Abstract
Glial cell line-derived neurotrophic factor (GDNF) plays important roles in protecting the damaged or dying dopamine neurons in the animal models of Parkinson’s disease (PD). This study was to determine the effect and mechanisms of GDNF on the apoptosis of neurons in 6-hydroxydopamine (6-OHDA) induced Parkinson’s disease model of rats. Healthy male Sprague–Dawley rats (220–240 g) were randomly divided into six groups (n = 10). 6-OHDA was used to establish the PD rat model. Tyrosine hydroxylase (TH) immunohistochemistry was used to assess the neuron loss in 6-OHDA-lesioned rats. TUNEL and western blot were used to identify the effects and mechanisms of GDNF in the rat model of PD. The numbers of TH-positive neurons in the 6-OHDA-injected lesioned substantia nigra (SN) decreased significantly compared with the Sham group. GDNF treatment effectively ameliorated the apoptosis of neuronal cells in SN induced by 6-OHDA. In addition, GDNF significantly increased serine protein kinase B (Akt) and glycogen synthase kinase 3 beta (GSK3β) phosphorylation induced by 6-OHDA. In contrast, application of LY294002 or triciribine reversed the roles of GDNF in PD models. The results implicated that the anti-apoptosis effects of GDNF in neurons might be mediated through PI3K/Akt/GSK3β pathway. Therefore, GDNF may be a promising agent for PD treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second common neurodegenerative disorder, which is characterized by the loss of the neurotransmitter dopamine in striatum or progressive degeneration of dopaminergic neurons in the pars compacta of substantia nigra (SN) [1]. Patients with PD have motor and behavioral disturbances, such as postural instability, a resting tremor and bradykinesia [2]. PD is not deadly, but can affect life quality of the patients, who need dedicated on-going care [3]. However, the causes of PD are still unclear, recent evidences show the involvement of mitochondria dysfunction, oxidative stress and apoptosis [4–6].
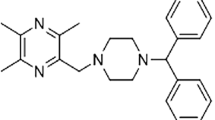
Glial cell line-derived neurotrophic factor (GDNF) belongs to a member of the transforming growth factor superfamily [7]. GDNF was originally found as a potent trophic agent which accelerates the survival and differentiation of dopaminergic neurons [8]. Moreover, GDNF can be effective in the treatment of neuronal degeneration for a lot of neurodegenerative diseases [9]. Recent investigations in the established 6-OHDA and MPTP primate and rodent models of PD revealed a substantial regeneration and neuroprotection effect by GDNF [10, 11]. In addition, GDNF has been demonstrated to be a promising PD therapy that can protect against neurotoxin-induced injury and facilitate the survival of dopamine neurons [12]. However, our understanding of the regulating networks of GDNF in PD is remained limited.
The phosphoinositide 3-kinase (PI3K)/threonine/serine protein kinase B (Akt) pathway has been demonstrated to play an important role in regulating cell proliferation, apoptosis and survival in various systems [13–16]. Moreover, Akt is a serine/threonine protein kinase, whose signaling is dependent on its phosphorylation by PI3K [17]. Besides, glycogen synthase kinase 3β (GSK3β) is a downstream target of the PI3K/Akt signaling pathway [18]. And the activity of GSK3β is regulated by the phosphorylation of two critical sites, Ser9 and Tyr216. Ser9 phosphorylation decreases GSK3β activity, while Tyr216 phosphorylation shows opposite results [19]. Phosphorylation of Ser9 is mediated by multiple signaling pathways, such as MAPK/p90RS, PI3K/Akt or PKC [20]. However, the mechanism for the regulation of phosphorylation at Tyr216 is less clear [21]. Furthermore, it has been demonstrated that PI3K/Akt signal pathway is proposed as an essential pathway in inhibiting neuronal loss [22]. In addition, PI3K/Akt signaling pathway plays a critical role in the pathogenesis of PD [23]. Hence, we hypothesize that the PI3K/Akt/GSK3β signalling pathway may be an important target for the treatment of PD.
In this study, by using 6-OHDA-induced rat model of PD, we demonstrated that the effect of GDNF in repressing neural apoptosis in the substantia nigra. More importantly, the anti-apoptosis mechanisms of GDNF might be related to the phosphorylation of PI3K/Akt/GSK3β pathway. Our data suggest potential therapeutic drug of GDNF in PD.
Materials and Methods
Parkinson’s Disease Animal Model
All animal treatments in present study were performed in accordance with the guidelines established by the National Institutes of Health for the care and use of laboratory animals and were approved by the Animal Care Committee of The First Affiliated Hospital of Zhengzhou University.
Sixty adult male Sprague–Dawley rats (HFK Bioscience, Beijing) weighing 220–240 g (10 weeks old) were divided randomly into six groups: Sham (n = 10), PD (6-OHDA, n = 10), PD + 10 μg GDNF (n = 10), PD + 50 μg GDNF (n = 10), PD + 100 μg GDNF (n = 10) and 100 μg GDNF (n = 10).
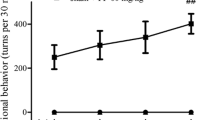
The stereotaxic lesion surgery was performed as described previously [24]. Briefly, the 6-OHDA solution (in 0.9% saline with 0.1% ascorbic acid, pH 5.5) was delivered to two injection sites on the right substantia nigra. Then 1 week after the unilateral 6-OHDA lesion, rats were treated with apomorphine (0.05 mg/kg, in a 0.2% solution of ascorbic acid, pH 5.5) once per week for subsequent 15 days to induce rotational behavior and animals with 210 rotations per 30 min were selected. After that, GDNF (in 10 mM citrate buffer with 150 mM NaCl, pH 5.0) at the doses of 10, 50 and 100 μg was intranasal administration for PD model of rats once daily for 10 days. To minimize respiratory distress and swallowing of the dose, the total volume (25 μl) of GDNF was delivered in 2.5 μl increments, alternating nostrils, every 4 min, as previously described [25].
Immunohistochemistry
Rats were anesthetized intraperitoneally with sodium pentobarbital (50 mg/kg) and the brains were removed and post-fixed. Immunohistochemistry was carried out by using free-floating brain slices (40 μm thickness) which encompassed the entire substantia nigra. And the brain slices were stained at 4 °C overnight with rabbit anti-TH (1:1000; Santa Cruz, USA) antibody. After that, slices were incubated for 1 h in biotinylated goat anti-rabbit IgG (1:1000; Invitrogen, USA). Then slices were incubated for 1 h in avidin–biotin horseradish peroxidase complex (1:200; Sigma, USA). Subsequently, DAB staining method was used to visualize TH protein.
TUNEL Assay
To demonstrate the anti-apoptotic effects of GDNF, TUNEL assay was performed according to the manual of Roche TUNEL kit. Briefly, section (40 μm) were incubated in 0.1% Triton X-100 for 8 min on ice for permeabilization. Then the sections were transferred to TUNEL reaction mixture and incubated in a humid chamber at 37 °C for 1 h. Finally, sections were visualized by using an Olympus IX73 inverted microscope equipped with fluorescence. And five visual fields were counted to calculate TUNEL positive cells.
Western Blot Analysis
Western blot analysis was carried out as described previously [26]. Briefly, the tissues were homogenized in lysis buffer for 30 min. Then equal amounts of protein were separated by 10% SDS–polyacrylamide gels and transferred to PVDF membranes. After that, the PVDF membranes were incubated with primary antibodies against pro caspase-3 (1:500; Cell signaling technology, USA), cleaved caspase-3 (1:500; Cell signaling technology, USA), Bax (1:1000; Santa Cruz Biotechnology, USA), Bcl-2 (1:1000; Santa Cruz Biotechnology, USA), phospho-Akt (Ser473) (1:1000; Cell Signaling Technology, USA), Akt (1:1000; Cell Signaling Technology, USA), phosph-GSK3β (Ser 9) (1:1000; Cell Signaling Technology, USA), GSK3β (1:1000; Cell Signaling Technology, USA) and β-actin (1:1000; Sigma, USA) overnight at 4 °C. Subsequently, the membranes were incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibodies. And protein was visualized by using an ECL system (Pierce Company, USA). Quantification of individual band was performed by using Gel Imaging Analyzer. And β-actin was used as an internal loading control.
Statistical Analysis
The data were expressed as the means ± SD. And the statistical significance was determined by a two-tailed Student’s t test. All statistical analyses were performed by SPSS (ver. 17.0, Somers, NY, USA). A P value less than 0.05 was considered as statistical significance.
Results
The Dopaminergic Neuron Loss in the Substantia Nigra (SN) in 6-OHDA-Lesioned Rats
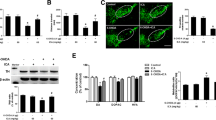
To investigate the neurotoxicity of 6-OHDA in Parkinson’s disease (PD) model, TH immunohistochemical staining was performed. It is well-known that TH is a rate limiting enzyme in the synthesis of dopaminergic neurons and is characterized as a specific marker for dopaminergic neurons [27]. The photographs of TH-positive neurons in the SN in PD and Sham groups were shown in Fig. 1a. And the statistical analysis indicated that the numbers of TH-positive neurons in the 6-OHDA-injected lesioned SN decreased significantly compared with the Sham group (Fig. 1b). The results demonstrated that the PD model was established successfully.
Immunohistochemical staining of TH protein and the number of TH positive neurons in the substantia nigra of unilateral 6-OHDA lesioned Parkinson rats. a Representative photographs of TH-positive neurons immunohistochemistry in Sham and PD groups. Magnification, ×100. b Quantitative analysis of TH positive cells in Sham and PD groups. *P < 0.05 versus Sham group
GDNF Attenuated the Apoptosis of the Neurons Induced by 6-OHDA in the Substantia Nigra
To examine the effects of GDNF on the 6-OHDA-induced apoptosis of the neurons in SN, we employed the TUNEL assay. Representative photographs of TUNEL-positive cells under different concentrations of GDNF were shown in Fig. 2a. Besides, in Fig. 2b, the TUNEL-positive cells in the PD model rats dramatically increased than those in the Sham-operated rats. However, the TUNEL positive cells decreased in the PD rats treated with GDNF in a dose-dependent manner. And in the middle and high dose groups (GDNF, 50 μg and GDNF, 100 μg), the number of TUNEL positive cells was significantly downregulated in the PD rats. In addition, high dose of GDNF alone did not affect the apoptosis of the neurons in Sham group.
Effects of GDNF treatment (10, 50 and 100 μg) on 6-OHDA-induced apoptosis in the substantia nigra. a Representative photoographs of TUNEL labeling in the substantia nigra. Magnification, ×100. b Quantitative analysis of TUNEL-positive cells in the substantia nigra. *P < 0.05 versus Sham group. # P < 0.05 versus PD group
Effects of GDNF on Apoptosis-Related Protein Induced by 6-OHDA in SN
To determine the effects of GDNF on 6-OHDA-induced apoptosis, we measured caspase-3, Bax and Bcl-2 expression levels in SN induced by 6-OHDA using western blot (Fig. 3a, b). The results showed 6-OHDA-induced toxicity significantly increased cleaved caspase-3 and Bax protein levels, while GDNF treatment can decrease cleaved caspase-3 and Bax protein levels in a dose-responsive manner (Fig. 3c, d). In contrast, as shown in Fig. 3e, compared with Sham group, Bcl-2 was significantly downregulated in the PD group. Besides, the treatment of 6-OHDA + GDNF 10, 50, 100 μg can increase the Bcl-2 protein expression levels.
Effects of GDNF treatment (10, 50 and 100 μg) on apoptosis-related proteins induced by 6-OHDA in the substantia nigra. a Expression of cleaved caspase-3 and pro caspase-3 proteins detected by western blot. b Bax and Bcl-2 protein expression detected by western blot. c The graphs demonstrated the statistical analysis of the expression ratios of cleaved caspase-3/pro caspase-3. d, e The statistical analysis of the protein expression levels of Bax and Bcl-2. And the relative optical density was normalized to β-actin. *P < 0.05 versus Sham group. # P < 0.05 versus PD group
GDNF Upregulated Akt and GSK3β Phosphorylation Induced by 6-OHDA in the SN
To detect the possible signaling pathways mediating the anti-apoptosis by GDNF, the phosphorylation levels of Akt and GSK3β in the SN were examined by western blot analysis. As shown in Fig. 4a, b, the phosphorylation levels of Akt and GSK3β were significantly decreased in the 6-OHDA-injected rats compared with that in Sham-operated rats. However, the decrease was notably alleviated in the PD rats receiving GDNF treatment. And after 50 or 100 μg GDNF treatment, the phosphorylation levels of Akt and GSK3β were significantly increased in the PD rats. Moreover, GDNF administration alone did not affect the phosphorylation of Akt or GSK3β in Sham group.
Effect of GDNF treatment (10, 50 and 100 μg) on Akt and GSK3β phosphorylation in the substantia nigra of unilateral 6-OHDA lesioned Parkinson rat. a The phosphorylation levels of Akt determined by western blot analysis. And the amount of p-Akt was quantitated by densitometric analysis. b The phosphorylation levels of GSK3β determined by western blot analysis. The amount of p-GSK3β was quantitated by densitometric analysis. The phosphorylated form was normalized versus the total form. *P < 0.05 versus Sham group. # P < 0.05 versus PD group
Anti-Apoptosis Effects of GDNF were Mediated by the PI3K/Akt/GSK3β Pathway
To demonstrate that the anti-apoptosis effects of GDNF were mediated by PI3K/Akt pathway, the rats were treated with 10 μM PI3K inhibitor, LY294002 or 5 μM Akt inhibitor, triciribine for 1 h. And representative photographs of TUNEL-positive cells were shown in Fig. 5a. The results showed that GDNF can significantly decrease the TUNEL-positive cells in the PD rats. However, LY294002 and triciribine remarkably abolished the decrease in the number of the TUNEL positive cells induced by GDNF in the PD rats (Fig. 5b).
Inhibition of PI3K or Akt blunted the anti-apoptosis effects of GDNF induced by 6-OHDA in the substantia nigra. The rats with PD were treated with PI3K inhibitor, LY294002 (10 μM) or Akt inhibitor, triciribine (5 μM) for 1 h after 100 μg GDNF treatment. Cell apoptosis was assessed by TUNEL staining in the substantia nigra. a Representative photographs of TUNEL-positive staining in the substantia nigra. Magnification, ×100. b Quantitative analysis of TUNEL-positive cells in the substantia nigra. *P < 0.05 compared between two groups
Next caspase-3, Bax and Bcl-2 expression levels in the SN were measured by western blot (Fig. 6a, b). The statistical analysis indicated LY294002 and triciribine blocked the downregulation of cleaved caspase-3 and Bax protein levels induced by GDNF in the PD rats (Fig. 6c, d). Conversely, LY294002 and triciribine decreased the upregulation of the protein expression levels of Bcl-2 induced by GDNF in the PD rats (Fig. 6e). These results revealed that the anti-apoptosis effects of GDNF in the PD rats were most likely mediated by the PI3K/Akt pathway.
Inhibition of PI3K or Akt attenuated the anti-apoptosis effects of GDNF on apoptosis-related proteins induced by 6-OHDA in the substantia nigra. a Expression of cleaved caspase-3 and pro caspase-3 proteins in the presence or absence of 10 μM LY294002 or 5 μM triciribine detected by western blot. b Bax and Bcl-2 protein expression in the presence or absence of LY294002 or triciribine detected by western blot. c The statistical analysis of the expression ratios of cleaved caspase-3/pro caspase-3 in (a). d, e The statistical analysis of the protein expression levels of Bax and Bcl-2 in (b). And the relative optical density was normalized to β-actin. *P < 0.05 compared between two groups
To further understand the role of PI3K/Akt/GSK3β signaling pathway in the anti-apoptosis by GDNF against 6-OHDA-induced neurotoxicity, we further assessed the phosphorylation of Akt and GSK3β in the presence and absence of PI3K inhibitor, LY294002 or Akt inhibitor, triciribine. As shown in Fig. 7a, b, Western blot analysis displayed that 6-OHDA markedly inhibited the phosphorylation of Akt and GSK3β. After treatment with GDNF, it alleviated the repression on Akt and GSK3β activation induced by 6-OHDA. However, this beneficial effect was abolished after treatment with LY294002 or triciribine. Thus, these results clearly demonstrated that the anti-apoptosis effects by GDNF on 6-OHDA-induced PD rats may be mediated partly through PI3K/Akt/GSK3β signaling pathway.
Inhibition of PI3K or Akt blocked the effect of GDNF on Akt and GSK3β phosphorylation in the substantia nigra induced by 6-OHDA. a The phosphorylation levels of Akt in the treatment with or without of LY294002 or triciribine determined by western blot. And the amount of p-Akt was quantitated by densitometric analysis. b The phosphorylation levels of GSK3β in the treatment with or without of LY294002 or triciribine determined by western blot analysis. The amount of p-GSK3β was quantitated by densitometric analysis. And the phosphorylated form was normalized versus the total form. *P < 0.05 compared between two groups
Discussion
6-OHDA is toxic in nervous system [28]. Because the neurotoxin of 6-OHDA can not cross the blood–brain barrier, its toxicity to the central nervous system is only by means of stereotaxic surgery [29]. Increasing evidences show 6-OHDA has become one of the neurotoxins to establish PD model in experimental animals [30–32]. After long term exposure to very low levels of a neurotoxin, recurrent apoptosis of small number of cells may be the way leads to the neurons die [33]. As free radicals have been demonstrated to give rise to Parkinson’s disease and 6-OHDA induces apoptosis via free radical production [34], apoptosis is one of the main causes to the generation of Parkinson’s disease.
The mechanisms of apoptosis are complex, which involves a cascade of reactions. A key step leading to apoptosis is the activation of caspase-3 [35]. During apoptosis, caspase-3 is considered as a central component of the proteolytic cascade, as it may cleave a variety of nuclear proteins, which may cause atypical apoptotic DNA fragmentation [36]. Kim et al. indicated the numbers of cleaved caspase-3-positive cells in the hippocampal dentate gyrus were increased in the mice with PD [37]. Hartmann et al. demonstrated the percentage of active caspase-3-positive neurons was significantly higher in PD patients than that in controls [38]. Our results showed that after the treatment of 6-OHDA, cleaved caspase-3 protein expression was notably higher in PD group than that in Sham group, which was consistent with previous studies. Moreover, Bcl-2 and Bax also contribute to the regulation of apoptotic cell death. Bcl-2 is anti-apoptotic, which protects against cell death and Bax is pro-apoptotic that promotes cell death [39]. And compared with the control rats, the protein expression of Bax in the brain increased significantly while that of Bcl-2 decreased significantly in the 6-OHDA treated rats [40]. In the present study, after the treatment of 6-OHDA, Bcl-2 expression was decreased and Bax expression was increased. These results suggested that 6-OHDA induced apoptosis of neurons.
GDNF has neuroprotective effects against a variety of neuronal insults [41, 42]. And GDNF reduces apoptosis in dopaminergic neurons in vitro [43]. Moreover, Tsybko found that the GDNF injection increased the anti-apoptotic protein Bcl-xl mRNA content in the hippocampi of mice [44]. In this study, the treatment of GDNF alleviated the number of TUNEL-positive cells, decreased cleaved caspase-3 and Bax protein levels and increased the Bcl-2 protein expression levels in PD model, demonstrating that GDNF protected the neurons against 6-OHDA-induced apoptosis.
Increasing evidences shows that Akt/GSK3β pathway plays a key role in preventing cellular apoptosis [45]. The activation of Akt is associated with cell proliferation [46]. Moreover, GSK3β, which is a constitutively active enzyme substrate of Akt, is inactivated by p-Akt [47]. Our observations showed that 6-OHDA treatment significantly dephosphorylated Akt and GSK3β, suggesting the 6-OHDA inactivated Akt but activated GSK3β, which was in agreement with previous studies [48]. However, our results clearly demonstrated that GDNF treatment attenuated the downregulation of Akt and GSK3β phosphorylation induced by 6-OHDA. More importantly, the PI3K inhibitor LY294002 and Akt inhibitor triciribine blocked the anti-apoptosis effects of GDNF on neurons and its regulation on Akt and GSK3β phosphorylation induced by 6-OHDA. Thus, PI3K/Akt/GSK3β pathway may play a key role in the anti-apoptosis by GDNF on neurons in 6-OHDA-induced PD model of rats (Fig. 8).
Conclusion
In conclusion, present study suggested that exposure to GDNF suppressed neurons apoptosis in 6-OHDA-induced rats, and the anti-apoptosis of GDNF may be partially by regulating the PI3K/Akt/GSK3β signaling pathway. Therefore, GDNF may serve as a therapeutic agent for patients with PD.
References
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909
Shimohama S, Sawada H, Kitamura Y, Taniguchi T (2003) Disease model: Parkinson’s disease. Trends Mol Med 9:360–365
Zhu G, Wang X, Wu S, Li X, Li Q (2014) Neuroprotective effects of puerarin on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced Parkinson’s disease model in mice. Phytother Res 28:179–186
Perier C, Bové J, Wu DC, Dehay B, Choi DK, Jacksonlewis V, Rathkehartlieb S, Bouillet P, Strasser A, Schulz JB (2007) Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson’s disease. Proc Natl Acad Sci USA 104:8161–8166
Nikam S, Nikam P, Ahaley SK, Sontakke AV (2003) Oxidative stress in Parkinson’s disease. Indian J Clin Biochem 24:98–101
Adams JD (2012) Parkinson’s disease-apoptosis and dopamine oxidation. Open J Apoptosis 1:1–8
Airaksinen MS, Saarma M (2002) The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3:383–394
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130–1132
Revishchin A, Moiseenko L, Kust N, Bazhenova N, Teslia P, Panteleev D, Kovalzon V, Pavlova G (2016) Effects of striatal transplantation of cells transfected with GDNF gene without pre- and pro-regions in mouse model of Parkinson’s disease. BMC Neurosci 17:1–15
Aron L, Klein R (2011) Repairing the parkinsonian brain with neurotrophic factors. Trends Neurosci 34:88–100
Drinkut A, Tillack K, Meka DP, Schulz JB, Kügler S, Kramer ER (2016) Ret is essential to mediate GDNF’s neuroprotective and neuroregenerative effect in a Parkinson disease mouse model. Cell Death Dis 7:e2359
Grondin R (2002) Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain 125:2191–2201
Kitagishi Y (2014) Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimers Res Ther 6:35–35
Chen L, Wei X, Hou Y, Liu X, Li S, Sun B, Liu X, Liu H (2014) Tetramethylpyrazine analogue CXC195 protects against cerebral ischemia/reperfusion-induced apoptosis through PI3K/Akt/GSK3β pathway in rats. Neurochem Int 66:27–32
Qi Z, Xu Y, Liang Z, Li S, Wang J, Wei Y, Dong B (2015) Baicalein alters PI3K/Akt/GSK3β signaling pathway in rats with diabetes-associated cognitive deficits. Int J Clin Exp Med 8:1993–2000
Sestito S, Nesi G, Daniele S, Martelli A, Digiacomo M, Borghini A, Pietra D, Calderone V, Lapucci A, Falasca M (2015) Design and synthesis of 2-oxindole based multi-targeted inhibitors of PDK1/Akt signaling pathway for the treatment of glioblastoma multiforme. Eur J Med Chem 105:274–288
Fayard E, Xue G, Parcellier A, Bozulic L, Hemmings BA (2010) Protein kinase B (PKB/Akt), a key mediator of the PI3K signaling pathway. Curr Top Microbiol Immunol 346:31–56
Liu F, Gong X, Zhang G, Marquis K, Reinhart P, Andree TH (2005) The inhibition of glycogen synthase kinase 3β by a metabotropic glutamate receptor 5 mediated pathway confers neuroprotection to Aβ peptides. J Neurochem 95:1363–1372
Noël A, Barrier L, Rinaldi F, Hubert C, Fauconneau B, Ingrand S (2011) Lithium chloride and staurosporine potentiate the accumulation of phosphorylated glycogen synthase kinase 3β/Tyr216, resulting in glycogen synthase kinase 3β activation in SH-SY5Y human neuroblastoma cell lines. J Neurosci Res 89:755–763
Luo J (2009) Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett 273:194–200
Yang Y, Wang H, Wang S, Xu M, Liu M, Liao M, Frank JA, Adhikari S, Bower KA, Shi X (2012) GSK3β signaling is involved in ultraviolet B-induced activation of autophagy in epidermal cells. Int J Oncol 41:1782–1788
Kim SN, Kim ST, Doo AR, Park JY, Moon W, Chae Y, Yin CS, Lee H, Park HJ (2011) Phosphatidylinositol 3-kinase/Akt signaling pathway mediates acupuncture-induced dopaminergic neuron protection and motor function improvement in a mouse model of Parkinson’s disease. Int J Neurosci 121:562–569
Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, Nishimura I, Wakamatsu K, Ito S (2005) Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci USA 102:13670–13675
Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci 15:120–132
Migliore MM, Vyas TK, Campbell RB, Amiji MM, Waszczak BL (2010) Brain delivery of proteins by the intranasal route of administration: a comparison of cationic liposomes versus aqueous solution formulations. J Pharm Sci 99:1745–1761
Cheng YF, Zhu GQ, Wang M, Cheng H, Zhou A, Wang N, Fang N, Wang XC, Xiao XQ, Chen ZW (2008) Involvement of ubiquitin proteasome system in protective mechanisms of Puerarin to MPP+-elicited apoptosis. Neurosci Res 63:52–58
Stanwood GD, Leitch DB, Savchenko V, Wu J, VAF, Anderson DJ, Stankowski JN, Aschner M, Mclaughlin BA (2009) Manganese exposure is cytotoxic and alters dopaminergic and GABAergic neurons within the basal ganglia. J Neurochem 110:378–389
Ossola B, Kääriäinen TM, Raasmaja A, Männistö PT (2008) Time-dependent protective and harmful effects of quercetin on 6-OHDA-induced toxicity in neuronal SH-SY5Y cells. Toxicology 250:1–8
Simola N, Morelli M, Carta AR (2007) The 6-Hydroxydopamine model of parkinson’s disease. Neurotox Res 11:151–167
Virgone-Carlotta A, Uhlrich J, Akram MN, Ressnikoff D, Chrétien F, Domenget C, Gherardi R, Despars G, Jurdic P, Honnorat J (2013) Mapping and kinetics of microglia/neuron cell-to-cell contacts in the 6-OHDA murine model of Parkinson’s disease. Glia 61:1645–1658
Thiele SL, Warre R, Nash JE (2012) Development of a unilaterally-lesioned 6-OHDA mouse model of Parkinson’s disease. J Vis Exp 60:e3234–e3234
Jing X, Shi H, Zhang C, Ren M, Han M, Wei X, Zhang X, Lou H (2014) Dimethyl fumarate attenuates 6-OHDA-induced neurotoxicity in SH-SY5Y cells and in animal model of Parkinson’s disease by enhancing Nrf2 activity. Neuroscience 286:131–140
Li Z, Hu Y, Zhu Q, Zhu J (2008) Neurotrophin-3 reduces apoptosis induced by 6-OHDA in PC12 cells through Akt signaling pathway. Int J Dev Neurosci 26:635–640
Bezard, Zhao (2005) Protective effect of green tea polyphenols on the SH-SY5Y cells against 6-OHDA induced apoptosis through ROS-NO pathway. Free Radic Biol Med 39:682–695
Mazumder S, Plesca D, Almasan A (2015) Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol 414:13–21
Porter AG, Jänicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104
Kim M, Cho KH, Shin MS, Lee JM, Cho HS, Kim CJ, Shin DH, Yang HJ (2014) Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson’s disease. Int J Mol Med 33:870–878
Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M (2000) Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci USA 97:2875–2880
Zong WX, Lindsten T, Ross AJ, Macgregor GR, Thompson CB (2001) BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev 15:1481–1486
Xu H, An D, Yin SM, Chen W, Zhao D, Meng X, Yu DQ, Sun YP, Zhao J, Zhang WQ (2015) The alterations of apoptosis factor Bcl-2/Bax in the early Parkinson’s disease rats and the protective effect of scorpion venom derived activity peptide. Chin J Applied Physiol 31:225–229
Arenas E, Trupp M, Åkerud P, Ibáñez CF (1995) GDNF prevents degeneration and promotes the phenotype of brain noradrenergic neurons in vivo. Neuron 15:1465–1473
Sharma HS (2006) Post-traumatic application of brain-derived neurotrophic factor and glia-derived neurotrophic factor on the rat spinal cord enhances neuroprotection and improves motor function. Acta Neurochir Suppl 96:329–334
Clarkson ED, Zawada WM, Freed CR (1996) GDNF reduces apoptosis in dopaminergic neurons in vitro. Neuroreport 7:145–149
Tsybko AS, Il’Chibaeva TV (2015) The effects of the glial cell line-derived neurotrophic factor (GDNF) on the levels of mRNA of apoptotic genes Bax and Bcl-xl in the brain of mice genetically predisposed to pathological behavior. Russ J Genet 5:407–412
Barre B, Perkins ND (2010) The Skp2 promoter integrates signaling through the NF-kappaB, p53, and Akt/GSK3beta pathways to regulate autophagy and apoptosis. Mol Cell 38:524–538
Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME (1997) Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275:661–665
Dal-Cim T, Molz S, Egea J, Parada E, Romero A, Budni J, Saavedra MDMD, Barrio LD, Tasca CI, López MG (2012) Guanosine protects human neuroblastoma SH-SY5Y cells against mitochondrial oxidative stress by inducing heme oxigenase-1 via PI3K/Akt/GSK-3β pathway. Neurochem Int 61:397–404
Gong L, Zhang QL, Zhang N, Hua WY, Huang YX, Di PW, Huang T, Xu XS, Liu CF, Hu LF (2012) Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson’s disease: linking to Akt/GSK3β signaling pathway. J Neurochem 123:876–885
Acknowledgements
This work was supported by The First Affiliated Hospital of Zhengzhou University and National Natural Science Foundation of China (81501109, U1504813).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yue, P., Gao, L., Wang, X. et al. Intranasal Administration of GDNF Protects Against Neural Apoptosis in a Rat Model of Parkinson’s Disease Through PI3K/Akt/GSK3β Pathway. Neurochem Res 42, 1366–1374 (2017). https://doi.org/10.1007/s11064-017-2184-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2184-1