Abstract
The study assessed involvement of Ca2+ signaling mediated by the metabotropic glutamate receptors mGluR1/5 in brain tolerance induced by hypoxic preconditioning. Acute slices of rat piriform cortex were tested 1 day after exposure of adult rats to mild hypobaric hypoxia for 2 h at a pressure of 480 hPa once a day for three consecutive days. We detected 44.1 ± 11.6 % suppression of in vitro anoxia-induced increases of intracellular Ca2+ levels and a fivefold increase in Ca2+ transients evoked by selective mGluR1/5 agonist, DHPG. Western blot analysis of cortical homogenates demonstrated a 11 ± 4 % decrease in mGluR1 immunoreactivity (IR), and in the nuclei-enriched fraction a 12 ± 3 % increase in IR of phospholipase Cβ1 (PLCβ1), which is a major mediator of mGluR1/5 signaling. Immunocytochemical analysis of the cortex revealed increase in the mGluR1/5 and PLCβ1 IR in perikarya, and a decrease in IR of the neuronal inositol trisphosphate receptors (IP3Rs). We suggest that enhanced expression of mGluR5 and PLCβ1 and potentiation of Ca2+ signaling may represent pro-survival upregulation of Ca2+-dependent genomic processes, while decrease in mGluR1 and IP3R IR may be attributed to a feedback mechanism preventing excessive intracellular Ca2+ release.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preconditioning of animals in vivo with episodes of mild hypoxia or ischemia of different forms, including mild hypobaric hypoxia (MHH), induces tolerance to subsequent severe hypoxia/ischemia and provides long-lasting morphological and behavioral neuroprotection [1–7]. This tolerance is accompanied by beneficial changes in the brain glutamatergic signaling that prevent excessive pathogenic up-regulation of Ca2+ transients induced by severe hypoxia [8]. However, the mechanisms of the delayed and long lasting tolerance to injurious hypoxia induced by hypoxic preconditioning are not sufficiently clear. Moreover different models of hypoxic preconditioning may have specific features.
Induction of tolerance uses complex signaling machinery. It is known from the literature that hypoxic or ischemic preconditioning increases the expression in the brain of the immediate early genes and different survival factors, including heat shock protein HSP70, antioxidants and antiapoptotic factors Bcl-2/Bclx1 and neuroprotective peptides [7, 9–14].
Hypoxia-inducible factor HIF1α [15–17] and transcription factors c-Fos, NGFI-A, pCREB and NFkB [18–21] have been suggested to be the main factors involved in preconditioning with ischemic as well as normobaric or hypobaric hypoxia. The majority of these mechanisms are initiated by Ca2+ dependent signal cascades, while the IP3R-mediated Ca2+ release (IICR) is an important source of Ca2+ supporting these activities. Consequently, there are grounds to expect that group I metabotropic glutamate receptors (GI mGluRs) and the PLC/IP3R/IICR-mediated branch of their canonical signaling might be involved in neuroprotective transcriptional brain responses to hypoxic preconditioning.
The effects of neuroprotective hypoxic procedures in vivo, and in particular of MHH, on the levels of proteins involved in the GI mGluRs-mediated Ca2+ signaling cascade in the brain cortex remains poorly understood. Sommer et al. [22] found no significant effect of in in vivo preconditioning with 2.5 min global forebrain ischemia on GI mGluRs expression in CA1 neurons of the Mongolian gerbil. Conversely, our previous studies demonstrated significant decreases in the intracellular membrane-bound Ca2+ level evoked by pharmacological stimulation of GI mGluRs in acute cortical slices obtained from rats submitted to preconditioning mild hypobaric hypoxia, which suggests potentiation of GI mGluR-mediated Ca2+ signaling [23, 24]. Interestingly, the pharmacological preconditioning of brain slices or primary neuronal cultures from adult rat brains with (S)-3,5-dihydroxyphenylglycine (DHPG), which is a selective agonist of GI mGluRs, was shown to induce tolerance to oxygen/glucose deprivation and to brain ischemia, suggesting that GI mGluRs may mediate the mechanisms of preconditioning [25–29]. However, it is important to differentiate between sensors or transducers involved in the induction of tolerance during the initial minutes to hours of preconditioning and those effectors which underlie tolerance over several days [16]. Here we focus on the latter.
The aim of this study was to evaluate the effects of hypoxic preconditioning on the GI mGluRs/PLC/IP3Rs/Ca2+ signal transduction pathway in the rat brain cortex and to determine whether the results conform to the hypothesis that GI mGluRs plays an instrumental role in induced brain tolerance. We investigated the effects of submitting the adult rats to MHH preconditioning. Changes in the levels of intracellular Ca2+ (Ca 2+i ) induced by in vitro anoxia or by application of the GI mGluRs agonist DHPG were measured in acute slices of piriform cortex. Moreover, protein expression of GI mGluR subtypes, PLCβ1 and IP3Rs was determined in this cortical region.
Materials and Methods
Animals
Adult male Wistar rats weighing 200–240 g were used. The animals were bred in the animal colony of the Mossakowski Medical Research Centre, Polish Academy of Sciences in Warsaw and in the Pavlov Institute of Physiology vivarium in Saint Petersburg. Rats were fed and watered ad libitum and kept on 12:12 h dark–light cycle at room temperature with constant humidity of approximately 60 %. Animal experiments were carried out according to domestic regulations and the European Community Council Directive of 24 November 1986 (86/609/EEC). Experimental protocols were approved by the local ethical committees.
Materials
Chemicals used were as follows: a selective mGluR1/5 agonist DHPG, the ratiometric calcium-sensitive fluorescent probe fura 2-AM and dimethyl sulphoxide (DMSO) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The following antibodies were used for immunohistochemistry: rabbit polyclonal antibodies against mGluR1 and mGluR5 (both 1:100) supplied by AbCam UK, antibodies against IP3R1 and PLCβ1 (both 1:50) and Rabbit ABC Staining System kit produced by Santa Cruz Biotechnology, Inc. USA, while secondary biotinylated antibodies (1:200), as well as Vectastain ABC Elite kit and 3,3-diaminobenzidine peroxidase substrate kit (1:100) were provided by Vector Laboratories USA. Chemicals for immunoblotting: rabbit polyclonal antibodies against mGluR1 (1:1000), secondary polyclonal antibody conjugated with alkaline phosphatase (1:5000) and antibodies against actin-β (1:100) were products of Sigma, USA, antibodies against GluR5 (1:2000) (UpState USA) and against PLCβ1 (1:200) were purchased from Santa Cruz Biotechnology, Inc. USA, and alkaline phosphatase kit III was from Vector Laboratories, USA.
Mild Hypobaric Hypoxia
Rats were exposed to hypobaric hypoxia in a 50 l “vacuum” box. The pressure inside the chamber was gradually reduced with pauses of a few minutes after every 133 hPa decrement. To avoid significant shifts in the pO2, pCO2, humidity and temperature during hypobaric exposure, the chamber was ventilated without pressure decrement. The rats were subject to hypobaric MHH sessions for 2 h at 480 hPa (equivalent to 5000 m asl), after which the pressure in the chamber was gradually restored to atmospheric. To induce hypoxic tolerance, the MHH sessions were applied once a day for three consecutive days. Control animals were submitted to the same procedures without hypobaric treatment. One day after the last session of MHH, or the sham procedure, the animals were anesthetized with chloral hydrate and decapitated. Their brains were rapidly removed for the preparation of acute cortical slices or for performing immunochemical and immunocytochemical analyses.
Piriform Cortex and Its Acute Slices
The histological preparations of piriform cortex were used for immunochemical analyses and the acute ex vivo slices were utilized for testing Ca2+ transients as in our previous papers [2, 3, 8, 23, 24, 30]. The piriform cortex was chosen because this structure—as well as the hippocampus, the cerebellum and the amygdala—is highly vulnerable to excitotoxicity and hypoxic damage [31]. Three types of glutamatergic neurons are distributed throughout layers II and III [32, 33] and the postsynaptic neurons of these layers are thought to be rich in GI mGluRs [34].
In ex vivo experiments the removed brains were bathed in ice-cold artificial cerebro-spinal fluid (ACSF), containing 124 mM NaCl, 5 mM KCl, 2.6 mM CaCl2, 1.24 mM KH2PO4, 1.3 mM MgSO4, 30 mM NaHCO3, 10 mM glucose, pH 7.4 and equilibrated with O2. Tangential slices (400 µm) microtomed from piriform cortices, placed between −1 and +2.5 mm from bregma, were mounted on net holders and placed in spectrophotometric quartz cells of 1.5 ml volume equipped with a mini perfusion system made in our laboratory. A peristaltic pump was used to perfuse ACSF buffered additionally with 24 mM Tris–HCl (pH 7.4) at a temperature of 37 °C through the system at a rate of 1.2 ml/min. Before measurements the slices were allowed to recover under these conditions for 2.5 h.
Measurement of Changes in Intracellular Ca2+ Levels Induced by In Vitro Anoxia or DHPG Application
Relative changes in Ca 2+i were measured as described previously [30] using the ratiometric calcium-sensitive fluorescent probe fura-2. After preincubation with 3.3 µM fura-2/AM, the levels of fura-2 fluorescence intensity was measured at 340 and 380 nm excitation and 510 nm emission wavelengths, using a Hitachi F-2000 spectrofluorimeter (Hitachi, Tokyo, Japan). The measurements were corrected for autofluorescence, and, after normalization, the ratio of fluorescence induced by excitation at wavelengths of 340 nm to those of 380 nm was calculated [8, 30]. Changes in this fluorescence ratio, corresponding to changes in Ca 2+i , were presented as the percentage of the baseline fura-2 fluorescence. In addition, to more precisely evaluate the effects of the treatments on calcium transients, the cumulative rise in fura-2 fluorescence over the basal level was calculated within fixed timeframes following anoxia or DHPG application. To evaluate the long-term neuroprotective efficacy of in vivo preconditioning with MHH, and to provide positive control to pharmacologically-induced calcium transients (see below), slices of control and preconditioned animals were submitted to anoxia in vitro. Anoxic conditions were induced by replacing the oxygen-containing superfusion fluid for 10 min with the medium previously saturated with N2, which was followed by restoration of the normoxic superfusion. Our previous studies demonstrated that this test procedure is a potent stimulus, inducing a sustained injurious Ca2+ overload in cortical slices of control animals [30]. To induce GI mGluRs-mediated Ca2+ transients, DHPG was applied via the superfusion medium to cortical slices of control and preconditioned animals at a final concentration of 100 µM twice, each for 2 min with a 40 min interval.
Immunocytochemical Detection of GI mGluR Subtypes, IP3Rs and PLCβ1
Iimmunoreactivities (IR) of mGluR1, mGluR5, IP3Rs and PLCβ1 proteins were detected in piriform cortex collected 24 h after preconditioning or sham procedures (5–7 animals in each group). The rats were decapitated and brains were rapidly excised and fixed in 4 % paraformaldehyde in 0.1 M PBS (pH 7.3) for 24 h. Samples were then paraffinized and sectioned according to a routine histological protocol. The 7 µm sections, taken around a line −2.8 mm from the bregma, were deparaffinized and incubated overnight at +4 °C with polyclonal rabbit antibodies against rat mGluR1, mGluR5, IP3Rs or PLCβ1. The sections were further processed using the rabbit ABC Staining System kit according to the standard protocol. Diaminobenzidine was used as a chromogenic substrate to visualize the immunopositive cells. Sections were mounted and assayed with an image analysis system consisting of a light microscope (Carl Zeiss, GmBH, Berlin, Germany), digital camera (Baumer Optronic, GmBH, Radeberg, Germany) and ImageJ MacBiophotonic software (NIH, Bethesda, MD, USA). GI mGluRs expression was quantified in neuronal cell bodies and in the neuropil, which is rich in synaptic endings. Standard fields of piriform cortex seen under 20× objective were outlined in layer II and III separately and their average optical density was measured against background. The obtained values were presented as the percentage of values measured in white matter. The IP3Rs immunoreactive cells were automatically quantified in fields of 400 × 80 µm for layer II and 400 × 160 µm for layer III. All the immunopositive cells were divided into two classes. Slightly labeled (class 1), was formed by objects ranged within 3 and 12 arbitrary units of grey scale above the background, and highly labeled (class 2) included objects with the staining intensity above 12 units. Values obtained from two fields in each hemisphere were averaged for each section. A similar approach was used to quantify the PLCβ1 immunopositive neurons, and, separately, their nuclei.
Western Blot
The quantitative Western blot analysis of the expression of mGluR1, mGluR5 and PLCβ1 proteins was performed in homogenates of piriform cortex of control and MHH treated animals. Cortical samples of 5–6 animals were prepared for each group as described by Wang et al. [35]. Briefly, the cortices from both hemispheres were collected 24 h after MHH or sham procedure and homogenized in a glass-Teflon homogenizer in ice cold buffer containing 25 mM trisHCl, 0.32 M sucrose and 1:100 v/v Protease Inhibitor Cocktail for general use (Sigma-Aldrich), containing 2 mM AEBSF, 0.3 μM Aprotinin, 116 μM Bestatin, 14 μM E-64, 1 μM Leupeptin and 1 mM EDTA, pH 7.4. After protein determination using the Bradford protein assay (Bio-Rad) [36], each sample (50 µg of protein) was subjected to 7 % SDS-polyacryl-amide gel electrophoresis. Samples were then transferred to nitrocellulose membranes and were blocked by 5 % milk-TBS containing 1 % Tween 20 (TBST). The membranes were incubated overnight with primary rabbit monoclonal antibodies against mGluR1, mGluR5, PLCβ1, or β-actin proteins, the latter in order to control the amounts of protein loaded onto the gel. After washing in TBST, the membranes were incubated for 2 h with monoclonal anti-rabbit conjugated secondary antibodies diluted in 2 % milk-TBST solution. The membranes were then washed with TBST and TBS and the IR was detected by alkaline phosphatase substrate kit III. The IR of PLCβ1 was determined in native homogenates as well as in their nuclei-free supernatants, which were obtained by centrifugation of the native homogenates at 1000×g for 10 min. Bands were scanned using Image Scanner II with Labscan 6.0 software (GE Helthcare Bio-Sciences, Uppsala, Sweden) and quantyfication was performed using image analysis software ImageQuant TL, Amersham Biosciences, Uppsala, Sweden.
Statistical Analysis
The results are presented as mean ± SEM. The statistical significance of differences between means taken at p < 0.05 was tested using Student t test statistics. The number of rats per experimental group was 6–9.
Results
MHH Preconditioning In Vivo Differentially Modifies Calcium Transients In Cortical Slices Induced by In Vitro Anoxia and Application of GI mGluRs Agonist
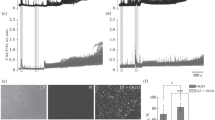
In the initial experiment we studied effects of preconditioning of rats with in vivo MHH on increases in Ca 2+i in brain slices induced by in vitro anoxia. As shown in Fig. 1, application of 10 min anoxia to control cortical slices induced 32.3 ± 0.6 % rise in Ca 2+i , with a rapid decrease almost to the baseline within 10–15 min of reoxygenation. This was followed by the secondary increase in Ca 2+i of 18.9 ± 0.6 % above baseline after 60 min of normoxic perfusion. MHH preconditioning reduced to 13.8 ± 0.9 % the pick level of the anoxic increase in Ca 2+i and also diminished the maximal secondary postanoxic rise in Ca 2+i to 9.8 ± 0.6 %. The mean anoxia-evoked cumulative increase in fura-2 fluorescence during 10 min of anoxia and 60 min of reoxygenation was significantly (p < 0.05) reduced after MHH preconditioning to only 438 ± 51 % (Fig. 1, area under solid line) compared to the control values of 783 ± 48 % (Fig. 1, area under dotted line), that indicates that MHH suppresses the calcium overload induced by anoxic test.
In vitro anoxia-evoked changes in intracellular calcium level in acute slices of rat piriform cortex of control (dotted line) and MHH preconditioned (solid line) animals. Black bar shows the time of anoxia. Values represent mean ± SEM (n = 8 for each curve). The differences between control and MHH values are significant (p < 0.05) at all time points except of 10, 15, 25 and 30 min
In subsequent experiments the cortical slices of the control and MHH preconditioned rats were submitted to the pharmacological stimulation of GI mGluRs. As presented in Fig. 2, repeated application of 100 µM DHPG to the superfusion medium induced relatively slight (1–2 %) and transient increases in Ca 2+i in the cortical slices of control rats. These calcium transients evoked by administration of DHPG were significantly potentiated in slices collected from the rats 24 h after MHH. The cumulative mean increase in fura-2 fluorescence induced after MHH by the first DHPG treatment (Fig. 2, area under solid line taken between 0 and 42 min) was 207.7 ± 19.4 %, and this value was significantly (p < 0.05) higher than 40 ± 8.3 % increase observed in control slices (Fig. 2, area under dotted line in the same time window). Thus, present data indicate that MHH enhances GI mGluRs signaling resulting in potentiation of the induction of calcium signals.
In vitro DHPG (100 μM)-evoked changes in intracellular calcium level in acute slices of rat piriform cortex of control (dotted line) and MHH preconditioned (solid line) animals. Black bars show duration of DHPG application. Values represent mean ± SEM (n = 7 for each curve). The differences between control and MHH values are significant (p < 0.05) starting with 7th minute
MHH Preconditioning Modifies Expression of GI mGluRs Proteins in Rat Piriform Cortex
Microscopic observations of the control rat cortex demonstrated uniform distribution of mGluR1 and mGluR5 IR in neuropil, contrasting with low IR in cell bodies (Fig. 3). Comparison of the mGluR1/5 IR in piriform cortex of control and MHH-pretreated groups of rats revealed no obvious differences in neuropil, but did show increased IR of both GI mGluRs subtypes in cytozol and/or perinuclear regions in a portion of cells. Panels b, d, f, h of Fig. 3 show representative pictures of increased somatic IR of both GI mGluR subtypes after MHH in layer II. In addition, in homogenates of piriform cortex of control and MHH-treated rats the total protein levels of GI mGluR subtypes were determined by Western blotting. Figure 4 shows representative blots (a) and the results of densitometric analysis of all the blots (b). The samples from the MHH-treated group showed a statistically significant (p < 0.05) decrease of mGluR1 IR to 88.9 ± 4.1 %, whereas the decrease in mGluR5 IR was not significant (Fig. 4).
Effect of MHH preconditioning on mGluR1 and mGluR5 immunoreactivity in principal neurons of piriform cortex. Representative photomicrographs of immunoreactivity of mGluR1 (a–d) and mGluR5 (e–h) in preparations obtained from control (a, b, e, f—CONTROL) or MHH treated (c, d, g, h—MHH) rats. Scale bar 50 µm for a, c, e and g see on e. Scale bar 10 µm for b, d, f and h see on f. Bottom panel represents densitometric quantification of mGluR1 (i) and mGluR5 (j) immunoreactivity in neuropil of layers II and III (nII and nIII columns, respectively) and cell bodies of layer II (cbII columns) in preparations of MHH treated (MHH) and sham (CONTROL) rats. Values represent mean ± SEM (n = 6 for each column). Asterisks mark result significantly different from control
MHH Preconditioning Increases Expression of PLCβ1 but Decreases Protein Levels of IP3Rs in Rat Piriform Cortex
In cortical slices of control rats the immunocytochemically assayed PLCβ1 protein had a typical plasmolemmal distribution (Fig. 5a, c). After MHH, PLCβ1 IR increased inside the cell bodies (Fig. 5e). In cortical layer II of the control rats morphometric analysis revealed low PLCβ1 IR in 64 ± 9 % of neurons, with those remaining representing high IR, while in the same cortical layer of MHH-pretreated rats 67 ± 7 % of neurons presented high cytoplasmic PLCβ1 IR (Fig. 5e). Control cortical preparations showed very low nuclear location of PLCβ1 IR in all the neurons; however, in MHH-pretreated rats 69 ± 4 % (layer II) and 48 ± 5 % (layer III) of neurons showed PLCβ1 IR in the nuclei (Fig. 5f). These differences between control and MHH-pretreated rats in the intensity and location of PLCβ1 IR were statistically significant (p < 0.05).
Effect of MHH preconditioning on PLCβ1 immunoreactivity in principal cortical neurons. Representative photomicrographs of PLCβ1 immunoreactivity in preparations obtained from control (a, c—CONTROL) and MHH treated (b, d—MHH) rats. Scale bars 50 µm (on a) for a and b and 10 µm (on c) for c and d. Bottom panel represents percentage of cell numbers in layers II and III with low and high degree of cytoplasmic (e) or nuclear (f) IR (1 and 2 classes, respectively) in control and MHH preparations. Values represent mean ± SEM (n = 5–7 for each column). Asterisks mark results significantly different from corresponding controls
Western blots representing expression of PLCβ1 protein in full homogenates of cortical samples (Fig. 6) showed a 12 ± 3 % increase in the level of this enzyme’s protein in MHH-preconditioned rats (p < 0.05). This effect completely disappeared after centrifugation of homogenates and subsequent blotting of their “nuclei-free” fractions (Fig. 6b), supporting observations of Fig. 5 that an increase in PLCβ1 IR occurs in the nuclear fraction.
Effect of MHH preconditioning on PLCβ1 immunoreactivity in cortical homogenates. a Representative Western blots, b results of the densitometric quantification of the blots obtained from total cortical homogenates [PLCβ1(t)] and those obtained from centrifuged, nuclei-free homogenates [PLCβ1(nf)]. Values represent mean ± SEM (n = 5–6 for each column). Asterisk marks results significantly different from control
As shown in Fig. 7, in the preparations of control animals the IP3Rs IR was generally revealed in cell bodies and proximal parts of dendritic stems contrasting to neuropil. The preparations of MHH-pretreated animals show a decrease of the IR, especially in dendritic stems and peripheral somatic regions (Fig. 7a). Morphometric analysis of the control preparations demonstrated equal proportions of neurons with low and high IP3Rs IR in layer II, whereas in layer III, 72 ± 8 % of neurons presented low IP3Rs IR. After MHH, the percentage of cells with high IR (class 2) decreased significantly (p < 0.05) to 24 ± 6 % in cortical layer II, with a corresponding increase of neurons with low IR. A similar trend noticed in layer III was statistically insignificant (Fig. 7b).
Effect of MHH preconditioning on IP3Rs immunoreactivity in principal cortical neurons. a Representative photomicrographs of IP3Rs immunoreactivity in cortical preparations obtained from the control (CONTROL) and MHH treated (MHH) rats. Scale bar 50 µm. b Percentage of cell numbers with low and high degree of IR (1 and 2 classes, respectively) in layers II and III of piriform cortex. Values represent mean ± SEM (n = 5–7 for each column). Asterisks mark results significantly different from corresponding controls
Discussion
The results of this study demonstrate that MHH preconditioning in vivo reduces Ca2+ transients induced by in vitro test anoxia in cortical slices, but potentiates DHPG-evoked increases in Ca 2+i . Immunocytochemical examination of piriform cortex in the MHH-treated group showed relative increases in the portion of neurons with high mGluR1, mGluR5 and PLCβ1 IR in soma or perikaryon. Western blotting of homogenates of piriform cortex in MHH-preconditioned rats shows a decrease in mGluR1 and an increase in PLC β1 protein levels; the latter effect being dependent on the presence of nuclei in homogenate. We consider these results to be compatible with the hypothetical role of GI mGluR signaling in the mechanisms of brain tolerance induced by MHH preconditioning. In turn a decrease in IP3R IR found in piriform cortex of the preconditioned group, may be attributed to negative feedback mechanisms preventing excessive IICR.
In the present study, we have used a well characterized model of preconditioning with episodes of MHH repeated over 3 days. Many previous studies have demonstrated that MHH applied for pre- and postconditioning provides neuroprotection which is detectable in in vivo, ex vivo and in vitro tests [3–5, 7, 10, 11, 13, 14, 20, 21, 37]. Here we utilized cortical slices and the ratiometric calcium sensitive fluorescent probe fura-2 for measuring Ca2+ transients evoked by anoxia or pharmacological stimulation of mGluR1/5. Previously, we used the same in vitro system, often supplemented with the application of the fluorescent probe chlortetracycline, which shows the level of intracellular bound calcium (Cab)—in studies characterizing Ca2+ transients induced by anoxia or glutamate receptor agonists [8, 23, 30, 38].
The results of this study demonstrate for the first time a significant suppression by MHH preconditioning in vivo of the primary and secondary increases in Ca 2+i induced by in vitro test anoxia (Fig. 1). This result resembles the protective effects of a rapid in vitro preconditioning with short 2-min anoxia which we observed previously [30], and is also consistent with previous works showing that ischemic preconditioning ameliorates excitotoxicity by down-regulating ionotropic NMDA- and AMPA-sensitive glutamate receptors [39, 40]. In addition, the current data support our previous study on the role of NMDA receptor-mediated Ca2+ influx to neurons in mediating anoxia-induced increases in Ca2+ in cortical slices [38]. Our data confirm that MHH preconditioning induces tolerance to anoxia and protects against the injurious Ca2+ overload of neurons. Furthermore, anoxia-induced increases in Ca 2+i provided a positive control to the experiments where we measured mGluR1/5 mediated calcium transients in neurons.
In contrast to that discussed above, where anoxia-induced increases in Ca 2+i result, mainly, from Ca2+ influx into cells via NMDA receptors [38], moderate increases in Ca 2+i evoked by administration of the mGluR1/5 agonist DHPG (Fig. 2) can be attributed to the activation of the signaling pathway, leading to release of Ca2+ from intracellular stores in endoplasmic reticulum (ER). The current results are consistent with our previous data demonstrating that application of DHPG to control cortical slices resulted in an increase in Ca 2+i with a corresponding decrease in Cab, reflecting mobilization of intracellular Ca2+ stores [8, 24]. The intracellular origin of Ca2+ transients in neurons challenged with DHPG was also demonstrated directly in other studies [41]. The present data show significant potentiation of DHPG-induced increases in Ca 2+i in cortical slices of MHH-preconditioned rats (Fig. 2), and this effect correlates with previous work showing potentiation of DHPG-evoked decrease in Cab in slices collected from animals preconditioned with MHH [24]. Based on these data we assume that preconditioning with MHH leads to potentiation of mGluR1/5-mediated signaling in the cortex, resulting in increased IICR. Bickler et al. [42] described potentiation of the NAD(P)H-triggered and IP3 receptor-mediated increase of the intracellular Ca2+ concentration and in phosphorylation of MAP kinases in the organotypic cultures of the hippocampal slices submitted to hypoxic preconditioning, which was interpreted as a potent upstream pro-survival signal for increased expression of anti-apoptotic genes. There are numerous data indicating neuroprotective effects of stimulation of GI mGluRs subtypes [26, 27, 43]. Enhanced expression of transcription factors and immediate early genes which are involved in nerve cell survival, have been directly associated with moderate increases of intracellular Ca2+ level mediated by GI mGluRs [44, 45]. Therefore, facilitation of mGluR1/5-mediated Ca2+ transients, which we have observed here, may be a component of the mechanism of tolerance induced by MHH preconditioning.
Considering the possible mechanisms of potentiation of GI mGluR-mediated Ca2+ signaling in the cerebral cortex of rats preconditioned with MHH, we assessed the levels of expression and cellular location of mGluR1, mGluRs5, PLCβ1 and IP3Rs proteins using immunochemical methods. Expression of GI mGluRs proteins in the MHH-treated group showed a relatively small (11.1 ± 4.1 %) reduction of mGluR1 IR in Western blotting of the cortical homogenates (Fig. 4); however, a significant increase in the number of principal pyramids with high levels of GI mGluRs (especially of mGluR5) IR in cell bodies and/or the perinuclear area were observed in the immunocytochemical assays (Fig. 3). Western blotting of the homogenates of piriform cortex provides information mainly about neuropil, which occupies more than 85 % of its volume, and therefore may not be sensitive enough to detect changes of intrasomatic expression of GI mGluRs demonstrated immunocytochemically.
A decrease of mGluR1 protein expression in Western blotting may reflect internalization of the receptor protein, triggered by its increased activation. There are known differences in the mechanisms and intensity of mGluR1 and mGluR5 endocytosis [46, 47], which may explain uneven changes in the expression of mGluR1 and mGluR5 proteins. The mGluR1 protein may play a major role in neurodegenerative mechanisms [48–50], whereas mGluRs5 is involved in the prosurvival signaling pathways, including expression and accumulation of BDNF [49, 51], phosphorylation of CREB and Elk-1 [45] and regulation of the members of the NFkB family [52]. Thus, the shift of balance between the subtypes of GI mGluR toward mGluR5 after MHH suggests that mGluR5 and Ca2+ signaling mediated by these receptors may be involved in the mechanisms of preconditioning.
Increased perinuclear GI mGluRs IR after MHH which was observed in the immunocytochemical examination may be attributed to the mechanisms of MHH-induced tolerance. Intraneuronal location of a portion of GI mGluR proteins in the ER and nuclear membranes is widely recognized [52, 53]. Possibly, MHH may activate de novo synthesis of GI mGluRs and their trafficking toward the nucleus and/or induce direct translocation of the receptors from synaptic to intracellular sites. This may facilitate nuclear IP3-mediated signaling using nucleolemmal PLCβ1 and IP3Rs. This supposition is supported by our immunochemical data, which evidenced increased expression of nuclear PLCβ1 after MHH (see below).
A canonical signaling pathway triggered by stimulation of GI mGluRs leads to the G protein-mediated activation of PLC. The results of our immunocytochemical examination showed a significant increase in the portion of neurons containing high cytoplasmic and nuclear PLCβ1 IR in the cortical preparations after MHH treatment (Fig. 5). Consistent with this finding, the results of Western blot analysis demonstrated that the increase in PLCβ1 IR was exclusive to nuclei-containing cortical homogenates of MHH-preconditioned rats (Fig. 6). For PLCβ1 immunoassaying commercial polyclonal rabbit antibody was used, which conjugates with both (a) and (b) splice isoforms of the enzyme; however, only PLCβ1(b) is ultimately located in the nucleus [54]. Our data showing PLCβ1 overexpression in the nuclei-containing, but not in the nuclei-free fraction of the homogenate, may reflect the activation of a, mainly, nuclear PLCβ1b/IP3/Ca2+ signaling pathway, which is known to contribute to posttranscriptional processing of the pro-survival gene expression [55]. Overexpression of PLCβ1 and PLCγ1 in the brain has been demonstrated after GI mGluRs activation by their agonist DHPG or by some antioxidants [43, 56], and this effect has been attributed to pro-survival cascades protecting cells from death induced by oxidative or ER stress [57, 58].
Activation of PLCβ1 by various neuronal Gq/11 protein-coupled receptors, including GI mGluRs, is followed by increased IP3 production (for a review see [59] ), leading to stimulation of IP3Rs and IICR. The changes in the expression or location of mGluR5 and PLCβ1 proteins in the cerebral cortex of rats preconditioned with MHH may explain potentiation of DHPG-evoked Ca2+ transients. Paradoxically, we found the opposite changes of expression of IP3Rs proteins after MHH, i.e. a decrease in the number of neurons with high IP3R IR in layers II and III of piriform cortex (Fig. 7). The mechanism of this effect is unclear. Down regulation of IP3Rs may result from suppression of IP3R mRNA and/or protein expression, or from activation of their degradation [60]. Suppression of IP3R mRNA was found in brains of rats [61] and gerbils [62, 63] after transient ischemic insults. Degradation consists of IP3Rs ubiqitination and proteosomal degradation in ER, which is caused by a long lasting increase of IP3 and Ca2+ levels induced by agonists of certain types of PLC-linked GPCRs [64].
We propose that MHH-mediated down regulation of IP3Rs should be attributed to neuroprotective compensatory mechanisms, since they may prevent potentially injurious excessive release of Ca2+ to cytosol from the ER stores. Strictly controlled potentiation of Ca2+ signaling, which plays a crucial role in the mechanisms of adaptive neuronal plasticity, including induced tolerance to hypoxia ischemia (for a review see [65] ), must be distinguished from the excessive and uncontrolled release of Ca2+ from ER in nerve cells and axons which leads to their damage and death [66]. Therefore, the interpretation of the effects of MHH preconditioning on the expression of GI mGluRs, PLCβ1, and IP3Rs proteins, and of the expected changes in the signal transduction pathways mediated by these proteins, should be related to their functional output, i.e. to alterations in the intracellular Ca2+ level and the resulting survival or death of neurons. Recently, using the same experimental techniques, we found that submitting not preconditioned rats to severe hypobaric hypoxia (SHH), consisting of one 3-h session of 240 hPa (equivalent to 11,000 m asl), which is known to induce brain damage [14], upregulates PLCβ1 IR much more strongly than MHH, but has no suppressive effect on IP3Rs IR [24]. We assume that the specific balance between the levels and activities of mGluRs, PLCβ1 and IP3Rs after MHH preconditioning results in moderate increases in Ca 2+i leading to activation of the pro-survival signaling [67], whereas changes observed after SHH may lead to excessive production of IP3 followed by potentially injurious enhancement of IICR to the cytosol and nucleus.
Our data suggests that in vivo MHH may result in detuning of GI mGluR-mediated Ca2+ signaling in the rat cortex, which is consistent with the hypothesis that GI mGluRs play a role in the mechanisms of induced brain tolerance to hypoxia; however, further studies are required to test this. Amongst other things, the role of mGluR1 versus mGluR5 should be directly tested using the selective antagonists of these receptors in both in vivo and in vitro experiments. Furthermore, ex vivo tests should compare the effect of MHH on hypoxia-sensitive and hypoxia-resistant brain regions.
Our study demonstrates that in vivo preconditioning of rats with MHH suppresses the anoxia-induced increases in Ca 2+i but potentiates DHPG-evoked Ca2+ transients in cortical slices. Western blot analysis of the homogenates of piriform cortex showed a decrease in mGluR1 IR and an increase in PLCβ1 IR, which appears to be related to the nuclear fraction. Immunocytochemical examination demonstrated relative increases in mGluR5 and PLCβ1 IR in soma or perikaryon and a decrease in IP3R IR in neurons of piriform cortex. These results are consistent with the hypothetical role of GI mGluR-mediated Ca2+ signaling in brain tolerance induced by hypoxic preconditioning. The enhanced expression of mGluR5 and PLCβ1 protein in perikarya and potentiation of Ca2+ signaling may represent pro-survival upregulation of Ca2+ dependent genomic processes, whereas a decrease in mGluR1 and IP3R IR may be attributed to negative feedback mechanisms preventing excessive intracellular Ca2+ release.
References
Schaller B, Graf R (2002) Cerebral ischemic preconditioning. An experimental phenomenon or a clinical important entity of stroke prevention? J Neurol 249:1503–1511
Samoilov MO, Lazarevich EW, Semenov DG, Mokrushin AA, Tyul’kova EI, Romanovskii DY, Milyakova EA, Dudkin KN (2003) The adaptive effects of hypoxic preconditioning of brain neurons. Neurosci Behav Physiol 33:1–11
Semenov DG, Miller OL, Samoilov MO (2004) Effect of in vivo hypoxic preconditioning on changes in intracellular calcium content induced by long term anoxia in rat brain slices. Bull Exp Biol Med 138:338–340
Rybnikova EA, Khozhai LI, Tyulkova EI, Glushchenko TS, Sitnik NA, Pelto-Huikko M, Otellin VA, Samoilov MO (2005) Expression of early gene proteins, structural changes in brain neurons in hypobaric hypoxia, and the correcting effects of preconditioning. Neurosci Behav Physiol 35:383–388
Rybnikova E, Vataeva L, Tyulkova E, Gluschenko T, Otellin V, Pelto-Huikko M, Samoilov M (2005) Mild hypoxia preconditioning prevents impairment of passive avoidance learning and suppression of brain NGFI-A expression induced by severe hypoxia. Behav Brain Res 160:107–114
Duszczyk M, Ziembowicz A, Gadamski R, Lazarewicz WJ (2006) Behavioral evaluation of ischemic damage of CA1 hippocampal neurons: effects of preconditioning. Acta Neurobiol Exp 66:311–319
Duszczyk M, Ziembowicz A, Gadamski R, Wieronska JM, Smialowska M, Lazarewicz JW (2009) Changes in the NPY immunoreactivity in gerbil hippocampus after hypoxic and ischemic preconditioning. Neuropeptides 43:31–39
Semenov DG, Samoilov MO, Lazarewicz JW (2008) Preconditioning reduces hypoxia-evoked alterations in glutamatergic Ca2+ signaling in rat cortex. Acta Neurobiol Exp 68:169–179
Chen J, Graham SH, Zhu RL, Simon RP (1996) Stress proteins and tolerance to focal cerebral ischemia. J Cereb Blood Flow Metab 16:566–577
Stroev SA, Tjulkova EI, Glushchenko TS, Rybnikova EA, Samoilov MO, Pelto-Huikko M (2004) The augmentation of brain thioredoxin-1 expression after severe hypobaric hypoxia by the preconditioning in rats. Neurosci Lett 370:224–229
Stroev SA, Gluschenko TS, Tjulkova EI, Spyrou G, Rybnikova EA, Samoilov MO, Pelto-Huikko M (2005) The effect of preconditioning on Cu, Zn-superoxide dismutase expression and enzyme activity in rat brain at the early period after severe hypobaric hypoxia. Neurosci Res 53:39–47
Arthur PG, Lim SC, Meloni BP, Munns SE, Chan A, Knuckey NW (2004) The protective effect of hypoxic preconditioning on cortical neuronal cultures is associated with increases in the activity of several antioxidant enzymes. Brain Res 1017:146–154
Rybnikova E, Tyulkova E, Pelto-Huikko M, Samoilov M (2002) Mild preconditioning hypoxia modifies nerve growth factor-induced gene A messenger RNA expression in the rat brain induced by severe hypoxia. Neurosci Lett 329:49–52
Rybnikova E, Sitnik N, Gluschenko T, Tjulkova E, Samoilov MO (2006) The preconditioning modified neuronal expression of apoptosis-related proteins of Bcl-2 superfamily following severe hypobaric hypoxia in rats. Brain Res 1089:195–202
Sharp FR, Ran R, Lu A, Tang Y, Strauss KI, Glass T, Ardizzone T, Bernaudin M (2004) Hypoxic preconditioning protects against ischemic brain injury. NeuroRx 1:26–35
Dirnagl U, Meisel A (2008) Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology 55:334–344
Taie S, Ono J, Iwanaga Y, Tomita S, Asaga T, Chujo K, Ueki M (2009) Hypoxia-inducible factor-1 alpha has a key role in hypoxic preconditioning. J Clin Neurosci 16:1056–1060
Blondeau N, Widmann C, Lazdunski M, Heurteau C (2001) Activation of the nuclear factor-kB is a key event in brain tolerance. J Neurosci 21:4668–4677
Meller R, Minami M, Cameron JA, Impey S, Chen D, Lan J-Q, Henshall DC, Simon RP (2005) CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab 25:234–246
Rybnikova E, Glushchenko T, Tulkova E, Churilova A, Jaroshevich O, Baranova K, Samoilov M (2008) Preconditioning induces prolonged expression of transcription factors pCREB and NF-kappa B in the neocortex of rats before and following severe hypobaric hypoxia. J Neurochem 106:1450–1458
Rybnikova E, Glushchenko T, Tyulkova E, Baranova K, Samoilov M (2009) Mild hypobaric hypoxia preconditioning up-regulates expression of transcription factors c-Fos and NGFI-A in rat neocortex and hippocampus. Neurosci Res 65:360–366
Sommer C, Roth SU, Kuhn R, Kiessling M (2000) Metabotropic glutamate receptor subtypes are differentially expressed after transient cerebral ischemia without, during and after tolerance induction in the gerbil hippocampus. Brain Res 872:172–180
Semenov DG, Belyakov AV, Samoilov MO (2010) Comparison of Ca2+ responses to stimulation of glutamate receptors in the rat cerebral cortex after hypobaric hypoxia of different severities. Neurosci Behav Physiol 40:1012–1016
Semenov DG, Belyakov AV, Glushchenko TS, Samoilov MO (2012) Participation of metabotropic glutamate receptors of brain in mechanisms of hypoxic signaling. Patologicheskaya Fiziologiya i Eksperimentalnaya Terapiya 3:11–19 (In Russian)
Schroder UH, Opitz T, Jager T, Sabelhaus CF, Breder J, Reymann KG (1999) Protective effect of group I metabotropic glutamate receptor activation against hypoxic/hypoglycemic injury in rat hippocampal slices: timing and involvement of protein kinase C. Neuropharmacology 38:209–216
Kalda A, Kaasik A, Vassiljev V, Pokk P, Zharkovsky A (2000) Neuroprotective action of group I metabotropic glutamate receptor agonists against oxygen-glucose deprivation-induced neuronal death. Brain Res 853:370–373
Werner CG, Scartabelli T, Pancani T, Landucci E, Moroni F, Pellegrini-Giampietro DE (2007) Differential role of mGlu1 and mGlu5 receptors in rat hippocampal slice models of ischemic tolerance. Eur J Neurosci 25:3597–3604
Scartabelli T, Gerace E, Landucci E, Moroni F, Pellegrini-Giampietro DE (2008) Neuroprotection by group ImGlu receptors in a rat hippocampal slice model of cerebral ischemia is associated with the PI3 K–Akt signaling pathway: a novel postconditioning strategy? Neuropharmacology 55:509–516
Nik Ramli NN, Omar N, Husin A, Ismail Z, Siran R (2015) Preconditioning effect of (S)-3,5-dihydroxyphenylglycine on ischemic injury in middle cerebral artery occluded Sprague-Dawley rats. Neurosci Lett 588:137–141
Semenov DG, Samoilov MO, Lazarewicz JW (2002) Calcium transients in the model of rapidly induced anoxic tolerance in rat cortical slices: involvement of NMDA receptors. Neurosignals 11:329–335
Candelario-Jalil E, Al-Dalain SM, Castillo R, Martínez G, Fernández OS (2001) Selective vulnerability to kainate-induced oxidative damage in different rat brain regions. J Appl Toxicol 21:403–407
Suzuki N, Bekkers JM (2011) Two layers of synaptic processing by principal neurons in piriform cortex. J Neurosci 31:2156–2166
Wiegand HF, Beed P, Bendels MH, Leibold C, Schmitz D, Johenning FW (2011) Complementary sensory and associative microcircuitry in primary olfactory cortex. J Neurosci 31:12149–12158
Wada E, Shigemoto R, Kinoshita A, Ohishi H, Mizuno N (1998) Metabotropic glutamate receptor subtypes in axon terminals of projection fibers from the main and accessory olfactory bulbs: a light and electron microscopic immunohistochemical study in the rat. J Comp Neurol 393:493–504
Wang X-D, Chen X-Q, Yang H-H, Hu G (1999) Comparison of the effects of cholinesterase inhibitors on [3H] MK-801 binding in rat cerebral cortex. Neurosci Lett 272:21–24
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Gamdzyk M, Makarewicz D, Słomka M, Ziembowicz A, Salinska E (2014) Hypobaric hypoxia postconditioning reduces brain damage and improves antioxidative defense in the model of birth asphyxia in 7-day-old rats. Neurochem Res 39:68–75
Semenov DG, Samoilov MO, Zielonka P, Lazarewicz JW (2000) Responses to reversible anoxia of intracellular free and bound Ca2+ in rat cortical slices. Resuscitation 44:207–214
Aizenman E, Sinor JD, Brimecombe JC, Herin GA (2000) Alterations of N-methyl-D-aspartate receptor properties after chemical ischemia. J Pharmacol Exp Ther 295:572–577
Tanaka H, Calderone A, Jover T, Grooms SY, Yokota H, Zukin RS, Bennett MV (2002) Ischemic preconditioning acts upstream of GluR2 down-regulation to afford neuroprotection in the hippocampal CA1. Proc Natl Acad Sci USA 99:2362–2367
Shen KZ, Johnson SW (2013) Group I mGluRs evoke K-ATP current by intracellular Ca2+ mobilization in rat subthalamus neurons. J Pharmacol Exp Ther 345:139–150
Bickler PE, Fahlman CS, Gray J, McKleroy W (2009) Inositol 1.4.5-triphosphate receptors and NAD(P)H mediate Ca2+- signaling required for hypoxic preconditioning of hippocampal neurons. Neuroscience 160:51–60
Baskys A, Bayazitov I, Fang L, Blaabjerg M, Poulsen FR, Zimmer J (2005) Group I metabotropic glutamate receptors reduce excitotoxic injury and may facilitate neurogenesis. Neuropharmacology 49:146–156
Choe ES, Wang JQ (2001) Group I metabotropic glutamate receptors control phosphorylation of CREB, Elk-1 and ERK via a CaMKII-dependent pathway in rat striatum. Neurosci Lett 313:129–132
Mao L, Wang JQ (2003) Group I metabotropic glutamate receptor-mediated calcium signaling and immediate early gene expression in cultured rat striatal neurons. Eur J Neurosci 17:741–750
Iacovelli L, Salvatore L, Capobianco L, Picascia A, Barletta E, Storto M, Mariggio S, Sallese M, Porcellini A, Nicoletti F, De Blasi A (2003) Role of G protein-coupled receptor kinase 4 and β- arrestin 1 in agonist-stimulated metabotropic glutamate receptor 1 internalization and activation of mitogen-activated protein kinases. J Biol Chem 278:12433–12442
Ribeiro FM, Ferreira LT, Paquet M, Cregan T, Ding Q, Gros R, Ferguson SSG (2009) Phosphorylation-independent regulation of metabotropic glutamate receptor 5 desensitization and internalization by G protein-coupled receptor kinase 2 in neurons. J Biol Chem 284:23444–23453
De Vry J, Horváth E, Schreiber R (2001) Neuroprotective and behavioral effects of the selective metabotropic glutamate mGlu(1) receptor antagonist BAY 36-7620. Eur J Pharmacol 428:203–214
Moroni F, Attucci S, Cozzi A, Meli E, Picca R, Scheideler MA, Pellicciari R, Noe C, Sarichelou I, Pellegrini-Giampietro DE (2002) The novel and systemically active metabotropic glutamate 1 (mGlu1) receptor antagonist 3-MATIDA reduces post-ischemic neuronal death. Neuropharmacology 42:741–751
Pellegrini-Giampietro DE (2003) The distinct role of mGlu1 receptors in post-ischemic neuronal death. Trends Pharmacol Sci 24:461–470
Viwatpinyo K, Chongthammakun S (2009) Activation of group I metabotropic glutamate receptors leads to brain-derived neurotrophic factor expression in rat C6 cells. Neurosci Lett 467:127–130
O’Riordan KJ, Huang I-C, Pizzi M, Spano PF, Boroni F, Egli R, Desai P, Fitch O, Malone L, Ahn YJ, Liou H-C, Sweatt JD, Levensoni JM (2006) Regulation of nuclear factor kappa B in the hippocampus by group I metabotropic glutamate receptors. J Neurosci 26:4870–4879
Jong YJ, Kumar V, Kingston AE, Romano C, O’Malley KL (2005) Functional metabotropic glutamate receptors on nuclei from brain and primary cultured striatal neurons. Role of transporters in delivering ligand. J Biol Chem 280:30469–30480
Bahk YY, Song H, Baek SH, Park BY, Kim H, Ryu SH, Suh PG (1998) Localization of two forms of phospholipase C-beta1, a and b, in C6Bu-1 cells. Biochem Biophys Acta 1389:76–80
Faenza I, Fiume R, Piazzi M, Colantoni A, Cocco L (2013) Nuclear inositide specific phospholipase C signalling-interactions and activity. FEBS J 280:6311–6321
Nagasawa K, Aoki H, Yasuda E, Nagai K, Shimohama S, Fujimoto S (2004) Possible involvement of group I mGluRs in neuroprotective effect of theanine. Biochem Biophys Res Commun 320:116–122
Nishida K, Yasuda E, Nagasawa K, Fujumoto S (2008) Altered levels of oxidation and phospholipase C isozyme expression in the brains of theanine-administered rats. Biol Pharmacol Bull 31:857–860
Yasuda E, Nagasawa K, Nishida K, Fujumoto S (2008) Decreased expression of phospholipase C-betta 1 protein in endoplasmic reticulum stress-loaded neurons. Biol Pharmacol Bull 31:719–721
Hermans E, Challiss RAJ (2001) Structural, signaling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem J 359:465–484
Patterson R, Boehning D, Snyder S (2004) Inositol 1, 4, 5-trisphosphate receptors as signals integrators. Ann Rev Biochem 73:437–465
Zhang SX, Zhang JP, Fletcher DL, Zoeller RT, Sun GY (1995) In situ hybridization of mRNA expression for IP3 receptor and IP3-3-kinase in rat brain after transient focal cerebral ischemia. Mol Brain Res 32:252–260
Xia J, Simonyi A, Sun GY (1998) Changes in IP3R1 and SERCA2b mRNA levels in the gerbil brain after chronic ethanol administration and transient cerebral ischemia-reperfusion. Mol Brain Res 56:22–28
Farwell W, Simonyi A, Scott H, Zhang JP, Carruthers V, Madsen R, Johnson J, Sun GY (1998) Effects of ischemic tolerance on mRNA levels of IP3R1, beta-actin, and neuron-specific enolase in hippocampal CA1 area of the gerbil brain. Neurochem Res 23:539–542
Wojcikiewicz RJH, Pearce MMP, Sliter DA, Wang Y (2009) When worlds collide: IP3 receptors and the ERAD pathway. Cell Calcium 46:147–153
Marini AM, Jiang X, Wu X, Pan H, Guo Z, Mattson MP, Blondeau N, Novelli A, Lipsky RH (2007) Preconditioning and neurotrophins: a model for brain adaptation to seizures, ischemia and other stressful stimuli. Amino Acids 32:299–304
Stirling DP, Cummins K, Wayne Chen SR, Stys P (2014) Axoplasmic reticulum Ca2+ release causes secondary degeneration of spinal axons. Ann Neurol 75:220–229
Duncan RS, Goad DL, Grillo MA, Kaja S, Payne AJ, Koulen P (2011) Control of intracellular calcium signaling as a neuroprotective strategy. Molecules 15:1168–1195
Acknowledgments
Authors thank Dr. Derek Martin, Edinburgh, U.K. for English editing of the manuscript. This study was supported by a research grants from RFBR Nos. 10-04-01134, 11-04-00677 and 12-04-31571 (Russia), and by statutory founds of the Mossakowski MRC of PAS (Poland).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Semenov, D.G., Belyakov, A.V., Glushchenko, T.S. et al. Hypobaric Preconditioning Modifies Group I mGluRs Signaling in Brain Cortex. Neurochem Res 40, 2200–2210 (2015). https://doi.org/10.1007/s11064-015-1708-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1708-9