Abstract
Perinatal brain insult mostly resulting from hypoxia–ischemia (H–I) often brings lifelong permanent disability, which has a major impact on the life of individuals and their families. The lack of progress in clinically—applicable neuroprotective strategies for birth asphyxia has led to an increasing interest in alternative methods of therapy, including induction of brain tolerance by pre- and particularly postconditioning. Hypoxic postconditioning represents a promising strategy for preventing ischemic brain damage. The aim of this study was to investigate the potential neuroprotective effect of hypobaric hypoxia (HH) postconditioning applied to 7-day old rats after H–I insult. The mild hypobaric conditions (0.47 atm) used in this study imitate an altitude of 5,000 m. We show that application of mild hypobaric hypoxia at relatively short time intervals (1–6 h) after H–I, repeated for two following days leads to significant neuroprotection, manifested by a reduction in weight loss of the ipsilateral hemisphere observed 14 days after H–I. HH postconditioning results in decrease in reactive oxygen species level observed in all experimental groups. The increase in superoxide dismutase activity observed after H–I is additionally enhanced by HH postconditioning applied 1 h after H–I. The increase observed 3 and 6 h after H–I was not statistically significant. Postconditioning with HH suppresses the glutathione concentration decrease evoked by H–I and increased glutathione peroxidase activity and this effect is not dependent on the time of postconditioning initiation. HH postconditioning had no effect on catalase activity. We show for the first time that HH postconditioning reduces brain damage resulting from H–I in immature rats and that the mechanism potentially involved in this effect is related to antioxidant defense mechanisms of immature brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neonatal encephalopathy (NE) occurring after perinatal hypoxic–ischemic (H–I) insult is a major contributor to global child mortality and morbidity. As many as a million deaths worldwide might be caused by perinatal asphyxia and almost 99 % arise in developing countries [1, 2]. The underlying mechanism for perinatal H–I brain injury is an interruption of placental blood flow followed by impaired gas exchange leading to deficits in oxygen and metabolic substrates delivery to the central nervous system. This initiates a cascade of intracellular events leading to neuronal cells death. One of the major injurious component of hypoxia and following reoxygenation is an oxidative stress caused by overproduction of reactive oxygen species (ROS) [3]. To counteract the toxic effects of ROS the mobilization of three main antioxidant enzymes is necessary. Superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase are enzymes which maintain the delicate balance of ROS in central nervous system, especially within the neonatal brain.
One of the proposed mechanisms of neuroprotection involves sublethal ROS production by hypoxic preconditioning. This does not damage the neurons but activates the SOD and GPx, additionally it also triggers expression of the erythropoietin (Epo) and hypoxia-inducible factor-1 α (HIF-1α), the proteins which prevent neurons from developing apoptosis or necrosis [4, 5]. It was also shown, that preconditioning with mild hypoxia increases the expression and activity of Cu, Zn-SOD during early stages of reoxygenation after severe hypobaric hypoxia in adult rats [6].
Induction of brain tolerance by pre- or postconditioning with subinjurious exposure to various stressors, which renders the brain less vulnerable to damaging exposure, is one of the experimental procedures that has been used to protect the developing brain [7–9]. The beneficial effects of ischemic preconditioning in experimental stroke therapy are well documented [8, 10, 11]. Additionally, the less invasive treatment of applying hypoxic preconditioning has been shown to result in protection against a subsequent sustained period of lethal hypoxia–ischemia [11–13].
Postconditioning represents another promising strategy to prevent ischemic brain damage. It was shown that ischemic postconditioning significantly reduces the number of dead neurons and infarct volume in experimental focal and global cerebral ischemia [14–16]. Improved brain metabolism, normalized cerebral blood flow and long term protection after ischemic postconditioning initiated at different times after the index ischemia has been reported in stroke and myocardial ischemia models [9, 17]. Besides ischemic postconditioning, the beneficial effects of hypoxic postconditioning to the heart have been described both in experiments in vivo and in vitro [18, 19]. Recently, Leconte et al. [20] showed the protective effect of delayed hypoxic postconditioning against cerebral ischemia in mice, thus offering the new potential for non-invasive neuroprotection in cerebral ischemia and perinatal asphyxia.
Experimental hypoxia may be induced either by replacing oxygen by nitrogen to achieve lower O2 concentration level (≤8 % and less) in the breathing gas mixture (normobaric hypoxia), or by decreasing the air pressure to reach the equivalent of 5 % (severe hypobaric hypoxia, 0.21–0.23 atm) or 10 % (mild hypobaric hypoxia, 0.47 atm) of normobaric oxygen. Although most of the beneficial effects of hypoxic pre- and postconditioning have been shown in normobaric hypoxia experiments, there are reports showing, that mild hypobaric hypoxia preconditioning protects rats from severe hypoxia [21] and induces brain ischemic tolerance [22, 23].
The aim of this study was to investigate the potential neuroprotective effect of mild hypobaric hypoxia (HH) postconditioning applied to 7-day old rats at different times after experimental H–I and to determine the effect of HH postconditioning on antioxidative defense in immature brain.
Experimental Procedure
All experiments described in this paper were approved by the 4th Local Ethical Committee in Warsaw, Poland, and performed in accordance with Polish governmental regulations (Dz.U.97.111.724) and the European Community Council Directive of 24 November 1986 (86/609/EEC). All efforts were made to minimize animal suffering and the number of animals used.
Induction of Cerebral Hypoxia–Ischemia
Neonatal cerebral H–I was induced according to Rice et al. [24]. Briefly, Wistar 7 day postnatal (PND7) rats weighting 12–18 g, were anaesthetized with halothane (4 % for induction, and 1.5–2.0 % for maintenance) in a mixture of nitrous oxide and oxygen (0.6:1) and the left common carotid artery was dissected and cut between double ligatures of silk sutures, or only exposed (sham control). After 60 min of recovery, animals were subjected to systemic hypoxia with 7.4 % oxygen in nitrogen for 75 min in a humidified chamber at 35 °C. The duration of hypoxia–ischemia typically is associated with infarction predominantly of the cerebral hemisphere ipsilateral to the carotid artery occlusion [13, 24]. After hypoxic treatment the pups were returned to the cages and housed at room temperature (22 °C) with a 12:12 h light–dark cycle and ample food and water.
Postconditioning Procedure
Animals were placed in a hypobaric chamber and mild HH was produced by decreasing the pressure to 0.47 atm (equivalent to 10 % normobaric oxygen or 5,000 m high-altitude), which was maintained for 60 min. Animals were divided into five groups: sham operated rats, rats subjected to H–I, and rats subjected to HH initiated at 1, 3 or 6 h after H–I episode. The treatment was repeated for the next 2 days in 24 h intervals. Sham operated rats served as control. Additional group of sham operated animals subjected to HH was created in experiments, which provided samples for determination of SOD activity and ROS and glutathione levels.
Evaluation of Brain Damage
Two weeks after H–I (PND 21) the pups were anaesthetised with a lethal dose of vetbutal and decapitated. The brains were removed and the cerebral hemispheres were weighed. Brain damage was assessed by comparing the weight of ipsilateral (left) and contralateral (right) hemispheres. The deficit in weight of the ipsilateral hemisphere was expressed as a percent of the weight of the contralateral hemisphere [25].
Histochemical evaluation of brain damage was performed on brains isolated 7 days after H–I. Animals were anesthetized with halothane and subjected to intracranial perfusion fixation with 4 % neutralized formalin (Sigma-Aldrich, St. Louis, Missouri, USA). The brains were then removed, postfixed for 4 h in the same fixative, and immersed for 2 days in a buffered 20 % sucrose solution at 4 °C. Sham operated animals were processed in the same way. The brains were frozen and 20 μm thick sections were cut with a cryostat (Microm HM 550). The sections were stained according to the Nissl protocol with 0.5 % Cresyl violet for histological assessment of neuronal cell damage.
Determination of ROS Level
The levels of ROS in brain hemispheres was measured using 2,7-dichlorofluorescein-diacetate (DCF-DA), a fluorogenic dye that measures hydroxyl, peroxyl and other ROS activity within the cell. DCF-DA is oxidized by ROS into 2′,7′-dichlorofluorescin (DCF), a highly fluorescent compound which can be detected by fluorescence spectroscopy. Briefly, the brains were collected 3 h after the last postconditioning session and tissues from the left and right hemispheres were homogenized separately in ice-cold 40 mM Tris–HCl buffer (pH 7.4). The resulting brain homogenates were incubated with DCF-DA (25 μM) for 30 min at 37 °C. The formation of the fluorescent product DCF was monitored by fluorescence spectrometer with excitation wavelength of 488 nm and emission wavelength of 525 nm. The relative fluorescence unit (RFU) was calculated per 1 mg protein in homogenate. The effect of H–I and HH was estimated by determination of ROS level in individual brains and standardized for variation in the basal level measured in brains from sham operated animals.
Determination of Antioxidant Enzymes Activity
The brains were collected 3 h after the last postconditioning session and the left and right hemispheres were homogenized separately in 20 mM HEPES buffer, pH 7.2, containing 1 mM EGTA, 210 mM mannitol, and 70 mM sucrose per gram tissue. Homogenates were centrifuged at 1,500×g for 5 min at 4 °C, then the supernatant was collected and the SOD activity was determined using Superoxide Dismutase Assay Kit (Cayman Chemical Company, USA), following the manufacturer provided manual.
For catalase activity measurement brain tissue was rinsed with PBS buffer, pH 7.4, to remove any blood cells and clots. Tissue was homogenized on ice in a cold 50 mM potassium phosphate buffer, pH 7.0, containing 1 mM EDTA, then centrifuged at 10,000×g for 15 min at 4 °C. Supernatant was collected and catalase activity was determined using Catalase Assay Kit (Cayman Chemical Company, USA) following the manufacture’s procedure.
Glutathione peroxidase (GPx) activity was measured in brain tissue from left and right hemisphere. Tissue was homogenized in cold buffer (50 mM Tris–HCl, pH 7.5, 5 mM EDTA and 1 mM DTT), centrifuged at 10,000×g for 15 min at 4 °C and the supernatant was collected for assay. GPx activity was measured using Glutathione Peroxidase Assay Kit (Cayman Chemical Company, USA).
Determination of Glutathione Concentration
Determination of glutathione concentration was carried out in tissues isolated from left and right hemispheres, which were homogenized separately in 25 mM HEPES, pH 7.4, containing 250 mM sucrose and then centrifuged at 1,000×g for 5 min at 4 °C. Supernatants were collected and the concentration of reduced glutathione was determined using a Glutathione Assay Kit, Fluorimetric (Sigma-Aldrich, USA), following the manufacturer’s procedure.
Statistical Analysis
Statistical analysis of the brain damage data was performed using paired t test. The other data was analyzed via a one way ANOVA, with a post hoc least significance test for significant differences between groups. Differences were considered significant with p value less than 0.05.
Results
Hypobaric Hypoxia Postconditioning Prevents Brain Damage
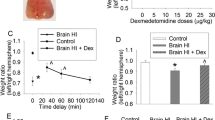
Figure 1 shows that H–I resulted in a weight deficit in the ipsilateral brain hemisphere of 38.0 % as compared to the contralateral hemisphere 2 weeks after the insult. Hypobaric treatment of the animals applied up to 6 h after termination of H–I produced significant neuroprotection (F3,60 = 10.6; p < 0.001). Where the HH was initiated 1 h after H–I the weight deficit of the ipsilateral hemispheres decreased to 12.9 % and was significantly different from H–I group (p < 0.001). Significant decreases in weight deficits were also observed when HH was applied 3 h (23.1 %) and 6 h (23.8 %) after H–I; however, there was no statistically significant difference between these two groups. HH applied to sham operated animals did not produce any changes in brain weight compared to sham controls (data not shown).
Effect of HH postconditioning initiated at different times after H–I on the degree of ipsilateral hemisphere weight loss in rat pups. The deficit in weight was expressed as a percent of the weight of the contralateral hemisphere. Results are presented as mean values ± SEM, n = 5. Differences statistically significant: *p < 0.05, **p < 0.01
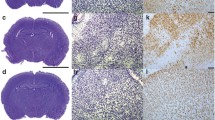
H–I induced brain injury in the ipsilateral (left) hemisphere. We observed marked cell loss in the cortex, and damage and disorganization of neurons in the CA1 region of hippocampus (Fig. 2c, i). HH postconditioning initiated 1 h after H–I abolished changes in the CA1 region of hippocampus and significantly decreased neuronal loss in the cortex (Fig. 2d, j). HH applied 3 and 6 h after H–I also decreased changes observed in studied areas, however neuroprotection seems to be less effective at HH applied 6 h after H–I.
The neuroprotective effect of HH postconditioning observed 7 days after H–I in CA1 region of the hippocampus (a–f) and in cerebral cortex (g–l). Microphotographs show the hemisphere ipsilateral to H–I; a, g sham operated; b, h sham operated + HH; c, i H–I; d, j HH initiated 1 h after H–I; e, k HH initiated 3 h after H–I; f, l HH initiated 6 h after H–I; both scale bars represent 50 μm
Hypobaric Hypoxia Postconditioning Reduces ROS Level After Hypoxia–Ischemia
The level of ROS was measured in both hemispheres 3 h after the last hypobaric session. H–I increased the level of ROS in the left, ischemic hemisphere up to six times (F1,10 = 31.8; p < 0.001; Fig. 3). HH applied in three 60 min sessions beginning 1 h and 3 h after H–I significantly decreased ROS level by 54 and 52 % respectively (p < 0.01). HH applied 6 h after H–I also tended to decrease ROS level in left hemisphere, although this result was not statistically significant. Neither H–I nor HH change the ROS level in right hemisphere.
Effect of Hypobaric Hypoxia Postconditioning on Antioxidant Enzymes Activity
SOD activity was determined in both hemispheres 3 h after the last hypobaric session. H–I increased ten times SOD activity only in the left (ipsilateral), ischemic hemisphere (F1,14 = 112.7; p < 0.001; Fig. 4). It is interesting to note that severe hypoxia (7.4 % oxygen in nitrogen applied for 75 min), which is one of the important elements of the hypoxia–ischemia model, did not change the activity of SOD in the contralateral hemisphere compared to the control. However, HH treatment applied to sham operated animals resulted in increase in SOD activity in both hemispheres up to six times compared to the control (data not shown). Postconditioning applied 1 h after H–I and repeated two more times in 24 h intervals between applications, resulted not only in an additional, 37 % increase in SOD activity in the left hemisphere compared to H–I alone (F1,8 = 8; p < 0.02) but also increased the SOD activity in right hemisphere (F1,13 = 55.7; p < 0.001). HH applied 3 and 6 h after H–I did not result in additional significant increase in SOD activity in left hemisphere compared to the H–I. The SOD activity increases observed in the right hemispheres in all HH postconditioned groups were identical to that produced by HH itself.
Activity of catalase increased significantly after H–I reaching values two times higher than the control (p < 0.05; Fig. 5a). HH treatment applied at all experimental times did not change increased activity of catalase. Neither H–I nor HH change the activity of catalase in right hemisphere.
H–I resulted in significant increase in GPx activity in left hemisphere (F1,12 = 8.05; p < 0.05), while the enzyme activity in right hemisphere reminded unchanged (Fig. 5b). HH postconditioning applied 1, 3 and 6 h after H–I increased the GPx activity in left hemisphere, however this increase was not statistically significant.
Effect of Hypobaric Hypoxia Postconditioning on GSH Level
Glutathione content was measured in both hemispheres at time points corresponding to those used for measurements of antioxidant enzymes. The GSH concentration determined in brains isolated from control rats ranged between 27.6 and 32.9 nmol/mg of protein in left and right hemispheres, respectively (Fig. 6). H–I resulted in a significant decrease of GSH in both hemispheres to 38 and 74 % of control respectively (p < 0.005 and p < 0.05 respectively). HH postconditioning applied 1, 3 or 6 h after H–I restored the GSH concentration in both hemispheres to the same level as the controls (Fig. 6).
Discussion
The beneficial effect of hypoxia postconditioning was reported in primary cultures of neonatal rat cardiomyocytes subjected to severe hypoxia [18, 19] and in focal cerebral ischemia in mice [20]. It was also shown that both mild HH preconditioning and postconditioning have beneficial effects preventing injury in rat brain resulted from severe HH and H–I [26–28]. Results presented in this paper show that mild HH initiated at short time after H–I reduces brain damage in rat puppies. It has been suggested that delayed hypoxic postconditioning started 24 h or later after ischemia or severe hypoxia is more effective than that started hours after the ischemic insult [20]. These results are related to adult animals and immature brain responds to H–I differently. Given the current knowledge, initiation of treatment in neonatal encephalopathy within the therapeutic window should start before 6 h of life [2]. It was also shown before, that only early blockade of oxidative stress at a relatively short window of opportunity results in neuroprotection [29]. Our results show that protection from brain damage appeared when hypoxic postconditioning was initiated only 1 h after H–I. Postconditioning applied 3 and 6 h after H–I also resulted in brain protection, although the loss of ipsilateral hemisphere mass was relatively higher. The observed neuroprotective effect of HH may result from repetition of hypobaric sessions for three times in 24 h intervals. This way the opportunity to block early oxidative stress and the consequence of delayed, secondary cerebral energy failure was created [30, 31].
Although the developing brain is generally considered as “resistant” to the damaging effects of H–I, nevertheless it exhibits periods of increased sensitivity to injury [32]. Yager et al. [33] have shown that the low concentration of glucose transporters in 7-day postnatal (P7) rats leads to a rapid fall in glucose concentration and depletion of cellular energy stores, which is observed by 90 min of H–I. Energy deficit results in depolarization of neurons and glia, and the release of excitatory amino acids. The composition and activity of immature NMDA receptors are different from those receptors identified in the adult brain, consequently P7 rats show enhanced sensitivity to excitotoxic insults [34]. Activation of NMDA receptors triggers an excitotoxic cascade which generates ROS and subsequent oxidative cell damage. SOD converts oxygen free radicals to H2O2, which can then be neutralised to H2O by the action of catalase or GPx. However, the immature brain has poor antioxidant capabilities and contains a high concentration of free iron [32], which increases the vulnerability of the immature brain to oxidative stress. Our experiments show that, although H–I resulted in increased SOD activity in the ipsilateral hemisphere, HH postconditioning increased this activity even further. HH itself resulted in increase in SOD activity, which was reported also by other authors [6, 35, 36], and did not result in increased ROS level, which is in agreement with reports of Arthur et al. [35]. This suggests that HH postconditioning may possibly allow for more efficient neutralization of ROS by SOD in the immature brain. HH postconditioning resulted in restoring of glutathione concentration to the values observed in the brains of control, sham operated animals, which may increase the ability of detoxification of accumulated H2O2 by GPx. Fullerton et al. [37] suggested that the reduced ability of the immature brain to neutralise H2O2 results from the limited capacity of antioxidant enzymes, especially GPx. In addition, the accumulation of H2O2 is more damaging to the immature brain due to the presence of high concentrations of free iron in the nervous system and the possibility of further free radical generation via the Fenton reaction [38]. Our results show that H–I results in increase in GPx activity, indicating that the defensive mechanisms in immature brain are activated. These results are in agreement with other studies [39, 40]. HH postconditioning applied at different times after H–I additionally increased the GPx activity and restored decreased GSH to the control level. It is possible that during mild hypoxia anaerobic metabolism is supporting ATP production, leading to an increased NADPH production and increasing reduction of oxidized glutathione [41]. Such a scenario may explain the neuroprotective effect of HH postconditioning.
Observed in our study restoration of GSH level suggests the increased ability of immature brain to neutralise free radicals production, which explains the presence of much lower concentration of ROS measured in the brains of postconditioned animals compared to H–I. However, it is also possible that HH postconditioning reduces ROS formation. Interestingly, in our experiments HH postconditioning did not have any additional effect on catalase activity, although this activity was already increased by H–I itself. These results may indicate that the mechanism of neuroprotection induced by HH does not involve catalase.
In conclusion, beneficial effect of HH postcondition on brain damage resulted from H–I in immature rats, involves the mobilisation of antioxidant mechanisms. The best effects of HH postconditioning are observed when the HH treatment is applied in a short time after H–I episode. Although the results indicate on the potential usefulness of HH postconditioning in therapy of birth asphyxia, the mechanism of observed neuroprotection needs more investigation.
Abbreviations
- H–I:
-
Hypoxia–ischemia
- HH:
-
Hypobaric hypoxia
- SOD:
-
Superoxide dismutase
- GPx:
-
Glutathione peroxidase
- GSH:
-
Reduced glutathione
References
Johnston MV, Fatemi A, Wilson MA, Northington F (2011) Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol 10(4):372–382
Wachtel EV, Hendricks-Muñoz KD (2011) Current management of the infant who presents with neonatal encephalopathy. Curr Probl Pediatr Adolesc Health Care 41:132–153
Gill MB, Peres-Polo JR (2008) Hypoxia ischemia mediated cell death in neonatal rat brain. Neurochem Res 33:2379–2389
Liu C, Chen S, Kamme F, Hu BR (2005) Ischemic preconditioning prevents protein aggregation after transient cerebral ischemia. Neuroscience 134:69–80
Chan PH (2001) Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21:2–14
Stroev SA, Gluschenko TS, Tjulkova EI, Rybnikova EA, Samoilov MO, Pelto-Huikko M (2005) The effect of preconditioning on the Cu, Zn superoxide dismutase expression and enzyme activity in rat brain at the early period after severe hypobaric hypoxia. Neurosci Res 53:39–47
Hagberg H, Dammann O, Mallard C, Leviton A (2004) Preconditioning and the developing brain. Semin Perinatol 28:389–395
Pignataro G, Scorziello A, Di Renzo G, Annunziato L (2009) Post-ischemic brain damage: effects of ischemic preconditioning and postconditioning and identification of potential candidates for stroke therapy. FEBS J 276:46–57
Zhao H (2007) The protective effect of ischemic postconditioning against ischemic injury: from the hart to the brain. J Neuroimmune Pharmacol 2:313–318
Kirino T (2002) Ischemic tolerance. J Cereb Blood Flow Metab 22:1283–1296
Sanders RD, Manning HJ, Robertson NJ, Ma D, Edwards AD, Hagberg H, Maze M (2010) Preconditioning and postinsult therapies for perinatal hypoxic-ischemic injury at term. Anesthesiology 113(1):233–249
Gustavsson M, Anderson MF, Mallard C, Hagberg H (2005) Hypoxic preconditioning confers long-term reduction of brain injury and improvement of neurological ability in immature rats. Pediatr Res 57:305–309
Vannucci RC, Towfighi J, Vannucci SJ (1998) Hypoxic preconditioning and hypoxic-ischemic brain damage in the immature rat: pathologic and metabolic correlates. J Neurochem 71:1215–1220
Zhao H, Sapolsky RM, Steinberg GK (2006) Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab 26:1114–1121
Pignataro G, Meller R, Inoue K, Ordonez AN, Ashley MD, Xiong Z, Simon RP (2008) In vivo and in vitro characterization of a novel neuroprotective strategy for stroke: ischemic postconditioning. J Cereb Blood Flow Metab 28:232–241
Wang JY, Shen J, Gao Q, Ye ZG, Yang SY, Liang HW, Bruce IC, Luo BY, Xia Q (2008) Ischemic postconditioning protects against global cerebral ischemia/reperfusion-induced injury in rats. Stroke 39:983–990
Ren C, Gao X, Niu G, Yan Z, Chen X, Zhao H (2008) Delayed postconditioning protects against focal ischemic brain injury in rats. PLoS ONE 3:e3851
Sun HY, Wang NP, Kerendi F, Halkos M, Kin H, Guyton RA, Vinten-Johansen J, Zhao ZQ (2005) Hypoxic postconditioning reduces cardiomyocyte loss by inhibiting ROS generation and intracellular Ca2+ overload. Am J Physiol Heart Circ Physiol 288(4):H1900–H1908
Wang HC, Zhang HF, Guo WY, Su H, Zhang KR, Li QX, Yan W, Ma XL, Lopez BL, Christopher TA, Gao F (2006) Hypoxic postconditioning enhances the survival and inhibits apoptosis of cardiomyocytes following reoxygenation: role of peroxynitrite formation. Apoptosis 11:1453–1460
Leconte C, Tixier E, Freret T, Toutain J, Saulnier R, Boulouard M, Roussel S, Schumann-Bard P, Bernaudin M (2009) Delayed hypoxic postconditioning protects against cerebral ischemia in the mouse. Stroke 40:3349–3355
Stroev SA, Tjulkova EI, Gluschenko TS, Rybnikova EA, Samoilov MO, Pelto-Huikko M (2004) The augmentation of brain thioredoxin-1 expression after severe hypobaric hypoxia by the preconditioning in rats. Neurosci Lett 370:224–229
Duszczyk M, Ziembowicz A, Gadamski R, Wieronska JM, Smialowska M, Lazarewicz JW (2009) Changes in the NPY immunoreactivity in gerbil hippocampus after hypoxic and ischemic preconditioning. Neuropeptides 43:31–39
Gong SJ, Chen LY, Zhang M, Gong JX, Ma YX, Zhang JM, Wang YJ, Hu YY, Sun XC, Li WB, Zhang Y (2012) Intermittent hypobaric hypoxia preconditioning induced brain ischemic tolerance by up-regulating glial glutamate transporter-1 in rats. Neurochem Res 37:527–537
Rice JE III, Vannucci RC, Brierley JB (1981) The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9:131–141
Makarewicz D, Duszczyk M, Gadamski R, Danysz W, Łazarewicz JW (2006) Neuroprotective potential of group I metabotropic glutamate receptor antagonists in two ischemic models. Neurochem Int 48(6–7):485–490
Churilova AV, Rybnikova EA, Gluschenko TS, Tyulkova EI, Samoilov MO (2010) Effects of moderate hypobaric hypoxia preconditioning on the expression of the transcription factors pCREB and NF-κB in rat hippocampus before and after severe hypoxia. Neurosci Behav Physiol 40:852–857
Galle AA, Jones NM (2013) The neuroprotective actions of hypoxic preconditioning and postconditioning in neonatal rat model of hypoxic-ischemic brain injury. Brain Res 1498:1–8
Rybnikova E, Vorobyev M, Pivina S, Samoilov M (2012) Postconditioning by mild hypoxic exposures reduces rat brain injury caused by severe hypoxia. Neurosci Lett 513:100–105
Mathai S, Gunn AJ, Backhaus RA, Guan J (2012) Window of opportunity for neuroprotection with antioxidant, allene oxide synthase, after hypoxia–ischemia in adult male rats. CNS Neurosci Ther 18:887–894
Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, Peebles D, Wylezinska M, Owen-Reece H, Kirkbride V, Cooper CE, Aldrige RF, Roth SC, Brown G, Deply DT, Reynolds OR (1994) Delayed (“secondary”) cerebral energy failure after acute hypoxia–ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res 36:699–706
Vannucci RC, Towfighi J, Vannucci SJ (2004) Secondary energy failure after cerebral hypoxia–ischemia in the immature rat. J Cereb Blood Flow Metab 24:1090–1097
Vannucci SJ, Hagberg H (2004) Hypoxia–ischemia in the immature brain. J Exp Biol 207:3149–3154
Yager JY, Brucklacher RM, Vannucci RC (1992) Cerebral energy metabolism during hypoxia–ischemia and early recovery in immature rats. Am J Physiol 262:H672–H677
Johnston MV (1995) Neurotransmitters and vulnerability of the developing brain. Brain Dev 17:301–306
Arthur PG, Lim SC, Meloni BP, Munns SE, Chan A, Knuckey NW (2004) The protective effect of hypoxic preconditioning on cortical neuronal cultures is associated with increases in the activity of several antioxidant enzymes. Brain Res 1017:146–154
Duan C, Yan F, Song X, Lu GW (1999) Changes of superoxide dismutase, glutathione peroxidase and lipid peroxides in the brain of mice preconditioned by hypoxia. Biol Signals Recept 8:256–260
Fullerton HJ, Ditelberg JS, Chen SF, Sarco DP, Chan PH, Epstein CJ, Ferriero DM (1998) Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann Neurol 44:357–364
Warner DS, Sheng H, Batinic-Haberle I (2004) Oxidants, antioxidants and the ischemic brain. J Exp Biol 207(Pt 18):3221–3231
Kumar A, Ramakrishna SV, Basu S, Rao GR (2008) Oxidative stress in perinatal asphyxia. Pediatr Neurol 38:181–185
Gulcan H, Ozturk IC, Arslan S (2005) Alterations in antioxidant enzyme activities in cerebrospinal fluid related with severity of hypoxic ischemic encephalopathy in newborns. Biol Neonate 88:87–91
Bounocore G, Perrone S, Bracci R (2001) Free radicals and brain damage in the newborn. Biol Neonate 79:180–186
Acknowledgments
This study was supported by the Ministry of Science and Higher Education Grant No. N N401 003935.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gamdzyk, M., Makarewicz, D., Słomka, M. et al. Hypobaric Hypoxia Postconditioning Reduces Brain Damage and Improves Antioxidative Defense in the Model of Birth Asphyxia in 7-Day-Old Rats. Neurochem Res 39, 68–75 (2014). https://doi.org/10.1007/s11064-013-1191-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1191-0