motif chemokine receptor 1
- CXCR4:
-
C-X-C motif chemokine receptor 4
- rrCX3CL1:
-
Recombinant rat CX3CL1 protein
- DAMGO:
-
[D-Ala2, N-MePhe4, Gly-ol]-enkephalin
- DOR:
-
delta opioid receptor
- ERK:
-
Extracellular regulated protein kinases
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GFAP:
-
Glial fibrillary acidic protein
- GLAST:
-
Glutamate-aspartate transporters
- GLT-1:
-
Glutamate transporter-1
- Iba-1:
-
Ionized calcium-binding adapter molecule 1
- IL-1β:
-
Interleukin-1β
- MPE:
-
Maximal possible antinociceptive effect
- MOR:
-
Mu opioid receptor
- NeuN:
-
Neuronal nuclei
- PAG:
-
Periaqueductal gray
- p38MAPK:
-
p38 mitogen-activated protein kinase
- PBS:
-
Phosphate buffer saline
- TNF-α:
-
Tumor necrosis factor α
References
Handley CA, Ensberg DL (1945) A comparison of amphetamine sulfate with other stimulants of the central nervous system in morphine respiratory depression. Anesthesiology 6(6):561–564
Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R (2008) Opioid complications and side effects. Pain Physician 11(2S):S105–S120
Sun S, Cao H, Han M, Li TT, Pan HL, Zhao ZQ, Zhang YQ (2007) New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain 129(1–2):64–75. doi:10.1016/j.pain.2006.09.035
Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O’Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR (2004) Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci 20(9):2294–2302. doi:10.1111/j.1460-9568.2004.03709.x
White FA, Bhangoo SK, Miller RJ (2005) Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov 4(10):834–844. doi:10.1038/nrd1852
Lin CP, Kang KH, Lin TH, Wu MY, Liou HC, Chuang WJ, Sun WZ, Fu WM (2015) Role of spinal CXCL1 (GROalpha) in opioid tolerance: a human-to-rodent translational study. Anesthesiology 122(3):666–676. doi:10.1097/ALN.0000000000000523
Ye D, Bu H, Guo G, Shu B, Wang W, Guan X, Yang H, Tian X, Xiang H, Gao F (2014) Activation of CXCL10/CXCR3 signaling attenuates morphine analgesia: involvement of Gi protein. J Mol Neurosci 53(4):571–579. doi:10.1007/s12031-013-0223-1
Parsadaniantz SM, Rivat C, Rostene W, Goazigo AR-L (2015) Opioid and chemokine receptor crosstalk: a promising target for pain therapy? Nat Rev Neurosci 16(2):69–78. doi:10.1038/nrn3858
Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ (1997) A new class of membrane-bound chemokine with a CX3C motif. Nature 385(6617):640–644
Lindia JA, McGowan E, Jochnowitz N, Abbadie C (2005) Induction of CX3CL1 expression in astrocytes and CX3CR1 in microglia in the spinal cord of a rat model of neuropathic pain. J Pain 6(7):434–438. doi:10.1016/j.jpain.2005.02.001
Hu JH, Yang JP, Liu L, Li CF, Wang LN, Ji FH, Cheng H (2012) Involvement of CX3CR1 in bone cancer pain through the activation of microglia p38 MAPK pathway in the spinal cord. Brain Res 1465:1–9. doi:10.1016/j.brainres.2012.05.020
Chen X, Geller EB, Rogers TJ, Adler MW (2007) The chemokine CX3CL1/fractalkine interferes with the antinociceptive effect induced by opioid agonists in the periaqueductal grey of rats. Brain Res 1153:52–57. doi:10.1016/j.brainres.2007.03.066
Hanisch U-K (2002) Microglia as a source and target of cytokines. Glia 40(2):140–155. doi:10.1002/glia.10161
Luo X, Tai WL, Sun L, Pan Z, Xia Z, Chung SK, Cheung CW (2016) Crosstalk between astrocytic CXCL12 and microglial CXCR4 contributes to the development of neuropathic pain. Mol Pain. doi:10.1177/1744806916636385
Morganti JM, Riparip L-K, Chou A, Liu S, Gupta N, Rosi S (2016) Age exacerbates the CCR2/5-mediated neuroinflammatory response to traumatic brain injury. J Neuroinflammation 13:80. doi:10.1186/s12974-016-0547-1
Holdridge SV, Armstrong SA, Taylor AMW, Cahill CM (2007) Behavioural and morphological evidence for the involvement of glial cell activation in delta opioid receptor function: implications for the development of opioid tolerance. Mol Pain 3:7. doi:10.1186/1744-8069-3-7
Cui Y, Liao X-X, Liu W, Guo R-X, Wu Z-Z, Zhao C-M, Chen P-X, Feng J-Q (2008) A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun 22(1):114–123. doi:10.1016/j.bbi.2007.07.014
Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC (2004) Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci 20(5):1150–1160. doi:10.1111/j.1460-9568.2004.03593.x
McNally GP, Westbrook RF (1998) Effects of systemic, intracerebral, or intrathecal administration of an N-methyl-D-aspartate receptor antagonist on associative morphine analgesic tolerance and hyperalgesia in rats. Behav Neurosci 112(4):966–978
Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR (2004) A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci 24(33):7353–7365. doi:10.1523/JNEUROSCI.1850-04.2004
Zhao CM, Guo RX, Hu F, Chen PX, Cui Y, Feng JQ, Meng JL, Mo LQ, Liao XX (2012) Spinal MCP-1 Contributes to the development of morphine antinociceptive tolerance in rats. Am J Med Sci 344(6):473–479. doi:10.1097/MAJ.0b013e31826a82ce
Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX (2006) Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res 1069(1):235–243. doi:10.1016/j.brainres.2005.11.066
Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K (2004) Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci 24(45):10211
Zhu ZP, Badisa RB, Palm DE, Goodman CB (2012) Regulation of rat MOR-1 gene expression after chronic intracerebroventricular administration of morphine. Mol Med Rep 5(2):513–516. doi:10.3892/mmr.2011.677
Heinisch S, Palma J, Kirby LG (2011) Interactions between chemokine and mu-opioid receptors: anatomical findings and electrophysiological studies in the rat periaqueductal grey. Brain Behav Immun 25(2):360–372. doi:10.1016/j.bbi.2010.10.020
Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR (2007) Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun 21(5):642–651. doi:10.1016/j.bbi.2006.11.003
Milligan ED, Sloane EM, Watkins LR (2008) Glia in pathological pain: a role for fractalkine. J Neuroimmunol 198(1–2):113–120. doi:10.1016/j.jneuroim.2008.04.011
Raghavendra V, Rutkowski MD, DeLeo JA (2002) The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci 22(22):9980–9989
Hutchinson MR, Coats BD, Lewis SS, Zhang Y, Sprunger DB, Rezvani N, Baker EM, Jekich BM, Wieseler JL, Somogyi AA, Martin D, Poole S, Judd CM, Maier SF, Watkins LR (2008) Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav Immun 22(8):1178–1189. doi:10.1016/j.bbi.2008.05.004
Wang Z, Ma W, Chabot JG, Quirion R (2009) Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J 23(8):2576–2586
Wang Z, Ma W, Chabot JG, Quirion R (2010) Calcitonin gene-related peptide as a regulator of neuronal CaMKII-CREB, microglial p38-NFκB and astroglial ERK-Stat1/3 cascades mediating the development of tolerance to morphine-induced analgesia. Pain 151(1):194–205
Wang Z, Ma W, Chabot JG, Quirion R (2010) Morphological evidence for the involvement of microglial p38 activation in CGRP-associated development of morphine antinociceptive tolerance. Peptides 31(12):2179–2184
Mao J, Sung B, Ji RR, Lim G (2002) Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci 22(18):8312–8323
Lin SL, Tsai RY, Shen CH, Lin FH, Wang JJ, Hsin ST, Wong CS (2010) Co-administration of ultra-low dose naloxone attenuates morphine tolerance in rats via attenuation of NMDA receptor neurotransmission and suppression of neuroinflammation in the spinal cords. Pharmacol Biochem Behav 96(2):236–245
Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR (2010) Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun 24(1):83–95
Eidson LN, Inoue K, Young LJ, Tansey MG, Murphy AZ (2017) Toll-like receptor 4 mediates morphine-induced neuroinflammation and tolerance via soluble tumor necrosis factor signaling. Neuropsychopharmacology 42(3):661–670
Narita M, Suzuki M, Narita M, Niikura K, Nakamura A, Miyatake M, Yajima Y, Suzuki T (2006) mu-Opioid receptor internalization-dependent and -independent mechanisms of the development of tolerance to mu-opioid receptor agonists: Comparison between etorphine and morphine. Neuroscience 138(2):609–619. doi:10.1016/j.neuroscience.2005.11.046
Arden JR, Segredo V, Wang Z, Lameh J, Sadée W (1995) Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged μ-opioid receptor expressed in HEK 293 cells. J Neurochem 65 (4):1636–1645. doi:10.1046/j.1471-4159.1995.65041636.x
Koch T, Schulz S, Pfeiffer M, Klutzny M, Schroder H, Kahl E, Hollt V (2001) C-terminal splice variants of the mouse mu-opioid receptor differ in morphine-induced internalization and receptor resensitization. J Biol Chem 276(33):31408–31414. doi:10.1074/jbc.M100305200
Chakrabarti S, Madia PA, Gintzler AR (2015) Selective upregulation of functional mu-opioid receptor splice variants by chronic opioids. J Neurochem. doi:10.1111/jnc.13519
Caputi FF, Lattanzio F, Carretta D, Mercatelli D, Candeletti S, Romualdi P (2013) Morphine and fentanyl differently affect MOP and NOP gene expression in human neuroblastoma SH-SY5Y cells. J Mol Neurosci 51(2):532–538. doi:10.1007/s12031-013-0019-3
Szentirmay AK, Kiraly KP, Lenkey N, Lacko E, Al-Khrasani M, Friedmann T, Timar J, Gyarmati S, Toth G, Furst S, Riba P (2013) Spinal interaction between the highly selective mu agonist DAMGO and several delta opioid receptor ligands in naive and morphine-tolerant mice. Brain Res Bull 90:66–71. doi:10.1016/j.brainresbull.2012.09.006
Xu J, Lu Z, Xu M, Rossi GC, Kest B, Waxman AR, Pasternak GW, Pan YX (2014) Differential expressions of the alternatively spliced variant mRNAs of the micro opioid receptor gene, OPRM1, in brain regions of four inbred mouse strains. PloS ONE 9(10):e111267. doi:10.1371/journal.pone.0111267
Taylor DA, Fleming WW (2001) Unifying perspectives of the mechanisms underlying the development of tolerance and physical dependence to opioids. J Pharmacol Exp Ther 297(1):11–18
Yan H, Yu LC (2013) Influences of calcitonin gene-related peptide on mu opioid receptors in nucleus accumbens neurons of rats. Neuropeptides 47(2):125–131. doi:10.1016/j.npep.2012.10.008
Raehal KM, Bohn LM (2011) The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology 60(1):58–65. doi:10.1016/j.neuropharm.2010.08.003
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333(6048):1456–1458. doi:10.1126/science.1202529
Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, Gross CT (2014) Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17(3):400–406. doi:10.1038/nn.3641
Limatola C, Ransohoff RM (2014) Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front Cell Neurosci 8:229. doi:10.3389/fncel.2014.00229
Gregg B, Lumeng CN, Bernal-Mizrachi E (2014) Fractalkine signaling in regulation of insulin secretion: Mechanisms and potential therapeutic implications? Islets 6(1):e27861. doi:10.4161/isl.27861
Umehara H, Bloom ET, Okazaki T, Nagano Y, Yoshie O, Imai T (2004) Fractalkine in vascular biology: from basic research to clinical disease. Arterioscler Thromb Vasc Biol 24(1):34–40. doi:10.1161/01.ATV.0000095360.62479.1F
Pello OM, Martínez-Muñoz L, Parrillas V, Serrano A, Rodríguez-Frade JM, Toro MJ, Lucas P, Monterrubio M, Martínez-A C, Mellado M (2008) Ligand stabilization of CXCR4/δ-opioid receptor heterodimers reveals a mechanism for immune response regulation. Eur J Immunol 38(2):537–549. doi:10.1002/eji.200737630
Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR (2011) Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev 63(3):772
Szabo I, Chen X-H, Xin L, Adler MW, Howard OMZ, Oppenheim JJ, Rogers TJ (2002) Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci 99(16):10276–10281. doi:10.1073/pnas.102327699
Chen X, Geller EB, Rogers TJ, Adler MW (2007) Rapid heterologous desensitization of antinociceptive activity between mu or delta opioid receptors and chemokine receptors in rats. Drug Alcohol Depend 88(1):36–41
Hu XM, Liu YN, Zhang HL, Cao SB, Zhang T, Chen LP, Shen W (2015) CXCL12/CXCR4 chemokine signaling in spinal glia induces pain hypersensitivity through MAPKs-mediated neuroinflammation in bone cancer rats. J Neurochem 132(4):452–463. doi:10.1111/jnc.12985
Funding
Funding was provided by the National Natural Science Foundation of China (Nos. 81471143; 81171259).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Peng, Y., Guo, G., Shu, B. et al. Spinal CX3CL1/CX3CR1 May Not Directly Participate in the Development of Morphine Tolerance in Rats. Neurochem Res 42, 3254–3267 (2017). https://doi.org/10.1007/s11064-017-2364-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2364-z