Abstract
Glutamate-induced excitotoxicity due to over-activation of glutamate receptors and associated energy depletion (phosphorylation and activation of AMPK) results in neuronal cell death in various neurological disorders. Restoration of energy balance during an excitotoxic insult is critical for neuronal survival. Ascorbic acid (vitamin C), an essential nutrient with well-known antioxidant potential, protects the brain from oxidative damage in various models of neurodegeneration. In this study, we reported the therapeutic efficacy of vitamin C in response to glutamate-induced excitation, resulting in energy depletion and apoptosis in the hippocampus of the developing rat brain. A single subcutaneous injection of glutamate at two different concentrations (5 and 10 mg/kg) in postnatal day 7 rat pups increased brain glutamate levels and increased the protein expression of neuronal apoptotic markers. Both doses of glutamate upregulated the ratio of pro-apoptotic Bax to anti-apoptotic Bcl-2, cytochrome-c release, caspase-3 activation and the expression of PARP-1. However, co-treatment of vitamin C (250 mg/kg) with glutamate decreased brain glutamate levels and reversed the changes induced by glutamate in the developing hippocampus. Interestingly, only a high dose of glutamate caused the phosphorylation and activation of AMPK and induced neuronal cell death, whereas a low dose of glutamate failed to mediate these effects. Vitamin C supplementation reduced the glutamate-induced phosphorylation of AMPK and attenuated neuronal cell death, as assessed morphologically by Fluoro Jade B in the hippocampal CA1 region of the developing brain. Taken together, our results indicated that glutamate in both concentrations is toxic to the immature rat brain, whereas vitamin C is pharmacologically effective against glutamate-induced neurodegeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glutamate is a major neurotransmitter, and glutamatergic neurons form the primary excitatory system in the brain. Despite the role of glutamate receptors in brain cognition, their overstimulation can induce neuronal apoptosis via excitotoxicity [1]. In addition, abnormal glutamate concentrations in the brain have been correlated with several neurodegenerative disorders, including Alzheimer’s disease [2, 3]. Prolonged over-activation of NMDA receptors can elevate intracellular calcium concentrations, collapse the mitochondrial membrane, activate caspases and induce the production of reactive oxygen species (ROS) and cell death [4–6]. Glutamate induces oxidative stress through its pro-oxidant activity and has been shown to stimulate mitochondrial production of free radicals after glutamatergic activation of N-methyl-D-aspartate (NMDA) receptors in neurons [7]. A series of in vitro studies using HT22 cells and cortical neurons showed that glutamate induces DNA fragmentation via caspase-3- or Bax/Bcl-2-dependent processes, subsequently resulting in apoptosis [8, 9].
Accumulating evidence has shown that glutamate excitotoxicity is associated with energy depletion. AMP-activated protein kinase (AMPK), an energy sensor and multifunctional metabolite in the brain, is activated by conditions of cellular energy depletion and oxidative stress [10, 11]. Furthermore, AMPK is activated when it is phosphorylated at Thr172 on its catalytic α subunit, and activated AMPK further phosphorylates key enzymes of lipid metabolism [12]. Transient activation of AMPK is beneficial; however, its sustained activation has been reported to induce apoptosis in several previous studies.
Ascorbic acid (vitamin C) is a strong antioxidant and has been shown to possess ROS scavenging properties [13]. All animals and plants require vitamin C as an essential nutrient for their metabolism, and vitamin C has been shown to protect living organisms against oxidative stress [14, 15]. A study performed by Sinclair indicates that oxidative stress may increase as a result of the enhanced production of ROS or via low intake of antioxidant substances, such as vitamin C [16]. Moreover, another work revealed that neurons maintained a considerably high amount of intracellular concentrations of vitamin C [17]. The physiological and biochemical characteristics of vitamin C are due to its electron donor property; thus, it is a strong reducing agent.
The hippocampus is an important brain region involved in a number of important functions, including cognition, learning and memory. In several animal models, such as hypoxia and ischemia, the hippocampus has been shown to be the most vulnerable brain region due to the over-activation of glutamate receptors, activation of caspases and subsequent cell death [18]. However, a previous study showed that vitamin C induces neuroprotection against glutamate and NMDA injury in cortical neurons [19]. These findings and those of others motivated the present study to explore the neuroprotective effects of vitamin C against two concentrations of glutamate in the hippocampus of the developing rat brain.
Materials and Methods
Animals and Drug Treatment
Postnatal day 7 Sprague–Dawley male rat pups with an average body weight of 18 g were used in the present study. The rat pups were randomly divided into six groups (n = 10 animals/group): (1) control (C); (2) glutamate 5 mg/kg (G5); (3) glutamate 10 mg/kg (G10); (4) glutamate 5 mg/kg plus vitamin C 250 mg/kg (G5C); (5) glutamate 10 mg/kg plus vitamin C 250 mg/kg (G10C); and (6) vitamin C (250 mg/kg). Glutamate in two different concentrations in 0.9 % saline solution was subcutaneously (sc) administered to the rat pups with a single injection. Vitamin C (250 mg/kg in 0.9 % saline solution) was administered (sc) as a co-treatment 30 min after glutamate administration and the animals were sacrificed 4–12 h after injection, where as the control group was treated with saline solution. These experimental procedures were approved (Approval ID: 125) by the local animal ethics committee (IACUC) of the Division of Applied Life Sciences, Department of Biology, Gyeongsang National University, South Korea.
Glutamate Assay
The Abnova (Taipei, Taiwan) Glutamate assay kit (Cat. # KA1670) was used to analyze glutamate levels in hippocampal brain tissue homogenates according to the manufacturer’s instruction.
Western Blotting Analysis
After 4 h of treatment, the animals (n = 5 animals/group) were sacrificed as described previously [20, 21] for western blot analysis. The brains were rapidly removed, and the hippocampal section was carefully dissected out and frozen on dry ice. The tissue was homogenized in 0.2 M phosphate buffered saline (PBS) in the presence of a protease inhibitor cocktail. The protein concentration was measured using the Bio-Rad protein assay. Equivalent amounts of protein (30 μg per sample) were electrophoresed on 10–15 % SDS-PAGE gels under reducing conditions and transferred onto a polyvinylidene difluoride (PVDF) membrane (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Different types of antibodies were used in immunoblotting to detect different proteins, including rabbit-derived (anti-actin, anti-Bcl-2 and anti-Bax), goat-derived (anti-cytochrome c) and mouse-derived (anti-PARP-1) polyclonal antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA), along with rabbit-derived [anti-caspase-3, anti-AMPK-α and p-AMPK-α (Thr172)] polyclonal antibodies from Cell Signaling Technology, Inc. Western blots were analyzed by densitometry using the computer-based Sigma Gel (SPSS, Inc., Chicago, USA) system.
Tissue Collection and Sample Preparation for Morphological Analysis
Animals (n = 5 animals/group) were sacrificed 12 h after treatment as described previously [22], and brain sections from control rats and rats subjected to glutamate followed by vitamin C and vitamin C alone were analyzed. Each group consisted of five pups per group, and the pups were transcardially perfused with 4 % ice-cold paraformaldehyde followed by 1 × PBS. The brains were post-fixed in 4 % paraformaldehyde overnight and then transferred to 20 % sucrose until they sank to the bottom of the tubes. O.C.T compound (A.O. USA) was used to freeze the brain, and 16-μm coronal sections were obtained using a Leica cryostat (CM 3050C, Germany). The sections were thawed and mounted onto probe-on plus charged slides (Fisher).
Immunofluorescence Assay of Caspase-3 and p-AMPK
Immunofluorescence assays was performed as previously described [22]. Briefly, tissue-containing slides were washed two times for 15 min in 0.01 M PBS, and then proteinase K solution was added to the tissue and incubated for 5 min at 37 °C. Then, the tissues were incubated for 90 min in blocking solution containing normal swine serum and 0.3 % Triton X-100 in PBS. Primary antibody (mouse polyclonal caspase-3 and rabbit polyclonal p-AMPK, each 1:100 in PBS Santa Cruz) were applied alternatively at 4 °C overnight. Subsequently, the secondary antibodies (rabbit anti-mouse FITC, goat anti-rabbit TRITC Santa Cruz 1:50 in PBS) were applied at room temperature for 90 min. The slides were twice washed with PBS for 5 min. For double staining, incubations were performed in parallel and counterstained with DAPI. Glass cover slips were mounted on glass slides with mounting medium. Images were captured using a confocal microscope (FluoView FV 1000 Olympus, Japan).
Enzyme Assays
The CycLex AMPK kinase assay kit was used to measure the activated AMPK levels in hippocampal brain homogenates of postnatal day 7 rats according to the manufacturer’s instructions. The AMPK activity of the brain homogenates was recorded as an increase in absorbance at 450 nm.
Fluoro-Jade B Staining
Fluoro-Jade B staining was performed according to the manufacturer’s protocol (Millipore, USA, Cat. #AG310, Lot #2159662) and reported previously by our lab [23]. Briefly, the brain tissue slides were air-dried overnight. First, the slides were immersed in a 1 % sodium hydroxide and 80 % ethanol solution for 5 min and then in 70 % alcohol for 2 min followed by 2 min in distilled water. The slides were transferred to a solution of 0.06 % potassium permanganate for 10 min and then rinsed with distilled water. Next, the slides were immersed in a 0.1 % acetic acid and 0.01 % Fluoro-Jade B solution for 20 min. The slides were then rinsed with distilled water and dried for 10 min. Glass coverslips were mounted onto glass slides with mounting medium. Images were captured using a FITC filter on a confocal laser-scanning microscope (FV 1000, Olympus, Japan).
Data and Statistical Analysis
Western blotting analyses were scanned and analyzed using densitometry via the computer-based Sigma Gel System (SPSS Inc., Chicago, IL). The density values were expressed as the mean ± SEM. For morphological studies, the computer-based Image J program was used to analyze integrated densities. We used one-way ANOVA followed by Dunnett’s post hoc test for comparison of all groups to control. The group differences were analyzed using two-tailed unpaired Student’s t test. p value less than 5 % is considered significant.
Results
Effect of Vitamin C on Glutamate Level in the Developing Rat Brain
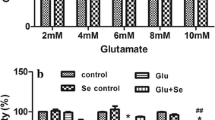
First, we analyzed the level of glutamate in the hippocampal homogenates of developing rat brains through the glutamate assay method after injecting glutamate exogenously at two different concentrations (5 and 10 mg/kg). Our results show that both concentrations of glutamate significantly increased the hippocampal glutamate levels (mM) (p < 0.01, p < 0.0001, F = 1,792, F = 2.468 respectively) 4 h after their administration. As mentioned earlier, vitamin C, which was coadministered along with two doses of glutamate, significantly (p < 0.05, p < 0.001 and F = 11.35, F = 8.654 for 5 and 10 mg/kg, respectively) reduced the glutamate level in the hippocampus of postnatal day 7 rat brains (Fig. 1a). Additionally, the treatment with vitamin C alone had no effect on the hippocampus glutamate levels, as evident from the data.
a The beneficial effect of vitamin C on glutamate levels in the hippocampus of the developing brain. Shown is the histogram of glutamate level (mM) in the hippocampus of postnatal day 7 rat brains. The young rats were treated with two different concentrations (5 and 10 mg/kg) of glutamate, vitamin C (250 mg/kg) with or without glutamate and (0.9 %) saline solution. The Abnova glutamate assay kit was used to measure glutamate levels in the young rat brain homogenates according to the manufacturer’s instructions. Shown are the b immunoblots of Bax and Bcl-2 proteins, c the histogram showing the relative density bars of Bax and Bcl-2 proteins, and d the histogram showing the Bax/Bcl-2 ratio after glutamate and vitamin C treatment in the hippocampus of postnatal day 7 developing rat brains. β-Actin was used as the loading control in each case. Each bar represents the mean ± SEM of three experiments (n = 5 animals/group). One-way ANOVA was used followed by Dunnett’s post hoc test for comparison of all groups to control (found p < 0.05 significant for all groups except for alone vitamin C). The group differences were analyzed using two-tailed unpaired Student’s t test. Significance: a p < 0.01, b p < 0.0001, c p < 0.05, and d p < 0.001, respectively
Vitamin C Reversed the Expressions of Dose-Dependent Glutamate-Induced Apoptotic Markers in the Postnatal Rat Brain
Exogenously administered glutamate in two different concentrations after increasing the brain glutamate levels was further assessed for its toxic effects in young rats. The western blot results indicate that both doses of glutamate not only caused the upregulation of the expression of pro-apoptotic Bax proteins and significantly downregulated anti-apoptotic Bcl-2 proteins (Fig. 1b, c), but glutamate also increased the Bax/Bcl-2 ratio (Fig. 1d). Compared with the low dose of glutamate (i.e., 5 mg/kg; p < 0.01, F = 1.96), the high dose of glutamate (i.e., 10 mg/kg; p < 0.0001, F = 11.13) showed a more significant effect in increasing the Bax/Bcl-2 ratio. However, animals that received vitamin C either as a co-treatment along with glutamate or alone shown a reduced expression of Bax, an increased expression of Bcl-2 protein and a decreased Bax/Bcl-2 ratio (Fig. 1b–d).
We next examined cytochrome c release upon treatment with glutamate and vitamin C using western blotting analyses. Our results showed that as a result of both doses of glutamate, a marked increase (p < 0.01, p < 0.0001 and F = 182.7, F = 515.5 for 5 and 10 mg/kg, respectively) in the release of cytochrome c was observed in developing rat brain, whereas its release was significantly (p < 0.05, p < 0.001 and F = 13.6, F = 2.21 for 5 and 10 mg/kg, respectively) inhibited by vitamin C in the hippocampus of postnatal day 7 rat brains (Fig. 2a, b). Additionally, vitamin C alone has also shown non-deleterious effects in terms of cytochrome c expression, as evident from the western blot analysis and density histogram (Fig. 2a, b). Similarly, we extended our study to analyze the effect of glutamate at two concentrations with vitamin C on the activated caspase-3 expression level. The results indicated that glutamate in both concentrations induced a significant (p < 0.01, p < 0.0001 and F = 64.67, F = 162.9 for 5 and 10 mg/kg, respectively) increase in the expression level of activated caspase-3 compared with the control, whereas vitamin C reduced the expression level of activated caspase-3 compared with both doses of glutamate alone in the hippocampus of the developing rat brain (Fig. 2c, d). Similarly, vitamin C alone also decreased caspase-3 expression as shown in Fig. 2c, d. Moreover, vitamin C coadministration also significantly (p < 0.05, p < 0.001 and F = 3.051, F = 33.22 for 5 and 10 mg/kg of glutamate, respectively) reduced the expression level of PARP-1 against the two concentrations of glutamate in the hippocampus of the developing rat brain (Fig. 3a, b).
Vitamin C inhibited glutamate-induced cytochrome-c release and caspase-3 activation in the hippocampus of young rats. a, b The representative immunoblot of cytochrome-c along with its relative density histogram and c, d the immunoblot of caspase-3 along with its relative density histogram showing that vitamin C inhibited cytochrome-c release and activation of caspase-3 against two different doses of glutamate in the hippocampus of the postnatal day 7 rat brain. After 4 h of drugs administration the animals were sacrificed. All of the groups and treatment details have been provided in the materials and methods section. β-actin is included as an internal reference control. Each bar represents the mean ± SEM of three experiments (n = 5 animals/group). Statistical analysis were analysed with one way ANOVA following Dunnett’s post hoc and Student’s t test. Significance: a p < 0.01, b p < 0.0001, c p < 0.05, and d p < 0.001, respectively
Vitamin C treatment reduced PARP-1 expression against both doses of glutamate in the hippocampus of young rats. Shown are the representative a, b immunoblots along with the relative density histogram of PARP-1. The immunoblots reveal that glutamate in both concentrations after 4 h induced PARP-1 activation, whereas vitamin C treatment reversed the changes in the postnatal day 7 rat brains. c, d Shown are the immunoblots and relative density histogram of p-AMPK-α/AMPK proteins. Densitometry analyses were analyzed through Sigma Gel, a computer based software. The hippocampal brain tissues of rat pups were collected 4 h after drug treatment. e Measurement of AMPK activity in the hippocampal brain homogenates from animals that received saline, glutamate alone (in two different concentrations), vitamin C alone, and in combination with two doses of glutamate. Methods were followed as described by the manufacturer. The experiments represents the mean ± SEM of three experiments (n = 5 animals/group). One way ANOVA followed by Dunnett’s post hoc test (comparing all groups with control) and Student’s t test (differences between the groups) were implicated. Significance: a p < 0.01, b p < 0.0001, c p < 0.05, and d p < 0.001, respectively
The Effect of Vitamin C Against Glutamate-Induced Phosphorylation of AMPK-α in the Developing Rat Brain
Previous studies have demonstrated an indirect correlation between excitotoxic insults and energy depletion; thus, we measured the expression levels of total AMPK and its activated and phosphorylated form p-AMPK-α in the hippocampus of the developing brain after administering glutamate alone in two different concentrations with and without vitamin C cotreatment. Our results indicated that although low doses of glutamate induced a significant increase in the expression of different apoptotic markers, it failed to phosphorylate and activate AMPK-α (whereas the total AMPK levels remained unchanged). However, these findings were in contrast to high doses of glutamate, which induced a significant (p < 0.0001, F = 7.511) phosphorylation and activation of AMPK-α at Thr172, suggesting that glutamate is involved in excitotoxicity in the developing brain; however, vitamin C significantly inactivated and dephosphorylated p-AMPK and reduced (p < 0.001, F = 6.329) the expression of p-AMPK-α in the hippocampus of the postnatal brain, indicating that it is involved in the restoration of energy balance against excitotoxic insult induced by high doses of glutamate (Fig. 3c, d).
To extend our knowledge on the activation of p-AMPK by glutamate, we performed an AMPK activity assay with the brain homogenates. The results were in accordance with the western blot, indicating that a low dose of glutamate was unable to phosphorylate and activate AMPK, whereas a high dose induced a significant (p < 0.0001, F = 13.19) activation and phosphorylation of AMPK at Thr172, as shown in Fig. 3e. Similarly, vitamin C treatment reversed the activated states of p-AMPK induced by high doses of glutamate in the hippocampus of the postnatal brain. Interestingly, vitamin C alone has no effect on the brain p-AMPK levels (Fig. 3e).
Morphological Assessment of Activated Caspase-3 and p-AMPK Colocalization After Drug Treatment in the Developing Rat Brain
The morphological investigation of caspase-3 and p-AMPK after glutamate and vitamin C treatment was further assessed through their colocalization in the hippocampus of the developing brain. The immunofluorescence images showed that as a result of a high dose (10 mg/kg) of glutamate, the expression levels of activated caspase-3 and p-AMPK were significantly (p < 0.0001) higher compared with the control group in the DG (Fig. 4a, b), CA1 (Fig. 4c, d) and CA3 (Fig. 4e, f) regions of the developing rat hippocampus. However, vitamin C co-treatment significantly (p < 0.001) reduced the colocalization of caspase-3 and p-AMPK and reduced their expressions in the above mentioned three regions (Fig. 4a–f) of the hippocampus of the postnatal day 7 brain. Also note that vitamin C (250 mg/kg) alone is not toxic to the immature rat hippocampus, as indicated by the lower expressions of caspase-3 and p-AMPK proteins as assessed morphologically and shown in Fig. 4a–f.
Vitamin C effect on p-AMPK-α and caspase-3 colocalization against a high dose (10 mg/kg) of glutamate in the hippocampal regions of postnatal day 7 rat brains. Shown are the immunofluorescence images and their integrated density histograms of the a, b DG, c, d CA1 and e, f CA3 regions of animals that received a high dose of glutamate alone, vitamin C alone or in combination with a high dose of glutamate after 12 h of drug treatment (n = 5 animals/group). The images of caspase-3 (FITC, green), p-AMPK (TRITC, red) and DAPI (merged). All technical details are described in the “Materials and Methods” section. The group differences were analyzed by using two-tailed unpaired Student’s t test. Significance: b p < 0.0001, and d p < 0.001, respectively (Color figure online)
Vitamin C Reduced the Glutamate-Induced Neurodegeneration in the Developing Rat Brain
Fluoro Jade B (FJB) staining was performed to observe the toxic effect of a high dose of exogenously administered glutamate on CA1 neurons in the developing brain. Our results showed that glutamate-treated animals exhibited a significantly (p < 0.0001, F = 8564) high number of FJB-positive neurons, whereas no FJB-positive neuronal cells were observed in saline-treated animals in the hippocampal CA1 region of the developing brain. In contrast, vitamin C-treated animals showed fewer (p < 0.001, F = 2.790) FJB-stained neuronal cells in the CA1 region of the immature brain, suggesting that vitamin C co-treatment reduced glutamate-induced neurodegeneration (Fig. 5a, b). Similarly, vitamin C alone had no toxic effect on the immature brain, as evident from the FJB photomicrographs images shown in Fig. 5a, b.
Vitamin C treatment reduced glutamate-induced neurodegeneration in the hippocampal CA1 region of the postnatal day 7 rat brain. a, b Shown are the FJB images counterstained with DAPI and its integrated density histogram in the hippocampal CA1 region of developing rats after 12 h of drug treatment. The majority of glutamate-induced FJB-positive degenerating neuronal cells are present in the CA1 region of the hippocampus, whereas vitamin C reduced the number of FJB-positive neurons. The images are representative of staining obtained in sections prepared from five animals/group. The FJB images represent the mean ± SEM of three experiments. Student’s t test was used for the statistical differences between the different groups. Significance: b p < 0.0001, and d p < 0.001, respectively
Discussion
Our data provided strong evidence for the protective effects of vitamin C against two doses of glutamate-induced neuronal degeneration in the hippocampus of the developing brain. This study addressed novel findings that exogenously administered glutamate in vivo induces the phosphorylation and activation of AMPK. Because of the immature nature of the blood brain barrier (BBB) in the developing stage, young rats are good model for inducing excitotoxicity by administering glutamate exogenously. Glutamate is a major excitatory neurotransmitter, which demonstrates many important functions in the brain, including synaptic plasticity and neuronal development, but at high concentrations, over-activation of its receptors results in toxicity, thereby inducing neuronal cell death [24–27].
Glutamate can induce its harmful effects via two mechanisms, excitotoxicity or oxidative toxicity. Several studies have demonstrated that overstimulation of glutamate receptors induces abnormal Ca2+ concentrations, which subsequently results in neuronal dysfunction and cell death [28–31]. In the developing fetal brain, two well-known processes exist for cellular proliferation and differentiation, and the Bcl-2 family of proteins plays a key role in programmed cell death [32–35]. In the present study, we measured glutamate levels in the hippocampal section of the developing brain after exogenous glutamate and vitamin C administration and also investigated the response and changes in the expression of pro- and anti-apoptotic proteins. Although we do not know the exact mechanism, these results clearly indicated that vitamin C not only reduced brain glutamate levels but also downregulated the ratio of pro- to anti-apoptotic proteins in the hippocampal region of neonatal day 7 brains. These results are consistent with a previously reported study in which vitamin C reduced the glutamate-induced increase in Bax/Bcl-2 ratio in the rat thymus and demonstrates a strong neuroprotective role [36]. In addition, our lab has also reported that vitamin C protects prenatal hippocampal neurons against ethanol- and PTZ-induced neurodegeneration [37].
Glutamate excitotoxicity has been shown to induce apoptosis in both neuronal and non-neuronal cells via a group of cysteine proteases known as caspases. Among these caspases, caspase-3 has been highlighted to be involved in neuronal cell death, and Ca2+ influx can activate it as a downstream event [38]. When activated, the pro-apoptotic protein Bax translocates into the mitochondria from the cytosol and has been shown to trigger cytochrome c release; this glutamate-induced mitochondrial cytochrome c release into the cytosol is an important step in apoptotic cell death [39] and subsequently causes the activation of the aspartate-specific cysteine protease caspase-3 in neuronal and non-neuronal cell death in vitro and in vivo [40]. Similarly, we demonstrated that both doses of glutamate-induced increases in the Bax/Bcl-2 ratio initiated the release of cytochrome c and the activation caspase-3. In addition, the natural antioxidant vitamin C was capable of inhibiting cytochrome c translocation and the activation of caspase-3 against both doses of glutamate. As previously reported, glutamate-dependent elevations in Ca2+ intracellular concentrations can induce toxicity via oxidative stress by producing ROS, thereby activating caspase-3 and cell death [41, 42]. This synergistic relationship between caspase-3 activation and ROS generation motivated the investigation of whether vitamin C inhibits caspase-3 via ROS scavenging; this question, however, requires further study.
Excitotoxicity-induced neuronal cell death is thought to be mediated via mitochondrial dysfunction because mitochondria are the powerhouse of cells [43]. Previous studies have demonstrated that AMPK acts as a multifunctional metabolic sensor in the brain, and its activation promotes cell death in vitro and in vivo in a number of different cells, including neurons [10, 11, 44–47], whereas its inhibition is very important for many models in several stressors. In this study, we observed a dramatic increase in the phosphorylation status of AMPK-α in the hippocampus of the developing brain upon exposure to a high dose of glutamate in the absence of an accompanying increase in total AMPK level. In contrast, vitamin C inhibited the glutamate-induced phosphorylation of AMPK-α in the hippocampus of the developing brain. This may be due to the enhanced susceptibility of developing neurons to excitotoxicity through several structural differentiation processes, including axon and dendrite elongation or synapse formation, that require additional energy. Our results are consistent with the hypothesis that long-term and over-activation of AMPK promotes cell death in the hippocampal CA1 region of the developing brain. Our results also suggest that the inhibition of glutamate-induced AMPK activation by vitamin C is likely to be an important component of its neuroprotective mechanism. Consistent with the previous studies, which revealed that despite its involvement in DNA repair, over-activation of (ADP ribose) polymerase-1 (PARP-1) in excitotoxicity induces neurodegeneration [48], and considering the toxic effects of glutamate, our results also indicated that the exposure of the developing brain at postnatal day 7 to glutamate significantly damaged neuronal DNA. This conclusion is evident from the increased expression of cleaved PARP-1 and FJB stained neuronal cells because FJB is a reliable marker for neuronal vulnerability [49], whereas vitamin C protected the hippocampus of the developing rat brain against glutamate toxicity by reducing the expression of cleaved PARP-1. Most importantly, the presence of FJB-positive neuronal cells in the hippocampal CA1 region suggests that vitamin C rescued neuronal DNA fragmentation (Figs. 3, 5).
In conclusion, our results suggested that glutamate is toxic to the developing brain at both concentrations (especially the high dose with energy depletion), and vitamin C can interfere with neurodegeneration induced by glutamate in the hippocampus of the developing brain. Moreover, vitamin C may represent a potential remedy for neurodegenerative conditions caused by the toxic effects of various neurotoxic drugs in new-borns or infants. However, further studies are required to assess the underlying neuroprotective mechanism of vitamin C comprehensively.
References
Sims NR, Zaidan E (1995) Biochemical changes associated with selective neuronal death following short-term cerebral ischaemia. Int J Biochem Cell Biol 27:531–550
Lipton SA, Rosenberg PA (1994) Excitatory amino acids as a final common pathway for neurologic disorder. N Engl J Med 330:613–622
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262:689–695
Fan MMY, Raymond LA (2007) N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in Huntington’s disease. Prog Neurobiol 81:272–293
Jung KH, Chu K, Lee ST, Park HK, Kim JH, Kang KM (2009) Augmentation of nitrite therapy in cerebral ischemia by NMDA receptor inhibition. Biochem Biophys Res Comm 378:507–512
Ndountse LT, Chan HM (2009) Role of N-methyl-D-aspartate receptors in polychlorinated biphenyl mediated neurotoxicity. Toxicol Lett 184:50–55
Patel M, Day BJ, Crapo JD, Fridovich I, McNamara JO (1996) Requirement for superoxide in excitotoxic cell death. Neuron 16:345–355
Zhang YM, Lu XF, Bhavnani BR (2003) Equine estrogens differentially inhibit DNA fragmentation induced by glutamate in neuronal cells by modulation of regulatory proteins involved in programmed cell death. BMC Neurosci 4:32
Zhang YM, Bhavnani BR (2005) Glutamate-induced apoptosis in primary cortical neurons is inhibited by equine estrogens via down-regulation of caspase-3 and prevention of mitochondrial cytochrome c release. BMC Neurosci 6:13
Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Writters LA (1999) Dealing with energy demand: the AMPK-activated protein kinase. Trends Biochem Sci 24:22–25
Ramamurthy S, Ronnett GV (2006) AMPK-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol 574:85–93
Hardie DG, Hawley SA, Scott JW (2006) AMP-activated protein kinase development of the energy sensor concept. J Physiol 574:7–15
Bode AM (1997) Metabolism of vitamin C in health and disease. Adv Pharmacol 38:21
Sebrel WH, Harris RS (1967) The vitamins: chemistry, physiology, pathology and methods. Academic Press, New York
Padayatti SJ, Katz A, Wang Y, Eck P, Kwon O, Lee J (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22:18–35
Sinclair AJ, Barnett AH, Lunec J (1990) Free radicals and antioxidant systems in health and disease. Br J Hosp Med 43:334–344
May MJ, Li L, Hayslett K, Qu Z (2006) Ascorbate transport and recycling by SH-SY5Y neuroblastoma cells: response to glutamate toxicity. Neurochem Res 31:785–794
Wang WP, Iyo AH, Miguel-Hidalgo J, Regunathan S, Zhu MY (2006) Agmatine protects against cell damage induced by NMDA and glutamate in cultured hippocampal neurons. Brain Res 1084:210–216
Majewska MD, Bell JA (1990) Ascorbic acid protects neurons from injury induced by glutamate and NMDA. NeuroReport 1:194–196
Shah SA, Yoon GH, Kim MO (2014) Protection of the developing brain with anthocyanins against ethanol-induced oxidative stress and neurodegeneration. Mol Neurobiol. doi:10.1007/s12035-014-8805-7
Shah SA et al (2013) Anthocyanins protect against ethanol-induced neuronal apoptosis via GABAB1 receptors intracellular signaling in prenatal rat hippocampal neurons. Mol Neurobiol 48:257–269
Shahid AS, Hae YL, Ray AB, Dae JY, Myeong OK (2014) Novel osmotin attenuates glutamate-induced synaptic dysfunction and neurodegeneration via the JNK/PI3K/Akt pathway in postnatal rat brain. Cell Death Dis 5:1–10
Ullah N, Naseer MI, Ullah I, Lee HY, Koh PO, Kim MO (2011) Protective effect of pyruvate against ethanol-induced apoptotic neurodegeneration in the developing rat brain. Neuropharmacology 61:1248–1255
Bleich S, Romer K, Wiltfang J, Kornhuber J (2003) Glutamate and the glutamate receptor system: a target for drug action. Int J Geriatr Psychiatry 18:S33–S40
Conn PJ (2003) Physiological roles and therapeutic potential of Metabotropic glutamate receptors. Ann N Y Acad Sci 1003:12–21
Choi DW (1987) Ionic dependence of glutamate neurotoxicity. J Neurosci 7:369–379
Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P (1995) Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 15:961–973
Choi DW (1992) Excitotoxic cell death. J Neurobiol 23:1261–1276
Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH (1991) Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA 88:6368–6371
Yamauchi M, Omote K, Ninomiya T (1998) Direct evidence for the role of nitric oxide on the glutamate-induced neuronal death in cultured cortical neurons. Brain Res 780:253–259
Sattler R, Tymianski M (2000) Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med 78:3–13
Bibel M, Barde YA (2000) Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev 14:19–2937
Dunty JWC, Chen SY, Zucker RM, Dehart DB, Sulik KK (2001) Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: implications for alcohol-related birth defects and neurodevelopmental disorder. Alcohol Clin Exp Res 25:1523–1535
Montoliu C, Valles S, Renau-Piqueras J, Guerri C (1994) Ethanolinduced oxygen radical formation and lipid peroxidation in rat brain: effect of chronic alcohol consumption. J Neurochem 63:1855–1862
Snider WD (1994) Functions of the neurotrophins during nervous system development: What the knockouts are teaching us. Cell 77:627–738
Pavlovic V, Pavlovic D, Kocic G, Sokolovic D, Sarac M, Jovic Z (2009) Ascorbic acid modulates monosodium glutamate induced cytotoxicity in rat thymus. Bratislavske´ lekarske listy 110:205–209
Naseer MI, Ullah N, Ullah I, Koh PO, Lee HY, Park MS, Kim MO (2011) Vitamin C protects against ethanol and PTZ-induced apoptotic neurodegeneration in prenatal rat hippocampal neurons. Synapse 65:562–571
Nicholls DG, Ward MW (2000) Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci 23:166–174
Liu X, Kim CN, Yang J, Jemmerson R, Wang X (1996) Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147–157
Nicholson DW, Thornberry NA (1997) Caspases: killer proteases. Trends Biochem Sci 8:299–306
Ekinci FJ, Linsley MD, Shea TB (2000) Amyloid-induced calcium influx induces apoptosis in culture by oxidative stress rather than tau phosphorylation. Mol Brain Res 76:389–395
Perskvist N, Long M, Stendahl O, Zheng L (2000) Mycobacterium tuberculosis promotes apoptosis in human neutrophils by activating caspase-3 and altering expression of Bax/Bcl-xL via an oxygen-dependent pathway. J Immunol 168:6358–6365
Nicholls DG, Ward MW (2000) Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci 23:166–174
Salt IP, Johnson G, Ashcroft SJ, Hardie DG (1998) AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J 335:533–539
Nakatsu Y, Kotake Y, Hino A, Ohta S (2008) Activation of AMP-activated protein kinase by tributyltin induces neuronal cell death. Toxicol Appl Pharmacol 230:358–363
Kim YM, Hwang TJ, Kwak DW, Lee YK, Park OJ (2007) Involvement of AMPK signaling cascade in capsaicin-induced apoptosis of HT-29 colon cancer cells. Ann N Y Acad Sci 1095:496–503
McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV (2005) Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem 280:20493–20502
Berger NA (1985) Poly (ADP-ribose) in the cellular response to DNA damage. Radiat Res 101:4–15
Anderson KJ, Miller KM, Fugaccia I, Scheff SW (2005) Regional distribution of fluoro-jade B staining in the hippocampus following traumatic brain injury. Exp Neurol 193:125–130
Acknowledgments
This study was supported by the Pioneer Research Centre Program of the National Research Foundation of Korea, which is funded by the Ministry of Science, ICT & Future Planning (2012-0009521).
Conflict of interest
It is hereby declared that we have no conflicts of interest in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Shahid Ali Shah and Gwang Ho Yoon have contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Shah, S.A., Yoon, G.H., Kim, HO. et al. Vitamin C Neuroprotection Against Dose-Dependent Glutamate-Induced Neurodegeneration in the Postnatal Brain. Neurochem Res 40, 875–884 (2015). https://doi.org/10.1007/s11064-015-1540-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1540-2