Abstract
Isorhynchophylline (IRN), an oxindole alkaloid, has been identified as the main active ingredient responsible for the biological activities of Uncaria rhynchophylla (Miq) Miq ex Havil. (Rubiaceae). Previous studies in our laboratory have revealed that IRN possesses potent neuroprotective effects in different models of Alzheimer’s disease. However, the antidepressant-like effects of IRN are remained unclear. The present study aims to evaluate the antidepressant-like effects of IRN. The antidepressant-like effects of IRN was determined by using animal models of depression including forced swimming and tail suspension tests. The acting mechanism was explored by determining the effect of IRN on the levels of monoamine neurotransmitters and the activities of monoamine oxidases. Intragastric administration of IRN at 10, 20 and 40 mg/kg for 7 days caused a significant reduction of immobility time in both forced swimming and tail suspension tests, while IRN did not stimulate locomotor activity in the open-field test. In addition, IRN treatment antagonized reserpine-induced ptosis and significantly enhanced the levels of monoamine neurotransmitters including norepinephrine (NE) and 5-hydroxytryptamine (5-HT), and the activity of monoamine oxidase A (MAO-A) in the hippocampus and frontal cortex of mice. These results suggest that the antidepressant-like effects of IRN are mediated, at least in part, by the inhibition of monoamine oxidases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depressive disorder is a prevalent psychiatric disorder, which affects about one-fifth of the world population, with a heavy social burden and a substantial lifetime risk [1]. Depression will be the second largest global disease burden, illustrating the severity and impact of the disorder [2]. The neurobiology of depression is still unclear; however, the efficacy of monoamine modulation in treatment of depression has been widely studied. Current treatments for depression often include selective serotonin-reuptake inhibitors (SSRIs), selective norepinephrine-reuptake inhibitors (SNRIs) and monoamine oxidase inhibitors (MAOIs). However, the efficacy of these antidepressants is inconsistent and many of them produce side-effects such as sedation, apathy and fatigue, sleep disturbance, cognitive impairment, sexual dysfunction, body weight gain etc. [3]. Therefore, it is particularly urgent to develop efficient and safe antidepressant drugs [4].
During recent years, the therapeutic effect of natural products on depressive disorders has drawn great research attention. In particular, several phytochemicals have been found to exhibit antidepressant-like effects, including evodiamine [5], curcumin [6, 7], icariin [8, 9], and ferulic acid [10]. Isorhynchophylline (IRN, the chemical structure is shown in Fig. 1), an oxindole alkaloid isolated from Uncaria rhynchophylla, has been identified as the main active ingredient responsible for the biological activities of U. rhynchophylla [11, 12]. U. rhynchophylla has been used traditionally to treat some central nervous system disorders including epilepsy and Alzheimer’s disease. Moreover, studies conducted elsewhere indicated that IRN possessed potent neuroprotective effect against the glutamate- and cerebral ischemia-induced neuronal damage or death [11, 13]. Furthermore, IRN has also been found to suppress 5-hydroxytryptamine (5-HT) receptor function [14, 15], and to promote the degradation of alpha-synuclein in neuronal cells via the induction of autophagy [16]. Recent studies by our research group has revealed that IRN reduced the neurotoxicity induced by beta-amyloid through inhibiting oxidative stress and tau protein hyperphosphorylation, as well as suppressing cellular apoptosis in vitro and in vivo [17–19]. Pharmacokinetic data revealed that IRN was readily absorbed into the body and could penetrate the blood–brain barrier [20], rendering it a promising therapeutic agent for the treatment of depression. However, the antidepressant-like effects of IRN are still unclear, in this study, we aimed to investigate the antidepressant-like effects of IRN by using the forced swimming test and the suspension tail test. The underlying mechanism of antidepressant-like properties is explored by testing the antagonizing effect against reserpine induced ptosis and measuring the contents of monoamine neurotransmitter including norepinephrine (NE), 5-HT and dopamine (DA), and the activities of monoamine oxidase A and B (MAO-A and MAO-B) in the hippocampus and frontal cortex of the mice.
Materials and Methods
Drugs and Chemical Reagents
Isorhynchophylline (IRN, purity ≥98%) was purchased from Chengdu Mansite Pharmaceutical Co. Ltd. (Chengdu, Sichuan, China). Its identity was confirmed by comparing its H1 NMR spectra with that published in the literature [21]. Moclobemide was purchased from Shanghai Xinyi Pharmaceutical Co. Ltd. (Shanghai, China). Reserpine solution was produced by Guangdong Bangming Pharmaceutical Co., Ltd. (Guangdong, China); Fluoxetine hydrochloride, 5-hydroxytrylamine (5-HT) and β-phenylethylamine (β-PEA) were obtained from Sigma-Aldrich (St. Louis, MO, USA). All other reagents and solvents used in the study were of analytical grade.
Animals
Male BALB/c mice (4–6 weeks, 18–22 g) were obtained from the Laboratory Animal Services Center, The Chinese University of Hong Kong. The animals were maintained on a 12 h light/dark cycle under controlled temperature (22 ± 2 °C) and humidity (50 ± 10%), and given standard diet and water ad libitum. They were allowed to acclimatize for 7 days before the experiments. The experimental procedures were approved by the Animal Experimentation Ethics Committee of The Chinese University of Hong Kong, and conformed to the Guidelines of the Principles of Laboratory Animal Care (NIH publication No. 80-23, revised 1996).
Drug Administration
Mice were randomly assigned into five groups of ten animals each: control, IRN (10 mg/kg), IRN (20 mg/kg), IRN (40 mg/kg) and fluoxetine (20 mg/kg) or moclobemide (20 mg/kg). IRN was suspended in 0.5% sodium carboxymethyl cellulose, while fluoxetine and moclobemide were dissolved in redistilled water. The drugs were given intragastrically daily between 9:30 and 10:30 a.m. for 7 days. All the drugs were administered in a volume of 10 mL/kg body weight. Sixty minutes after the last dose, mice in each group were used to perform the behavioral tests. Immediately after the behavioral tests, all the mice were sacrificed by cervical dislocation and the brains were quickly harvested for biomedical measurement.
Forced Swimming Test
The forced swimming test was carried out according to the method described by Porsolt et al. [22] with modifications. Briefly, 60 min after the last drug administration, mice were forced to swim in a transparent glass vessel (height: 25 cm; diameter: 14 cm; containing 10 cm of water at 24 ± 1 °C) for 6 min (test). A mouse was judged to be immobile when it ceased struggling and remained floating motionless in the water, making only small movements necessary to keep its head above water. The total duration of immobility (s) was recorded during the last 4 min of the 6-min testing period.
Tail Suspension Test
The tail suspension test was performed based on the method of Steru et al. [23]. Briefly, mice were suspended 50 cm above the floor by means of an adhesive tape, placed approximately 1 cm from the tip of the tail. Animals were partitioned to avoid interference during the test. The total duration of immobility was recorded during a test period of 6 min. Mice were considered immobile only when they hung passively and completely motionless.
Open-Field Test
The ambulatory behaviour was assessed in an open-field test as described previously [24]. The open field arena used was a wooden box measuring 40 × 60 cm and 50 cm height with the floor divided into 12 equal squares. At the start of each trial a mouse was placed in the left corner of the field and was allowed to freely explore the arena for 5 min. The number of squares crossed with all paws (crossing) was counted in a 6 min session. The arena floor was cleaned between the trials with a 10% ethanol solution and the test was carried out in a temperature, noise, and light controlled room.
Reversal of Reserpine-Induced Ptosis in Mice
The test was performed according to the method described by Bourin et al. [25] with modifications. Reserpine (2.5 mg/kg) was given intraperitoneally to the animals and ptosis was evaluated 120 min after reserpine treatment. Animals were placed on a shelf (20 cm above the tabletop) and the degree of ptosis was rated according to the following rating scale: zero, eyes open; one, eyes one quarter closed; two, eyes half closed; three, eyes three-quarters closed; and four, eyes completely closed [26].
Monoamine Oxidase (MAO) Assay
Immediately after the forced swimming test, all the mice were sacrificed by cervical dislocation and the brains were quickly harvested and the hippocampus and the frontal cortex were dissected, frozen in liquid nitrogen and stored at −80 °C for posterior biochemical analysis. MAO activity in the hippocampus and the frontal cortex was measured following the procedures described previously [27, 28]. Briefly, the hippocampus and the frontal cortex were suspended in ten volume of cold sodium phosphate buffer (200 mM, pH 7.4) containing 320 mM sucrose and homogenized at 4 °C for 30 s by using a motor-driven pellet pestle. The mixture was centrifuged at 600×g for 10 min at 4 °C to remove nucleus and cell debris. The mitochondrial fraction was obtained by further centrifugation at 15,000×g for 30 min at 4 °C and resuspended in buffer. Protein concentration was determined by the Lowry method [29] using bovine serum albumin as the standard. The protein concentration of the mitochondrial fraction was diluted to 1 mg/mL. The MAO assay mixture contained 400 μL of mitochondrial protein in the phosphate buffer. 5-Hydroxytryptamine (5-HT, 4 mM) or β-phenylethylamine (β-PEA, 2 mM) was added as specific substrate for monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B), respectively. The final volume of the reaction mixture was 3 mL. The reaction mixture was incubated at 37 for 60 min, followed by the addition of HCl (600 μL, 1 M). The reaction products were extracted by 4 mL of butylacetate or cyclohexane for the assay of MAO-A and MAO-B, respectively. The organic phase was collected and the absorbance was measured at 280 nm (for MAO-A) and 242 nm (for MAO-B), respectively. Blank samples were prepared by adding 600 μL of 1 M HCl prior to reaction, and worked up subsequently in the same manner.
Determination of Monoamine Neurotransmitter Levels
Immediately after the tail suspension test, all the mice were sacrificed by cervical dislocation and the brains were quickly harvested and the hippocampus and the frontal cortex were dissected, frozen in liquid nitrogen and stored at −80 °C for posterior biochemical analysis. The levels of NE, 5-HT and DA in the hippocampus and the frontal cortex were measured by ELISA according to the manufacturer’s (Beckman Coulter, Fullerton, CA, USA) instructions. A 30 mg portion of the homogenate was diluted in 300 µL normal saline (0.9%) for detection. We assayed NE, 5-HT and DA in supernatant fluid using the ELISA kit (Abnova Coporation, Taipei, Taiwan) after centrifugation of homogenized tissue for 10 min at 14,000×g at 4 °C. Dispensed antigen standards and samples were added to each well of 96-well plates precoated with primary antibodies. After adding biotin conjugate reagent and enzyme conjugate reagent into each well, the plates were incubated at 37 °C for 30 min. Then the plates were rinsed five times with distilled water. Within 30 min of the chromogenic reaction, the absorbance was measured at 450 nm using FLUOstar OPTIMA microplate reader (BMG Labtech, Offenbury, Germany).
Statistical Analysis
Data were expressed mean ± SEM. Multiple group comparisons were performed using one-way analysis of variance (ANOVA) followed by post-hoc Bonferroni test to detect inter-group differences. For non-parametric data, Kruskal–Wallis test was conducted. GraphPad Prism software (Version 5.0; GraphPad Software, Inc., San Diego, CA) was used to perform the statistical analysis. A difference was considered statistically significant if the p value was <0.05.
Results
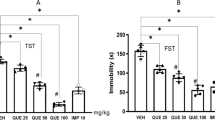
Effects of IRN on the Immobility Time in the Forced Swimming Test
Figure 2 showed the effects of IRN on the immobility time in the forced swimming test. Treatment with IRN (10, 20 and 40 mg/kg) significantly decreased the duration of immobility [F(4, 45) = 35.850, p < 0.01, p < 0.001 and p < 0.001, respectively], when compared with the control group. Treating the mice with mocloxetine at daily dose of 20 mg/kg for 7 days caused a reduction of immobility time (p < 0.001) in the forced swimming test.
Effects of IRN on the Immobility Time in Tail Suspension Test
As shown in Fig. 3, the treatment regimen with IRN at doses of 20 and 40 mg/kg also significantly decreased the immobility time in the tail suspension test [F(4, 45) = 21.554, p < 0.001 and p < 0.001, respectively], when compared with the control. Fluoxetine (20 mg/kg) treatment could also reduce the immobility time (p < 0.001) in tail suspension test.
Effects of IRN on Open Field Test
The effect of IRN on ambulatory activity was evaluated by open-field behavior test. As shown in Fig. 4, IRN (10, 20 and 40 mg/kg) treatment groups showed similar the number of crossings [F(4, 45) = 0.775] and rearings [F(4, 45) = 0.860] to the vehicle control group. Similarly, fluoxetine (20 mg/kg) treatment group didn’t change the number of crossings and rearings in the open-field test as compared with the vehicle control group.
Effects of IRN on Reserpine-Induced Ptosis
The effect of IRN on reserpine-induced ptosis in mice was shown in Fig. 5. Treating the mice with IRN at daily doses of 20 or 40 mg/kg for 7 days significantly antagonized the reserpine-induced ptosis [F(4, 45) = 12.514, p < 0.01 and p < 0.001, respectively] when compared with the vehicle control group. Similarly, treatment with fluoxetine (20 mg/kg) also significantly antagonized ptosis induced by reserpine (p < 0.001).
Effects of IRN on the Enzyme Activities of Monoamine Oxidase
The effects of IRN and moclobemide on the enzyme activities of monoamine oxidases (MAO-A and MAO-B) in mouse cerebrum were shown in Table 1. Comparing with the vehicle control group, treatment with IRN at doses of 20 and 40 mg/kg significantly inhibited the activity of MAO-A [F(4, 45) = 6.942, p < 0.05 and p < 0.001, respectively] in the hippocampus of mice, and [F(4, 45) = 9.512, p < 0.05 and p < 0.01, respectively] in the frontal cortex of mice. IRN did not appear to inhibit the activity of MAO-B in the hippocampus [F(4, 45) = 1.219] and frontal cortex [F(4, 45) = 0.659] of mice. In addition, treatment with moclobemide (a reversible inhibitor of MAO-A) at 20 mg/kg inhibited the activity of MAO-A but not MAO-B in the hippocampus and frontal cortex of mice.
Effect of IRN on the Levels of 5-HT, NE and DA in Brain of Mice
The effects of IRN and fluoxetine on the levels of 5-HT, NE and DA in the hippocampus of mice were shown in Table 2. The mice treatment of IRN (20 and 40 mg/kg) significantly elevated the contents of 5-HT [F(4, 45) = 14.265, p < 0.05 and p < 0.001, respectively] and NE [F(4, 45) = 16.513, p < 0.001 and p < 0.001, respectively] in the hippocampus of mice, as compared with the vehicle control group. Moreover, treatment with fluoxetine (20 mg/kg) could also significantly increase the levels of 5-HT (p < 0.001) and NE (p < 0.001) in the hippocampus of mice.
On the other hand, the effects of IRN and fluoxetine on the levels of 5-HT, NE and DA in the frontal cortex of mice were shown in Table 3. The mice treatment of IRN (20 and 40 mg/kg) significantly elevated the contents of 5-HT [F(4, 45) = 14.265, p < 0.001 and p < 0.001, respectively] and NE [F(4, 45) = 8.610, p < 0.01 and p < 0.001, respectively] in the frontal cortex of mice, as compared with the vehicle control group. Equally, treatment with fluoxetine (20 mg/kg) could also significantly increase the levels of 5-HT (p < 0.001) and NE (p < 0.001) in the frontal cortex of mice. However, no significant changes in the level of DA were observed after IRN or fluoxetine treatment in the hippocampus [F(4, 45) = 0.082] and frontal cortex [F(4, 45) = 0.295] of mice.
Discussion
Animal models are widely used in pre-clinical antidepressant evaluation and to provide insights into the neuropathology of depression. Both displaying a depressive behavior of despair or hopelessness, the tail suspension and forced swimming tests are thought to be highly predictable for antidepressant effects in humans [30]. The two depressive models are widely used for the screening of antidepressant activity [22, 31]. Our results demonstrated that treating mice with IRN (10–40 mg/kg) for 7 days could significantly reduce the immobility time in both the forced swimming and tail suspension tests (Figs. 2, 3).
In these behavioral tests, false-positive results can be obtained for agents that stimulate locomotor activity [32]. Therefore, the effect of IRN on locomotion was evaluated by the open-field test. The results showed that IRN or fluoxetine treatment did not change the number of crossings and rearings, suggesting that the reduction of immobility time elicited by IRN treatment in the forced swimming test and tail suspension test was not associated with stimulating motor activity.
It is well known that monoamine neurotransmitters such as NE and 5-HT in central nervous system play a key role in the pathophysiology of depression and the alterations in the central nervous system are associated with the mechanisms of action underlying the therapeutic activity of antidepressant drugs [33–35]. Therefore, reversal of reserpine-induced ptosis, hypothermia and suppression of locomotor activity in mice by treatment of IRN was performed in the present study to explore the monoamine mechanisms in the brain of mice. Reserpine can irreversibly inhibit the vesicular uptake of monoamines, including NE, DA and 5-HT and its metabolites. As a consequence, ptosis is observed in animals suffering from a depletion of monoamines [25, 36]. The symptom can be reversed by major classes of antidepressant drugs. The results obtained in the present study indicated that treating mice with IRN markedly antagonized the ptosis induced by reserpine. Moreover, IRN treatment could also significantly elevate the levels of 5-HT and NE in the hippocampus and frontal cortex of mice. Such an observation suggested that the antidepressant-like effect of IRN may be caused by the preservation of monoamine neurotransmitters.
MAO plays a major role in the pathogenesis of psychiatric disorders, particularly depression. MAO inhibitors are known to enhance the availability of biogenic amines (5-HT, NE or DA) at the synapse [37]. Two MAO isozymes are distinguished on the basis of their substrate preferences and sensitivity to inhibition by the MAO inhibitors, MAO type A (MAO-A) and MAO type B (MAO-B) [38]. MAO-A preferentially metabolizes 5-HT and NE, which are the monoamines most closely linked to depression. MAO-B inhibition in the human brain principally reduces the catabolism of DA and NE [39]. In order to verify whether the increase in monoamines, 5-HT and NE after IRN administration resulted from the inhibition of MAO activity, we measured the MAO activity in the hippocampus and frontal cortex of mice. The results showed that IRN (20 and 40 mg/kg) treatment significantly inhibited the activity of MAO-A, while it had no effect on MAO-B activity. Generally, preservation of monoamine neurotransmitters can be achieved either by inhibiting their reuptake or through the monoamine oxidase mechanism. Therefore, these findings suggested that the antidepressant-like effect of IRN may be mediated by inhibition the activity of MAO-A.
In conclusion, IRN exerts antidepressant-like effect in the force swimming test and tail suspension test in mice. The results demonstrated that the antidepressant-like effect of IRN is mediated, at least in part, via modulating the central monoaminergic neurotransmitter system and inhibition of the activity of MAO-A. Further investigations are needed to reveal the antidepressant-like effect mechanisms of IRN.
References
Kessler RC (2012) The costs of depression. Psychiatr Clin North Am 35:1–14
Manji HK, Drevets WC, Charney DS (2010) The cellular neurobiology of depression. Nat Med 7:541–547
Covington HE 3rd, Vialou V, Nestler EJ (2010) From synapse to nucleus: novel targets for treating depression. Neuropharmacology 58:683–693
Laakmann G, Dienel A, KIeser M (1998) Clinical significance of hyperforin for the efficacy of Hypericum extracts on depressive disorders of different severities. Phytomedicine 5:435–442
Jiang ML, Zhang ZX, Li YZ, Wang XH, Yan W, Gong GQ (2015) Antidepressant-like effect of evodiamine on chronic unpredictable mild stress rats. Neurosci Lett 588:154–158
Xu Y, Ku BS, Yao HY et al (2005) The effects of curcumin on depressive-like behaviors in mice. Eur J Pharmacol 518:40–46
Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH et al (2005) Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav 82:200–206
Liu B, Xu C, Wu X, Liu F, Du Y, Sun J et al (2015) Icariin exerts an antidepressant effect in an unpredictable chronic mild stress model of depression in rats and is associated with the regulation of hippocampal neuroinflammation. Neuroscience 294:193–205
Gong MJ, Han B, Wang SM, Liang SW, Zou ZJ (2016) Icariin reverses corticosterone-induced depression-like behavior, decrease in hippocampal brain-derived neurotrophic factor (BDNF) and metabolic network disturbances revealed by NMR-based metabonomics in rats. J Pharm Biomed Anal 123:63–73
Chen J, Lin D, Zhang C (2015) Antidepressant-like effects of ferulic acid: involvement of serotonergic and norepinergic systems. Metab Brain Dis 30:129–136
Kang TH, Murakami Y, Takayama H, Kitajima M, Aimi N, Watanabe H et al (2004) Protective effect of rhynchophylline and isorhynchophylline on in vitro ischemia-induced neuronal damage in the hippocampus: putative neurotransmitter receptors involved in their action. Life Sci 76:331–343
Yuan D, Ma B, Yang JY (2009) Anti-inflammatory effects of rhynchophylline and isorhynchophylline in rat N9 microglial cells and the molecular mechanism. Int Immunopharmacol 9:1549–1554
Shimada Y, Goto H, Itoh T, Sakakibara I, Kubo M, Sasaki H et al (1999) Evaluation of the protective effects of alkaloids isolated from the hooks and stems of Uncaria sinensis on glutamate-induced neuronal death in cultured cerebellar granule cells from rats. J Pharm Pharmacol 51:715–722
Kanatani H, Kohda H, Yamasaki K (1985) The active principle of the branchlets and hook of Uncaria sinensis Oliv. examined with a 5-hydroxytryptamine receptor-binding assay. J Pharm Pharmacol 37:401–404
Matsumoto K, Morishige R, Murakami Y (2005) Suppressive effects of isorhynchophylline on 5-HT2A receptor function in the brain: behavioural and electrophysiological studies. Eur J Pharmacol 517:191–199
Lu JH, Tan JQ, Durairajan SS (2012) Isorhynchophylline, a natural alkaloid, promotes the degradation of alpha-synuclein in neuronal cells via inducing autophagy. Autophagy 8:98–108
Xian YF, Lin ZX, Mao QQ, Ip SP, Su ZR, Lai XP (2012) Protective effect of isorhynchophylline against β-amyloid-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol 32:353–360
Xian YF, Lin ZX, Mao QQ, Zhao M, Hu Z, Ip SP (2012) Bioassay-guided isolation of neuroprotective compounds from Uncaria rhynchophylla against beta-amyloid-induced neurotoxicity in PC12 cells. Evid Based Complement Alternat Med 2012:802625
Xian YF, Mao QQ, Wu JC, Su ZR, Chen JN, Lai XP et al (2014) Isorhynchophylline treatment improves the amyloid-β-induced cognitive impairment in rats via inhibition of neuronal apoptosis and tau protein hyperphosphorylation. J Alzheimers Dis 39:331–346
Huang B, Wu Q, Wen G, Lu Y, Shi J (2001) The distribution of isorhynchophylline in the tissues of the rats and the determination of its plasma half-time. Acta Academiae Medicinae Zunyi 24:119–120
Haginiwa J, Sakai S, Aimi N, Yamanaka E, Shinma N (1973) Studies of plants containing indole alkaloids. 2. On the alkaloids of Uncaria rhynchophylla Miq. Yakugaku Zasshi 93:448–452
Porsolt RD, Pichon MLE, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressant. Arch Int Pharmacodyn Ther 229:327–336
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85:367–370
Herrera-Ruiz M, García-Beltrán Y, Mora S (2006) Antidepressant and anxiolytic effects of hydroalcoholic extract from Salvia elegans. J Ethnopharmacol 107:53–58
Bourin M, Poncelet M, Chermat R, Simon P (1983) The value of the reserpine test in psychopharmacology. Arzneimittelforschung 33:1173–1176
Sánchez-Mateo CC, Bonkanka CX, Prado B, Rabanal RM (2007) Antidepressant activity of some Hypericum reflexum L. fil. Extracts in the forced swimming test in mice. J Ethnopharmacol 112:115–121
Yu ZF, Kong LD, Chen Y (2002) Antidepressant activity of aqueous extracts of Curcuma longa in mice. J Ethnopharmacol 83:161–165
Zhou BH, Li XJ, Yang D (2006) Effect of apocynum venetum on the activity of MAO in mice. China Pharmacist 9:689–692
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Fuchs E, Fliugge G (2006) Experimental animal models for the simulation of depression and anxiety. Dialogues Clin Neurosci 8:323–333
Yin C, Gou L, Liu Y (2011) Antidepressant-like effects of l-theanine in the forced swim and tail suspension tests in mice. Phytother Res 25:1636–1639
Bourin M, Fiocco AJ, Clenet F (2001) How valuable are animal models on defining antidepressant activity? Hum Psychopharmacol 16:9–21
Jans LA, Riedel WJ, Markus CR, Blokland A (2007) Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry 12:522–543
Savegnago L, Jesse CR, Pinto LG, Rocha JB, Nogueira CW, Zeni G (2007) Monoaminergic agents modulate antidepressant-like effect caused by diphenyl diselenide in rats. Prog Neuropsychopharmacol Biol Psychiatry 31:1261–1269
Yi LT, Li YC, Pan Y (2008) Antidepressant-like effects of psoralidin isolated from the seeds of Psoralea corylifolia in the forced swimming test in mice. Prog Neuropsychopharmacol Biol Psychiatry 32:510–519
Dhingra D, Sharma A (2006) Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog Neuropsychopharmacol Biol Psychiatry 30:449–454
Bryant SG, Brown CS (1986) Current concepts in clinical therapeutics: major affective disorders. Part 1. Clin Pharm 5:304–318
Johnston JP (1968) Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol 17:1285–1297
Foley P, Gerlach M, Youdim MB, Riederer P (2000) MAO-B inhibitors: multiple roles in the therapy of neurodegenerative disorders. Parkinsonism Relat Disord 6:25–47
Acknowledgements
This study was partially supported by a Seeding Fund from the School of Biomedical Sciences, Faculty of Medicine, The Chinese University of Hong Kong (Project Number: 2015.1.081).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xian, YF., Fan, D., Ip, SP. et al. Antidepressant-Like Effect of Isorhynchophylline in Mice. Neurochem Res 42, 678–685 (2017). https://doi.org/10.1007/s11064-016-2124-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2124-5