Abstract
Purpose
There is lack of comprehensive analysis evaluating the impact of clinical, molecular, imaging, and surgical data on survival of patients with gliomatosis cerebri (GC). This study aimed to investigate prognostic factors of GC in adult-type diffuse glioma patients.
Methods
Retrospective chart and imaging review was performed in 99 GC patients from adult-type diffuse glioma (among 1,211 patients; 6 oligodendroglioma, 16 IDH-mutant astrocytoma, and 77 IDH-wildtype glioblastoma) from a single institution between 2005 and 2021. Predictors of overall survival (OS) of entire patients and IDH-wildtype glioblastoma patients were determined.
Results
The median OS was 16.7 months (95% confidence interval [CI] 14.2–22.2) in entire patients and 14.3 months (95% CI 12.2–61.9) in IDH-wildtype glioblastoma patients. In entire patients, KPS (hazard ratio [HR] = 0.98, P = 0.004), no 1p/19q codeletion (HR = 10.75, P = 0.019), MGMTp methylation (HR = 0.54, P = 0.028), and hemorrhage (HR = 3.45, P = 0.001) were independent prognostic factors on multivariable analysis. In IDH-wildtype glioblastoma patients, KPS (HR = 2.24, P = 0.075) was the only independent prognostic factor on multivariable analysis. In subgroup of IDH-wildtype glioblastoma with CE tumors, total resection of CE tumor did not remain as a significant prognostic factor (HR = 1.13, P = 0.685).
Conclusions
The prognosis of GC patients is determined by its underlying molecular type and patient performance status. Compared with diffuse glioma without GC, aggressive surgery of CE tumor in GC patients does not improve survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomatosis cerebri (GC) is defined as a diffuse glioma that exhibits an infiltrative growth pattern affecting at least three lobes of the brain while grossly maintaining the underlying normal brain architecture [1, 2]. Initially recognized as a distinct tumor type in the 2007 World Health Organization (WHO) classification, GC was later excluded in the subsequent 2016 and 2021 WHO classifications, due to the integrated diagnostic approach incorporating molecular genetics [3, 4]. Currently, GC is considered as a growth pattern within various types of diffuse gliomas, rather than a distinct pathological entity. However, the incidence of GC is relatively higher than anticipated, occurring in up to 8.2% of adult-type diffuse gliomas, and individuals with GC generally exhibit poorer prognosis when compared to diffuse glioma of corresponding tumor type [5].
Despite its relatively high incidence and poor prognosis, there is a lack of comprehensive evaluation on prognostic factors of adult-type diffuse glioma patients with GC. Previous investigations on prognostic factors of GC were conducted prior to the 2021 WHO classification. Majority of these studies did not reflect molecular information of adult-type diffuse gliomas and compared different tumor types which are now biologically distinct and separate tumors [6, 7], while other population-based studies on GC lacked information of tumor types [8]. Moreover, previous studies have not adequately addressed the recent paradigm shift concerning the extent of resection (EOR) in gliomas [9]. Specifically, these studies have failed to distinguish between contrast-enhancing (CE) and non-enhancing (NE) tumors, thereby overlooking potential differences in survival outcomes associated with the resection of CE or NE tumors. Although achievement of supramaximal resection (additional removal of NE tumors along with total resection of CE borders) is not feasible in GC due to its diffusely infiltrative nature, total resection of CE tumor is sometimes feasible in GC patients. Nevertheless, the impact of total resection of CE tumor remains unclear in GC patients.
In this study, we conducted a comprehensive analysis incorporating clinical, molecular, imaging and surgical data in GC patients to investigate independent prognostic factors.
Methods
Study population and demographic data
The study was conducted retrospectively, and informed consent from the patients were waived by the institutional review board of our institution (Approval no.: 4–2023-0045). From January 2005 to December 2021, a total of 1,473 adult patients diagnosed with diffuse glioma from our institution were initially recruited for this study.
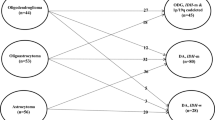
Inclusion criteria comprised: 1) grade 2 to 4 diffuse gliomas confirmed by histopathology, 2) known IDH mutation, 1p/19q codeletion, and O6-methylguanine-DNA methyltransferase promoter (MGMTp) methylation status, and 3) aged ≥ 18 years. Exclusion criteria included: 1) not fulfilling the radiologic and/or histologic diagnostic criteria for GC (n = 1,112), 2) lack of diagnostic information necessary to assign a specific WHO diagnosis, leading to diagnosis of IDH-wildtype diffuse glioma, not otherwise specified (n = 112), 3) diagnostic work-up results not readily allowing a WHO diagnosis, despite successful performance of diagnostic testings, leading to diagnosis of IDH-wildtype diffuse glioma, not elsewhere classified (n = 21), 4) follow-up loss within 3 months (n = 93), and 5) presence of H3 K27M alteration in midline-located tumors, leading to diagnosis of diffuse midline glioma, H3 K27-altered (n = 36).
A total of 99 patients with GC were included as our study cohort. Figure 1 shows the patient flowchart.
Molecular analysis
All patients were diagnosed according to the 2021 WHO classification [4]. Both innumohistochemical (IHC) analysis and peptide nucleic acid-mediated clamping polymerase chain reaction (PCR) were performed to detect IDH1/2 mutation. In IDH1/2-negative patients on IHC analysis, IDH1/2 status was confirmed by PCR. MGMTp methylation was evaluated by methylation-specific PCR. Fluorescent in situ hybridization analysis was performed to detect 1p/19q codeletion. All patients underwent evaluation for IDH1/2 mutation, 1p/19q co-deletion, and MGMTp methylation status.
ATRX loss and p53 expression were assessed by IHC analysis. ATRX loss was defined as less than 10% expression of positive tumor cells and more than 50% of nuclei stained for p53 was considered positive expression for p53. The presence of H3 K27M mutant protein was evaluated in midline located tumors. TERTp (telomerase reverse transcriptase promoter) mutation (C228T and C250T) was determined using a pyrosequencing assay. Since 2015, targeted next-generation sequencing (NGS) was performed using Illuminal Trusight Tumor 170 panel including EGFR gene amplification and chromosome + 7/-10 [10, 11].
In 32 (32.3%) patients, IDH mutation status was determined based on IHC and PCR results without targeted NGS results. ATRX loss, p53 protein expression, TERTp mutation, EGFR amplification, chromosome + 7/-10, and TP53 information were available in 88 (88.9%), 70 (70.7%), 81 (81.8%), 71 (71.7%), 39 (39.4%) and 68 (68.7%) patients, respectively.
MRI Protocol
Preoperative and postoperative brain magnetic resonance images (MRI) including T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), post-contrast 3D T1-weighted images, post-contrast FLAIR and diffusion weighted images were acquired in a 3-T unit (Achieva or Ingenia; Philips Healthcare). Detailed parameters for MRI protocols are listed in Supplementary Material S1.
Imaging analysis
GC was defined in cases in which radiological and/or pathological findings were present: 1) T2-weighted and FLAIR images showing diffuse infiltration of tumor involving three or more consecutive lobes with relative preservation of the underlying anatomical architecture, and 2) pathological analysis confirming glial cell proliferation indicative of an infiltrative glioma [12].
Baseline preoperative MRIs were reviewed by two neuroradiologists (Y.W.P. and S.S.A., with 11 and 18 years of experience) in consensus. In preoperative MRI, the type of GC (either type 1 or type 2), location (supratentorial or infratentorial), presence of contrast enhancement, necrosis, hemorrhage, cystic change, presence of diffusion restriction, leptomeningeal metastases, and proportion of CE tumor > 5% were labeled. Type 1 GC encompassed gliomas characterized as GC without a discernable CE mass, while type 2 GC comprised of GC with formation of a discrete CE mass, according to the previous criteria [2].
Evaluation of extent of resection (EOR)
Bi-dimensional perpendicular measurement of the CE and NE tumor was performed in baseline and immediate postoperative imaging taken within 48 h. The EOR of CE and NE tumors was each labeled as either gross total removal (GTR) or non-GTR, and further categorized as GTR of both CE and NE tumors, GTR of CE tumor and non-GTR of NE tumor, non-GTR of both CE and NE tumor, and biopsy reflecting recent recommendations integrating gliomas with or without contrast enhancement [13, 14].
Clinical data analysis
Clinical data including age, sex, date of diagnosis, initial Karnofsky performance status (KPS), date of death or last follow-up were collected from the electronic medical record. All patients underwent adjuvant treatment conforming to the recommended guideline according to each tumor type [15, 16]. In case of radiotherapy, radiation fields included the extent of gliomatosis cerebri. Overall survival (OS) was defined as the duration from the initial surgery until death or last follow-up date.
Statistical analysis
Univariable and multivariable Cox proportional hazards regression modeling for OS was performed in the entire GC patients and subgroup of GC patients with IDH-wildtype glioblastoma. Variables of interest on the univariable Cox proportional hazards regression models (P < 0.05) were included in the multivariable Cox proportional hazards regression modeling using backward elimination according to the likelihood ratio. Subgroup analyses was additionally performed in GC patients with CE tumor portion in IDH-wildtype glioblastoma, to determine if GTR of CE tumor improves survival. Survival rates were determined using the Kaplan–Meier method and curves which were compared using the log-rank test. For continuous variables, the cutoff point for Kaplan–Meier plots was determined using survMisc package. Statistical significance was set at P < 0.05. The data was analyzed using R version 4.3.2 including survMisc package [17, 18].
Results
Patient characteristics
Among 1,211 adult-type diffuse glioma patients, 99 adult-type diffuse glioma patients with GC (age range 18–89 years, 45 females and 54 males) were included. The median follow-up period was 54.0 months (95% confidence interval [CI] 33.2–121.1). Majority of the tumor types were IDH-wildtype glioblastomas (n = 77, 77.8%), followed by IDH-mutant astrocytomas (n = 16, 16.2%) and oligodendrogliomas (n = 6, 6.0%). GTR of both CE and NE tumors was not achieved in any of the patients due to the extensive infiltrative nature of GC. All patients underwent standard treatment according to the molecular type and grade [15]. The detailed patient characteristics of the study cohort are summarized in Table 1.
Survival analysis in entire GC patients
The median OS was 16.7 months (95% CI 14.2–22.2) in entire patients. On univariable analysis, older age, sex, higher KPS, CNS WHO grade 4, IDH-wildtype, no 1p/19q codeletion, MGMTp methylation, GC type 2, presence of contrast enhancement, proportion of CE tumor > 5%, necrosis, diffusion restriction, cystic change, and hemorrhage were significant predictors of OS. On multivariable Cox analysis, higher KPS (HR = 0.98, P = 0.004), no 1p/19q codeletion (HR = 10.75, P = 0.019), MGMTp methylation (HR = 0.54, P = 0.028), and hemorrhage (HR = 3.45, P = 0.001) remained as independent prognostic factors. The univariable and multivariable Cox analysis results are show in Supplmentary Table 1. Figure 2 shows the Kaplan-Meir curves in entire GC patients according to molecular type of tumors (log-rank test, P < 0.001). Figure S1 shows Kaplan-Meir curves in entire GC patients according to KPS (log-rank test, P = 0.001), 1p/19q codeletion (log-rank test, P = 0.001), MGMTp methylation (log-rank test, P = 0.005), and hemorrhage (log-rank test, P < 0.001).
Survival analysis in GC patients with IDH-wildtype Glioblastoma
The median OS was 14.3 months (95% CI 12.2–61.9) in IDH-wildtype glioblastoma patients. On univariable analysis in GC patients with IDH-wildtype glioblastoma (n = 77), older age, higher KPS, presence of CE tumor, proportion of CE tumor > 5%, diffusion restriction, and hemorrhage were significant predictors of OS. On multivariable analysis, high KPS (HR = 0.98, P = 0.042) was the only independent prognostic factor for OS. The univariable and multivariable Cox analysis results are show in Table 2. Figure 3(a) shows the Kaplan-Meir curve in GC patients with IDH-wildtype glioblastoma according to KPS (log-rank test, P = 0.027).
a Kaplan–Meier curve for overall survival of GC patients with IDH-wildtype glioblastoma based on KPS. b Kaplan–Meier curve for overall survival of GC patients with IDH-wildtype glioblastoma and contrast enhancement based on EOR. CE = contrast-enhancing; CI = confidence interval; EOR = extent of resection; GC = gliomatosis cerebri; GTR = gross total resection; IDH = isocitrate dehydrogenase; KPS = Karnofsky Performance status; NE = non-enhancing; OS = overall survival
On univariable analysis of subgroup of IDH-wildtype glioblastoma patients with CE tumor (n = 69), older age and higher KPS were significant predictors of OS. On multivariable analysis, higher KPS (HR = 0.98, P = 0.044) was the only independent prognostic factor for OS. Of note, EOR showed no significant association with OS (Table 3, P = 0.168; HR = 1.21, P = 0.541 for non-GTR of both CE and NE tumors, HR = 2.03, P = 0.060 for biopsy, reference group as GTR of CE and non-GTR of NE tumors). The median OS were 15.6 (95% CI 11.6–61.9), 12.8 (95% CI 11.1–45.5), and 11.2 (95% CI 3.3–15.5) months for GTR of CE and non-GTR of NE tumor, non-GTR of both CE and NE tumors, and biopsy, respectively (log-rank test, P = 0.157). Figure 3(b) shows the Kaplan–Meier curve in GC patients with IDH-wildtype glioblastoma and CE tumor according to EOR.
Discussion
Our study comprehensively analyzes the prognosis of GC patients reflecting the recent WHO classification encompassing clinical, molecular and imaging data. In the entire GC patients, higher KPS, presence of 1p/19q codeletion, presence of MGMTp methylation, and absence of hemorrhage were favorable prognostic factors. In subgroup analysis of IDH-wildtype glioblastoma patients, only higher KPS remained as an independent prognostic factor. Of note, GTR of CE tumor did not remain as an independent prognostic factor. Our findings suggest that the prognosis of GC is determined by its underlying molecular type reflecting the 2021 WHO classification. Furthermore, aggressive surgery of CE tumors may not be the preferable surgical approach in GC patients and thus the recommended surgical strategy of supramaximal safe resection in diffuse gliomas may not be applied in GC patients.
Previous studies on GC before the molecular era were restricted by the lack of integrated diagnosis comprising molecular information as well as small sample size [12, 19,20,21]. Previous studies lacked molecular information such as IDH mutation or 1p/19q codeletion status which are essential in diagnosing molecular types of adult-type diffuse gliomas [22], and may have analyzed misclassified tumor types based solely on histopathology. Thus, the prognostic implication of GC in line with the current 2021 WHO classification in adult-type diffuse glioma remains to be unveiled. Moreover, results from population-based studies should be interpreted with caution [8, 23]; population-based database prevents direct access to imaging, which may include inconsistently defined GC. Herein the imaging review of experienced neuroradiologists as well as clear and consistent definition of GC likely improved the reliability of our results.
Previous studies on patients with GC showed that performance status and molecular profile significantly affects prognosis [6, 7, 20, 24,25,26], which is also a consistent finding in glioma patients without GC. This finding is in line with our study results showing higher KPS, presence of 1p/19q codeletion, and presence of MGMTp methylation associated with longer OS in adult-type diffuse glioma patients with GC. Presence of 1p/19q codeletion indicates the diagnosis of oligodendroglioma, which is well-acknowledged for its relatively favorable prognosis [27], while MGMTp methylation is a well-known molecular marker in gliomas predicting better response to alkylating chemotherapeutic agents [28, 29]. In terms of imaging findings, presence of hemorrhage was significantly associated with poor prognosis in our study. Intratumoral hemorrhage may reflect the underlying tumor aggressiveness; it may be a result of tumor coagulopathy, or arise from dysplastic neoangiogenic vessels traversing necrotic areas or from large vessels that are invaded by the tumor [30,31,32].
In IDH-wildtype glioblastoma patients with GC, only preoperative KPS remained as a significant prognostic factor in our study. Of note, GTR of CE tumor did not show significant impact on survival within IDH-wildtype glioblastoma patients with CE tumor. To date, it is unclear whether extent of resection provides any survival benefit in GC. Majority of studies on GC mostly lacked results on surgical approach, owing to the fact that GC patients in these datasets predominantly underwent biopsy and analysis of the impact of surgical approach on survival was not possible [7, 20, 33,34,35,36]. Previous studies that provided results on surgical approach show discrepant results; several single-institutional studies [21, 37, 38] or population-based analysis [23] showed that extent of resection did not provide any survival benefit, while two meta-analyses showed that surgical resection was associated with improved outcomes [25, 39]. These studies were performed prior to the molecular classification, and lacks separate labeling of CE and NE tumors. Recent surgical guidelines separately label the EOR of CE and NE tumors, and recommend aggressive surgery of CE tumor when feasible [13, 40]. In patients with GC, neurosurgeons from our institution aim total resection of CE tumor when amenable. Thus, only 19.2% of GC patients from our dataset underwent biopsy while 36.4% of patients underwent GTR of CE tumor, enabling reliable prognostic analyses of extent of resection. However, this aggressive surgical approach failed to reach statistical significance on survival in GC patients. Our finding may provide solid evidence to the recommendation that IDH-wildtype patients with GC should be biopsied only [40].
This study has several limitations. First, this study is a retrospective single center study with a relatively small sample size. Despite the large number of adult-type diffuse glioma patients the sample size of patients with GC was small owing to the relatively low incidence of GC. Second, a substantial proportion (19.2%) of patients underwent limited diagnostic biopsy, which may possibly lead in undersampling of tumor. Third, we did not conduct volumetric assessment and analyses of EOR.
Conclusion
In conclusion, the prognosis of GC patients is determined by its underlying molecular type and performance. Compared with supramaximal resection recommended in IDH-wildtype glioblastoma patients without GC, aggressive surgery of CE tumor portion in GC patients may not improve survival and thus further studies should be conducted to address this issue.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable response.
References
Nevin S (1938) Gliomatosis cerebri. Brain 61:170–191. https://doi.org/10.1093/brain/61.2.170
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. https://doi.org/10.1007/s00401-007-0243-4
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Ranjan S, Warren KE (2017) Gliomatosis Cerebri: Current Understanding and Controversies. Front Oncol 7:165. https://doi.org/10.3389/fonc.2017.00165
Taillibert S, Chodkiewicz C, Laigle-Donadey F, Napolitano M, Cartalat-Carel S, Sanson M (2006) Gliomatosis cerebri: a review of 296 cases from the ANOCEF database and the literature. J Neurooncol 76:201–205. https://doi.org/10.1007/s11060-005-5263-0
Chen S, Tanaka S, Giannini C, Morris J, Yan ES, Buckner J, Lachance DH, Parney IF (2013) Gliomatosis cerebri: clinical characteristics, management, and outcomes. J Neurooncol 112:267–275. https://doi.org/10.1007/s11060-013-1058-x
Carroll KT, Hirshman B, Ali MA, Alattar AA, Brandel MG, Lochte B, Lanman T, Carter B, Chen CC (2017) Management and Survival Patterns of Patients with Gliomatosis Cerebri: A SEER-Based Analysis. World Neurosurg 103:186–193. https://doi.org/10.1016/j.wneu.2017.03.103
Karschnia P, Young JS, Dono A, Häni L, Sciortino T, Bruno F, Juenger ST, Teske N, Morshed RA, Haddad AF, Zhang Y, Stoecklein S, Weller M, Vogelbaum MA, Beck J, Tandon N, Hervey-Jumper S, Molinaro AM, Rudà R, Bello L, Schnell O, Esquenazi Y, Ruge MI, Grau SJ, Berger MS, Chang SM, van den Bent M, Tonn J-C (2022) Prognostic validation of a new classification system for extent of resection in glioblastoma: A report of the RANO resect group. Neuro Oncol 25:940–954. https://doi.org/10.1093/neuonc/noac193
Sahm F, Schrimpf D, Jones DT, Meyer J, Kratz A, Reuss D, Capper D, Koelsche C, Korshunov A, Wiestler B, Buchhalter I, Milde T, Selt F, Sturm D, Kool M, Hummel M, Bewerunge-Hudler M, Mawrin C, Schüller U, Jungk C, Wick A, Witt O, Platten M, Herold-Mende C, Unterberg A, Pfister SM, Wick W, von Deimling A (2016) Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol 131:903–910. https://doi.org/10.1007/s00401-015-1519-8
Na K, Kim HS, Shim HS, Chang JH, Kang SG, Kim SH (2019) Targeted next-generation sequencing panel (TruSight Tumor 170) in diffuse glioma: a single institutional experience of 135 cases. J Neurooncol 142:445–454. https://doi.org/10.1007/s11060-019-03114-1
Herrlinger U, Jones DT, Glas M, Hattingen E, Gramatzki D, Stuplich M, Felsberg J, Bähr O, Gielen GH, Simon M (2016) Gliomatosis cerebri: no evidence for a separate brain tumor entity. Acta Neuropathol 131:309–319
Karschnia P, Dono A, Young JS, Juenger ST, Teske N, Häni L, Sciortino T, Mau CY, Bruno F, Nunez L, Morshed RA, Haddad AF, Weller M, van den Bent M, Beck J, Hervey-Jumper S, Molinaro AM, Tandon N, Rudà R, Vogelbaum MA, Bello L, Schnell O, Grau SJ, Chang SM, Berger MS, Esquenazi Y, Tonn JC (2023) Prognostic evaluation of re-resection for recurrent glioblastoma using the novel RANO classification for extent of resection: A report of the RANO resect group. Neuro Oncol 25:1672–1685. https://doi.org/10.1093/neuonc/noad074
Karschnia P, Dietrich J, Bruno F, Dono A, Juenger ST, Teske N, Young JS, Sciortino T, Häni L, van den Bent M, Weller M, Vogelbaum MA, Morshed RA, Haddad AF, Molinaro AM, Tandon N, Beck J, Schnell O, Bello L, Hervey-Jumper S, Thon N, Grau SJ, Esquenazi Y, Rudà R, Chang SM, Berger MS, Cahill DP, Tonn JC (2023) Surgical management and outcome of newly diagnosed glioblastoma without contrast enhancement ('low grade appearance’) - a report of the RANO resect group. Neuro Oncol. https://doi.org/10.1093/neuonc/noad160
Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L, French P, Hegi ME, Jakola AS, Platten M, Roth P, Rudà R, Short S, Smits M, Taphoorn MJB, von Deimling A, Westphal M, Soffietti R, Reifenberger G, Wick W (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18:170–186. https://doi.org/10.1038/s41571-020-00447-z
Kim YZ, Kim C-Y, Lim J, Sung KS, Lee J, Oh H-J, Kang S-G, Kang S-H, Kong D-S, Kim SH, Kim S-H, Kim SH, Kim YJ, Kim EH, Kim IA, Kim HS, Roh TH, Park J-S, Park HJ, Song SW, Yang SH, Yoon W-S, Yoon HI, Lee S-T, Lee S-W, Lee YS, Wee CW, Chang JH, Jung T-Y, Jung HL, Cho JH, Choi SH, Choi HS, Hong JB, Lim DH, Chung D-S (2019) The Korean Society for Neuro-Oncology (KSNO) Guideline for Glioblastomas: Version 2018.01. Brain Tumor Res Treat 7:1–9
R Development Core Team (2010) R: A language and environment for statistical computing. 4.3.2 edn. R Fundation for Statistical Computing; 2019 Vienna, Austria. https://www.R-project.org
Davis C (2014) SurvMisc: Miscellaneous functions for survival data. R package version 0.4.2 edn. https://github.com/dardisco/survMisc
Rajz GG, Nass D, Talianski E, Pfeffer R, Spiegelmann R, Cohen ZR (2012) Presentation patterns and outcome of gliomatosis cerebri. Oncol Lett 3:209–213. https://doi.org/10.3892/ol.2011.445
Vates GE, Chang S, Lamborn KR, Prados M, Berger MS (2003) Gliomatosis cerebri: a review of 22 cases. Neurosurgery 53:261–271. https://doi.org/10.1227/01.neu.0000073527.20655.e6
Perkins GH, Schomer DF, Fuller GN, Allen PK, Maor MH (2003) Gliomatosis cerebri: improved outcome with radiotherapy. Int J Radiat Oncol Biol Phys 56:1137–1146. https://doi.org/10.1016/s0360-3016(03)00293-1
Kurokawa R, Kurokawa M, Baba A, Ota Y, Pinarbasi E, Camelo-Piragua S, Capizzano AA, Liao E, Srinivasan A, Moritani T (2022) Major Changes in 2021 World Health Organization Classification of Central Nervous System Tumors. Radiographics 42:1474–1493. https://doi.org/10.1148/rg.210236
Georgakis MK, Spinos D, Pourtsidis A, Psyrri A, Panourias IG, Sgouros S, Petridou ET (2018) Incidence and survival of gliomatosis cerebri: a population-based cancer registration study. J Neurooncol 138:341–349. https://doi.org/10.1007/s11060-018-2802-z
Doig D, Thorne L, Rees J, Fersht N, Kosmin M, Brandner S, Jäger HR, Thust S (2023) Clinical, Imaging and Neurogenetic Features of Patients with Gliomatosis Cerebri Referred to a Tertiary Neuro-Oncology Centre. J Personalized Med 13:222
Georgakis MK, Tsivgoulis G, Spinos D, Liaskas A, Herrlinger U, Petridou ET (2018) Prognostic Factors and Survival of Gliomatosis Cerebri: A Systematic Review and Meta-Analysis. World Neurosurg 120:e818–e854. https://doi.org/10.1016/j.wneu.2018.08.173
Berzero G, Di Stefano AL, Ronchi S, Bielle F, Villa C, Guillerm E, Capelle L, Mathon B, Laurenge A, Giry M, Schmitt Y, Marie Y, Idbaih A, Hoang-Xuan K, Delattre J-Y, Mokhtari K, Sanson M (2020) IDH-wildtype lower-grade diffuse gliomas: the importance of histological grade and molecular assessment for prognostic stratification. Neuro Oncol 23:955–966. https://doi.org/10.1093/neuonc/noaa258
Liang J, Lv X, Lu C, Ye X, Chen X, Fu J, Luo C, Zhao Y (2020) Prognostic factors of patients with Gliomas – an analysis on 335 patients with Glioblastoma and other forms of Gliomas. BMC Cancer 20:35. https://doi.org/10.1186/s12885-019-6511-6
Weller M, Stupp R, Reifenberger G, Brandes AA, van den Bent MJ, Wick W, Hegi ME (2010) MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol 6:39–51. https://doi.org/10.1038/nrneurol.2009.197
Kinslow CJ, Rae AI, Taparra K, Kumar P, Siegelin MD, Grinband J, Gill BJA, McKhann GM, Sisti MB, Bruce JN, Canoll PD, Iwamoto FM, Horowitz DP, Kachnic LA, Neugut AI, Yu JB, Cheng SK, Wang TJC (2023) MGMT Promoter Methylation Predicts Overall Survival after Chemotherapy for 1p/19q-Codeleted Gliomas. Clin Cancer Res 29:4399–4407. https://doi.org/10.1158/1078-0432.Ccr-23-1295
Zimmerman RA, Bilaniuk LT (1980) Computed tomography of acute intratumoral hemorrhage. Radiology 135:355–359. https://doi.org/10.1148/radiology.135.2.7367626
Wakai S, Yamakawa K, Manaka S, Takakura K (1982) Spontaneous intracranial hemorrhage caused by brain tumor: its incidence and clinical significance. Neurosurgery 10:437–444. https://doi.org/10.1227/00006123-198204000-00004
Choi G, Park DH, Kang SH, Chung YG (2013) Glioma mimicking a hypertensive intracerebral hemorrhage. J Korean Neurosurg Soc 54:125–127. https://doi.org/10.3340/jkns.2013.54.2.125
Kaloshi G, Everhard S, Laigle-Donadey F, Marie Y, Navarro S, Mokhtari K, Idbaih A, Ducray F, Thillet J, Hoang-Xuan K, Delattre JY, Sanson M (2008) Genetic markers predictive of chemosensitivity and outcome in gliomatosis cerebri. Neurology 70:590–595. https://doi.org/10.1212/01.wnl.0000299896.65604.ae
Park S, Suh YL, Nam DH, Kim ST (2009) Gliomatosis cerebri: clinicopathologic study of 33 cases and comparison of mass forming and diffuse types. Clin Neuropathol 28:73–82. https://doi.org/10.5414/npp28073
Kong DS, Kim ST, Lee JI, Suh YL, Lim DH, Kim WS, Kwon KH, Park K, Kim JH, Nam DH (2010) Impact of adjuvant chemotherapy for gliomatosis cerebri. BMC Cancer 10:424. https://doi.org/10.1186/1471-2407-10-424
Inoue T, Kumabe T, Kanamori M, Sonoda Y, Watanabe M, Tominaga T (2010) Prognostic factors for patients with gliomatosis cerebri: retrospective analysis of 17 consecutive cases. Neurosurg Rev 34:197–208. https://doi.org/10.1007/s10143-010-0306-1
Kim K, Chie EK, Park HJ, Kim DG, Jung HW, Park SH, Kim IH (2014) Exclusive radiotherapy for gliomatosis cerebri: long-term follow-up at a single institution. Clin Transl Oncol 16:829–833. https://doi.org/10.1007/s12094-013-1156-4
Anghileri E, Schettino C, Pollo B, Farinotti M, Silvani A, Paterra R, Patanè M, DiMeco F, Bruzzone MG, Eoli M, Cuccarini V (2020) Gliomatosis cerebri (GC) or GC-like? A picture to be reconsidered in neuro-oncology based on large retrospective analysis of GC series. Neurol Sci 41:2111–2120. https://doi.org/10.1007/s10072-020-04288-7
Georgakis MK, Tsivgoulis G, Pourtsidis A, Petridou ET (2019) Gliomatosis Cerebri Among Children and Adolescents: An Individual-Patient Data Meta-analysis of 182 Patients. J Child Neurol 34:394–401. https://doi.org/10.1177/0883073819836551
Young JS, Morshed RA, Hervey-Jumper SL, Berger MS (2023) The surgical management of diffuse gliomas: Current state of neurosurgical management and future directions. Neuro Oncol 25:2117–2133. https://doi.org/10.1093/neuonc/noad133
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1I1A1A01071648).
Author information
Authors and Affiliations
Contributions
Conception and design: Yae Won Park.
Collection and assembly of data: Yae Won Park, Seo Hee Choi, Narae Lee, Sung Soo Ahn, Jong Hee Chang, Se Hoon Kim, Seung-Koo Lee.
Data analysis and interpretation: Ilah Shin, Yae Won Park.
Statistical analysis: Ilah Shin, Yae Won Park.
Manuscript writing: Ilah Shin, Yae Won Park.
Final approval of the manuscript: Ilah Shin, Yae Won Park, Seo Hee Choi, Narae Lee, Sung Soo Ahn, Jong Hee Chang, Se Hoon Kim, Seung-Koo Lee.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Informed consent from the patients was waived by the institutional review board of Yonsei University, College of Medicine (Approval no.: 4–2023-0045).
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shin, I., Sim, Y., Choi, S.H. et al. Revisiting prognostic factors of gliomatosis cerebri in adult-type diffuse gliomas. J Neurooncol 168, 239–247 (2024). https://doi.org/10.1007/s11060-024-04656-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-024-04656-9