Abstract
Purpose
Primary brain neoplasms are the most common solid tumors in pediatric patients and seizures are a common presenting symptom. Surgical intervention improves oncologic outcomes and seizure burden. A better understanding of factors that influence seizure outcomes in the surgical management of primary brain tumors of childhood can guide treatment approach thereby improving patient quality of life.
Methods
We performed a systematic analysis using articles queried from PubMed, EMBASE, and Cochrane published from January 1990 to August 2022 to determine predictors of seizure outcomes in pediatric patients undergoing resection of primary brain tumors.
Results
We identified 24 retrospective cohort studies, one prospective cohort study, and one mixed retrospective and prospective study for the systematic analysis. A total of 831 pediatric patients were available for analysis. 668 (80.4%) patients achieved seizure freedom after surgery. Complete tumor resection increased the likelihood of a seizure-free (Engel I) outcome compared to subtotal resection (OR 7.1, 95% CI 2.3–21.9). Rates of Engel I seizure outcomes did not significantly differ based on factors such as age at seizure onset, duration of epilepsy, gender, tumor laterality, or age at surgery, but trended towards significance for improved outcomes in temporal lobe tumors.
Conclusion
Primary brain tumors in the pediatric population are commonly associated with seizures. Resection of these lesions reduces seizure burden and is associated with high rates of seizure freedom. Complete resection, compared to subtotal resection, significantly increases the likelihood of seizure-free outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary central nervous system (CNS) malignancies are the most common pediatric solid tumors, comprising 17–22% of all pediatric malignancies, and are the most common cause of cancer death in the pediatric population [1, 2]. Primary brain tumors frequently present with seizures and cause epilepsy with a prevalence of 10–19% [1, 3]. Epilepsy leads to poor quality of life and long-term cognitive impairment [4]. These patients present a unique challenge as the clinician must address oncologic as well as epileptic treatment goals. Thus, management of lesional epilepsy in pediatric patients involves a multidisciplinary approach, with treatment options including antiseizure medications (ASMs), surgical resection, and adjunctive treatments ranging from radiation to vagus nerve stimulation [5,6,7].
In adult patients with lesional epilepsy, surgery improves seizure outcomes as quantified by the Engel outcome scale [8]. The degree of tumor resection has consistently emerged as the most reliable predictor of achieving complete seizure freedom across several studies [7, 9, 10]. While resection reduces seizure recurrence rates in the adult population across many tumor subtypes, our understanding of the effects of resection on pediatric seizure recurrence and outcomes remains limited and requires further characterization [11].
To our knowledge, this is the first systematic review analyzing post-resection seizure outcomes in pediatric patients with primary brain neoplasms. In particular, this analysis seeks to answer questions at the intersection between “tumor surgery” and “epilepsy surgery” with the hopes of illuminating factors to guide preoperative workup, patient counseling, operative plan, and post-operative follow up. Specifically, the primary goal of this study is to identify the relationship between extent of surgical resection in primary brain neoplasms and seizure outcomes in pediatric patients and further, to determine seizure outcomes after surgical resection within different subgroups of this population.
Methods
Article selection and data extraction
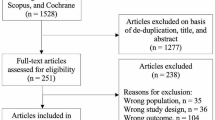
A systematic review of the literature using PubMed, EMBASE, and Cochrane was conducted using the dedicated search terms “pediatric OR children,” “epilepsy OR seizure,” “tumor OR lesion,” “resection OR surgery,” and “Engel.” Initially, 669 articles published from January 1990 and August 2022 were returned for potential inclusion. Inclusion criteria included patients less than or equal to 18 years of age, with tumor-related lesional epilepsy, and who underwent surgical resection, with a reported postoperative seizure or Engel outcome. Exclusion criteria included non-English articles, case reports, systematic reviews, and articles with fewer than 10 eligible patients. The detailed process of manuscript screening is illustrated in Fig. 1, based on guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
For an article to be considered, all eligible patients must have outcomes evaluated by the modified Engel classification system dichotomized based on seizure freedom (Engel class I) or persistent seizures (≥ 1 post-operative seizure, Engel class II to IV) [8]. If only a subset of patients fit the inclusion and exclusion criteria in an article, we extracted individual patient data for those who met criteria. Patient data were stratified based on the following variables: age at surgery, age of seizure onset, duration of epilepsy, sex, tumor hemisphere, location, histopathology, WHO grade, seizure frequency, extent of resection, and follow-up length. For comparison with subtotal resection, gross total resection included supramaximal resection except when explicitly stated. Due to evolution of terminology over time, seizure semiology descriptions were aggregated. For example, “complex partial” was grouped with “focal with impaired awareness.”
Statistical analysis
The rates of postoperative seizures and seizure-free outcomes were determined for all patients and were categorized according to specific variables of interest. Patient data were collapsed across all reports to generate summary statistics. First, significance testing was performed using paired t-tests for continuous variables, Fisher’s Exact test for 2 × 2 proportional categories, and χ2 testing for seizure outcomes across > 2 groups. Bonferroni-Holm correction was applied to account for multiple comparisons. These tests were run assuming that studies were independent samples from similar populations. To model these assumptions explicitly, meta-analysis was performed on variables with at least 5 prior reports across two separate conditions via linear mixed effects regression models. Forest plots were generated on variables of interest. For categorical values the odds ratio (OR) was calculated and for continuous variables the mean difference was analyzed. Heterogeneity across studies was evaluated using the Cochran Q statistic. The possible effect of publication bias was visualized using funnel plots and quantified via Egger’s regression test. Significance was assessed at p < 0.05 or confidence intervals that did not include the null hypothesis. Data preprocessing was performed in both MATLAB (MATLAB and Statistics Toolbox Release 2022b, The MathWorks, Inc., Natick, Massachusetts, United States) using custom code and R Studio (RStudio Team 2020) using the metafor package [12].
Results
Twenty-six studies were included for analysis (Fig. 1), all of which reported postoperative seizure rates and associated factors of interest in pediatric patients with seizures associated with primary epilepsy-associated brain tumors [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. Twenty-four studies were retrospective cohort studies, one study was a prospective cohort study, and one study was a mixed retrospective and prospective cohort study.
Flowchart summarizing the manuscript selection process. Identification criteria required manuscripts with primary clinical data regarding the incidence of preoperative and postoperative seizures in patients with primary brain tumors. Exclusion criteria included duplicate records, only abstracts, records before 1990, insufficient detail to determine precise seizure rates, or previously included data
A total of 831 patients were identified with a range of 9 to 108 patients per study (Table 1). All patients had seizures pre-operatively. 80.4% of the patients were seizure free at last follow-up with a seizure-free rate ranging from 55 to 100% (Supplementary Fig. 1). Within the studies reporting sex (230 patients), 66% of patients were male, with similar seizure outcome rates across genders (p > 0.5, paired t-test). There was no difference noted in age of surgery, age of seizure onset, length of follow up, or even duration of epilepsy (p > 0.2 for all, paired t-test). Seizure semiology was mostly split between focal with impaired awareness (35.5%) and focal without impaired awareness (52.2%). No difference in seizure outcome was noted between subtypes, even when grouping in focal versus generalized to increase power (p > 0.2, χ2 test and Fisher’s exact test, respectively). Seizures occurred daily in approximately 40% of the patients, but their seizure outcome rates were not significantly different than patients with less frequent seizures (p > 0.5, χ2 test and Fisher’s exact test). For patients that had post operative AED use reported (186 patients across 8 studies), 60.5% of those with Engel 1 outcomes were on no AEDs at all, corresponding to a “cure” of their epilepsy (Table 1).
Meta-analysis was performed for all variables that were reported in at least five studies. Gender was nearly equivalent for patients with seizure freedom (Fig. 2a), with an odds ratio (OR) of 0.93 (p = 0.24, random effects model). Seizure-free patients on average were 1.4 years younger (Fig. 2b). However, this did not achieve significance (p = 0.24, random effects model), possibly due to significant heterogeneity in the reported studies (Cochran’s Q test, p = 0.006). Patients achieving seizure freedom were diagnosed at about the same age (Fig. 2c), at mean difference of 0.59 years older, and had a similar duration of epilepsy (Fig. 2d), with a mean difference of 0.67 years shorter duration (p > 0.3 for both, random effects model). Again, there was significant heterogeneity across studies for these outcomes of interest as well (p < 0.0001 for both, Cochran’s Q test). No significant publication bias was noted across these variables, as visualized on the associated Funnel Plots (Egger’s regression test, p > 0.05 for all) (Supplementary Fig. 2).
Meta-analyses examining demographic factors associated with Engel 1 as compared to Engel 2–4 outcomes as demonstrated by Forest Plots. a) Odds ratios for males versus female patients. Larger values indicate higher Engel 1 rate for males. b-d) Mean difference in age at surgery, seizure onset, or duration of surgery of patients with Engel 1 outcomes minus patients with Engel 2–4 outcomes. Values are in years. Significance is determined by overlap of error bars with the null hypothesis (OR of 1 or mean difference of 0)
We next examined surgical and lesional factors that could influence patient outcomes (Table 2). Within the reported populations, lesions were three times more likely to be within the temporal lobes, but there was no clear difference in laterality. Resection of temporal lesions had an 85% chance of leading to seizure freedom, compared to 79% in extratemporal locations. Similarly, 88% of patients with left sided lesions reported seizure freedom, versus 91% of those with lesions on the right. Notably, neither of these outcomes were significant (p > 0.4, Fisher’s exact test for both), suggesting that tumor location does not play a large role in determining seizure outcomes. Phase 1 monitoring including inpatient EEG with video monitoring, functional MRI, PET, SPECT, MEG, and/or neuropsychological evaluation was reported for 65 patients, of which the vast majority (94%) were concordant with the MRI. Gross total resection (GTR) of the tumor was achieved in 71.4% of patients, with 23.1% reported as supramaximal resection. Notably, 87.6% of cases with GTR achieved Engel I outcomes, compared to only 59.5% of cases with subtotal resection (p < 0.0001, Fisher’s exact test). Interestingly, there seemed to be no benefit to supramaximal resection, which demonstrated a 90.2% Engel 1 outcome rate and was not significantly different than GTR (p > 0.6, Fisher’s exact test). A wide variety of tumor pathologies were reported; however, glioneuronal tumors were unequivocally the most common, comprising 82% of all lesions. Seizure freedom rates reached 85% in these patients, compared to a range between 67 and 100% in patients whose lesion were of alternate pathologies such as astrocytomas or oligodendrogliomas. Seizure outcomes were not dependent on histological subtype (p > 0.5, χ2 test). Consistent with the prevalence of brain tumors in the pediatric population, all tumor grades reported were WHO grades I or II, with no impact on seizure outcomes (p > 0.5, Fisher’s exact test).
In contrast to basic statistical testing, meta-analysis demonstrated that temporal tumor location was associated with more than double the rates of seizure freedom when compared to extratemporal location, with an OR that approached significance (OR 2.2, p = 0.07 random effects model) (Fig. 3a). Tumor laterality did not affect outcomes (p > 0.6, random effects model, Fig. 3b). Notably, seizure-free outcome was seven times more common in patients who achieved GTR (Fig. 3c) (OR 7.0, 95% CI 2.3–22.0, p < 0.001, random effects model). Again, no benefit was seen in supramaximal resection (p > 0.8, random effects model, Fig. 3d). None of these metrics had significant heterogeneity (p > 0.1 for all, Cochran’s Q test) or publication bias (Egger’s regression test, p > 0.05 for all, Supplementary Fig. 3) across studies.
Meta-analyses examining tumor factors associated with Engel 1 as compared to Engel 2–4 outcomes as demonstrated by Forest Plots. a) Odds ratios for temporal lesions versus extratemporal lesions, with larger values favoring better outcomes in temporal lesions. c-d) Similar plots comparing left versus right, total versus subtotal, and total versus supramaximal, with larger values favoring the former. Significance determined by overlap of error bars with the null hypothesis (OR of 1)
Discussion
We present here the first systematic review of postoperative seizure outcome in pediatric patients undergoing surgery for primary brain neoplasms, based on data from 26 reported studies in the literature. Based on each patient’s last follow-up, seizure freedom was achieved in 80.4% of patients. This study sought to identify differences in seizure outcomes between sex, tumor location, tumor hemisphere, extent of resection, age of surgery, age of seizure onset, and duration of epilepsy. Based on our analysis, complete resection increases the likelihood of a seizure-free outcome in comparison to subtotal resection of the tumor.
Interest in the outcomes of surgical management of pediatric epilepsy has continued to grow over the last decade. However, many antecedent reports grouped together disparate seizure etiologies and therefore a broad range of surgical interventions including resection, hemispherotomies, and corpus callosotomies [39,40,41]. Here, we sought to evaluate the factors affecting seizure control specifically in epilepsy-associated primary brain neoplasms. Unique to this population, clinicians must integrate and balance oncological and epilepsy related considerations to optimize patient outcomes. Similar to previous literature [11, 42], patients in which GTR was achieved had not only improved tumor control but also significantly improved seizure outcomes. Yet even within this population, Engel 1 outcomes were only achieved in 88% of patients. While the seizure onset zone is often to be concordant with imaging, it is possible that peri-tumoral tissue may also be irritative requiring additional resection beyond the borders of the tumor itself for optimal seizure control [42,43,44]. Interestingly, supramaximal resection did not seem to offer a statistically significant benefit in seizure outcomes, suggesting that “peri-tumoral” is not enough of a criteria for defining other potential seizure foci. In our study a mere 7.8% (65/831) of the included patients reported the outcomes of Phase 1 monitoring and in fact a small subset (6.2%) were discordant. Further work can focus on the role of seizure onset zone mapping for primary brain neoplasms and may shed more light on the role of Phase 1 (or even Phase 2) evaluation for post operative seizure control [45].
Recent trends towards stereotactic Laser Interstitial Thermal Therapy (LITT) rather than resection provide an alternative for tumor and seizure control [46]. Unfortunately, our inclusion criteria required all studies to include open resection cases, none of which reported seizure outcomes for tumors treated with LITT. While LITT may be at a theoretical disadvantage due to limitations to smaller lesions, and a lack of clear patient selection guidelines, its potential for multifocal, minimally invasive ablations already makes it an promising tool in the world of epilepsy [47]. While the different indications may make a direct comparison between LITT and open resection difficult, future work can focus on describing the seizure related and oncological outcomes of LITT for primary CNS tumors in the pediatric population.
In addition to the extent of resection, related factors such as tumor location, histopathologic diagnosis, and tumor grade may influence seizure outcomes after resection. In the epilepsy literature, the odds of seizure freedom after surgery are better for temporal [48] and right sided lesions [49]. Besides tumor location, pre-operative tumor histopathology and grade can influence post-resection seizure outcomes, as resection of low grade glioneuronal tumors and gliomas is associated with increased seizure freedom compared to higher grade gliomas and metastatic brain lesions [11, 50, 51]. In our study, tumor laterality did not influence outcomes, but temporal lesions trended towards significance with a twofold increase in the odds of seizure freedom relative to extra-temporal locations. Additionally, we found no statistical difference in seizure outcomes based on histopathological diagnosis and tumor grade. One possibility for this difference is that previous studies have included patients across the age spectrum. In general, pediatric epilepsy patients have more favorable surgical outcomes than adults, reducing the gap in outcomes across groups [52]. High grade gliomas are rarer in the pediatric population, which may minimize the differences seen across tumor subtypes within low grade lesions. For example, our study heavily features lower grade glioneuronal tumors. As such, it is reasonable to suggest that these factors play a lesser role in the pediatric population.
The patient gender, age of seizure onset, age at surgery, seizure semiology, and duration of epilepsy in the pediatric population were also variables of interest in our study. Our analyses revealed no correlation between these basic features and seizure outcomes, which is consistent with a prior study looking at surgical management of pediatric epilepsy [53]. Literature on age at time of surgery has demonstrated mixed results, with some finding a correlation between age and seizure outcomes [54] and others finding no relationship [42]. While logically, a longer duration of epilepsy should worsen outcomes, it did not seem to have a significant impact. One possibility is that the range of epilepsy duration was not clinically significant, as pediatric low-grade tumors undergo malignant transformation far less frequently than those in the adult population, (estimated at 6.7% over 15 years) [55, 56]. All of these results are likely confounded by the lesion subtype and location, which differ across age groups. In fact, perhaps as a corelate of this variety, our metanalysis revealed statistically significant heterogeneity across studies for age at the time of surgery, age of first seizure, and duration of epilepsy. At the very least, our study suggests that GTR of tumor can provide excellent seizures outcomes regardless of many factors that may theoretically connotate a poor outcome and should be an explicit goal of surgery when feasible.
Our study shares limitations common to all meta-analyses. As demonstrated by statistically significant heterogeneity in many of our analyses, retrospective data collection spanning different patient populations makes it difficult to refine our statements. Additionally, due to the summary nature of the statistics reported in prior literature, it is not possible to control for confounding factors. This is especially difficult in the pediatric population, which experiences a wide variety of lesions whose prevalence varies based on factors such as age, ethnicity, and medical history. However, despite these limitations, this paper clearly demonstrates gross total resection leads to high rates of seizure freedom in pediatric patients with neoplastic lesional epilepsy. Of course, while this study focused on postoperative seizure outcomes, surgical decision making depends on many other outcomes of interest, such as oncologic, cognitive and behavioral results, which were not evaluated here.
Conclusions
Brain tumors are the most common neoplasms in the pediatric population, with epilepsy being a common presenting symptom and key factor in patient quality of life. This is the first systematic review analyzing the factors affecting seizure outcomes in pediatric patients after surgical resection of primary brain neoplasms. With a total patient population of 831, 80.4% of patients were found to be completely seizure-free at last follow-up appointment. Based on our meta-analysis, patients with complete resection of tumors were 7 times more likely to achieve seizure-freedom in comparison to patients with subtotal resection. Our findings have implications in surgical management and prognosis of brain tumor-associated epilepsy in pediatric patients. Future work may focus on other functional implications of tumor resection, such as cognitive and behavioral outcomes.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sánchez Fernández I, Loddenkemper T (2017) Seizures caused by brain tumors in children. Seizure 44:98–107. https://doi.org/10.1016/j.seizure.2016.11.028
US Department of Health and Human Services; National Cancer Institute (1999) Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995
Fattal-Valevski A, Nissan N, Kramer U, Constantini S (2013) Seizures as the clinical presenting symptom in children with brain tumors. J Child Neurol 28:292–296. https://doi.org/10.1177/0883073812445786
Englot DJ, Magill ST, Han SJ et al (2016) Seizures in supratentorial meningioma: a systematic review and meta-analysis. JNS 124:1552–1561. https://doi.org/10.3171/2015.4.JNS142742
Moosa ANV (2019) Antiepileptic drug treatment of Epilepsy in Children. CONTINUUM: Lifelong Learning in Neurology 25:381–407. https://doi.org/10.1212/CON.0000000000000712
González HFJ, Yengo-Kahn A, Englot DJ (2019) Vagus nerve stimulation for the treatment of Epilepsy. Neurosurg Clin North Am 30:219–230. https://doi.org/10.1016/j.nec.2018.12.005
Guan J, Karsy M, Ducis K, Bollo RJ (2016) Surgical strategies for pediatric epilepsy. Transl Pediatr 5:55–66. https://doi.org/10.21037/tp.2016.03.02
Engel J Jr (1992) Update on surgical treatment of the epilepsies. Clin Exp Neurol 29:32–48
Khajavi K, Comair YG, Wyllie E et al (1999) Surgical Management of Pediatric Tumor-Associated Epilepsy. J Child Neurol 14:15–25. https://doi.org/10.1177/088307389901400102
Choi JY, Chang JW, Park YG et al (2004) A retrospective study of the clinical outcomes and significant variables in the Surgical treatment of temporal lobe Tumor Associated with intractable seizures. Stereotact Funct Neurosurg 82:35–42. https://doi.org/10.1159/000076659
Englot DJ, Chang EF, Vecht CJ (2016) Epilepsy and brain tumors. Handbook of clinical neurology. Elsevier, pp 267–285
Viechtbauer W (2010) Conducting Meta-analyses in R with the metafor Package. J Stat Soft. https://doi.org/10.18637/jss.v036.i03. 36:
Babini M, Giulioni M, Galassi E et al (2013) Seizure outcome of surgical treatment of focal epilepsy associated with low-grade tumors in children: clinical article. PED 11:214–223. https://doi.org/10.3171/2012.11.PEDS12137
Battaglia D, Chieffo D, Tamburrini G et al (2012) Posterior resection for childhood lesional epilepsy: neuropsychological evolution. Epilepsy Behav 23:131–137. https://doi.org/10.1016/j.yebeh.2011.11.014
Benifla M, Rutka JT, Otsubo H et al (2008) Long-term seizure and social outcomes following temporal lobe surgery for intractable epilepsy during childhood. Epilepsy Res 82:133–138. https://doi.org/10.1016/j.eplepsyres.2008.07.012
Benifla M, Bennet-Back O, Shorer Z et al (2017) Temporal lobe surgery for intractable epilepsy in children: what to do with the hippocampus? Seizure 52:81–88. https://doi.org/10.1016/j.seizure.2017.09.020
Çataltepe O, Turanli G, Yalnizoglu D et al (2005) Surgical management of temporal lobe tumor—related epilepsy in children. J Neurosurgery: Pediatr 102:280–287. https://doi.org/10.3171/ped.2005.102.3.0280
Chang EF, Christie C, Sullivan JE et al (2010) Seizure control outcomes after resection of dysembryoplastic neuroepithelial tumor in 50 patients: clinical article. PED 5:123–130. https://doi.org/10.3171/2009.8.PEDS09368
Cohen NT, Ziobro JM, Depositario-Cabacar DF et al (2020) Measure thrice, cut twice: on the benefit of reoperation for failed pediatric epilepsy surgery. Epilepsy Res 161:106289. https://doi.org/10.1016/j.eplepsyres.2020.106289
Cossu M, Lo Russo G, Francione S et al (2008) Epilepsy surgery in children: results and predictors of outcome on seizures. Epilepsia 49:65–72. https://doi.org/10.1111/j.1528-1167.2007.01207.x
Daszkiewicz P, Kowalczyk P, Roszkowski M (2018) Surgical treatment of neuronal-glial tumors of mesial-basal part of temporal lobe: long term outcome and control of epilepsy in pediatric patients. Neurol Neurochir Pol 52:2–8. https://doi.org/10.1016/j.pjnns.2017.04.001
Englot DJ, Han SJ, Rolston JD et al (2014) Epilepsy surgery failure in children: a quantitative and qualitative analysis: clinical article. PED 14:386–395. https://doi.org/10.3171/2014.7.PEDS13658
Erturk O, Ozkara C, Yalcinkaya C et al (2014) Epilepsy surgery in children with lesional partial epilepsies. Turkish Neurosurg. https://doi.org/10.5137/1019-5149.JTN.11342-14.1
Fallah A, Weil AG, Sur S et al (2015) Epilepsy surgery related to pediatric brain tumors: Miami Children’s hospital experience. PED 16:675–680. https://doi.org/10.3171/2015.4.PEDS14476
Fay-McClymont TB, Hrabok M, Sherman EMS et al (2012) Systematic review and case series of neuropsychological functioning after epilepsy surgery in children with dysembryoplastic neuroepithelial tumors (DNET). Epilepsy Behav 23:481–486. https://doi.org/10.1016/j.yebeh.2011.12.011
Freitag H, Tuxhorn I (2005) Cognitive function in Preschool Children after Epilepsy surgery: rationale for early intervention. Epilepsia 46:561–567. https://doi.org/10.1111/j.0013-9580.2005.03504.x
Gaggero R, Consales A, Fazzini F et al (2009) Epilepsy associated with supratentorial brain tumors under 3 years of life. Epilepsy Res 87:184–189. https://doi.org/10.1016/j.eplepsyres.2009.08.012
Giulioni M, Galassi E, Zucchelli M, Volpi L (2005) Seizure outcome of lesionectomy in glioneuronal tumors associated with epilepsy in children. J Neurosurgery: Pediatr 102:288–293. https://doi.org/10.3171/ped.2005.102.3.0288
Günbey C, Bilginer B, Oğuz KK et al (2022) Lesional resective epilepsy surgery in childhood: comparison of two decades and long-term seizure outcome from a single center. Epilepsy Res 181:106882. https://doi.org/10.1016/j.eplepsyres.2022.106882
He C, Hu L, Chen C et al (2021) Clinical characteristics of low-grade tumor‐related epilepsy and its predictors for surgical outcome. Ann Clin Transl Neurol 8:1446–1455. https://doi.org/10.1002/acn3.51387
Hosoyama H, Matsuda K, Mihara T et al (2017) Long-term outcomes of epilepsy surgery in 85 pediatric patients followed up for over 10 years: a retrospective survey. J Neurosurgery: Pediatr 19:606–615. https://doi.org/10.3171/2016.12.PEDS16197
Iannelli A, Guzzetta F, Battaglia D et al (2000) Surgical treatment of temporal Tumors Associated with Epilepsy in Children. Pediatr Neurosurg 32:248–254. https://doi.org/10.1159/000028946
Kakkar A, Majumdar A, Kumar A et al (2016) Alterations in BRAF gene, and enhanced mTOR and MAPK signaling in dysembryoplastic neuroepithelial tumors (DNTs). Epilepsy Res 127:141–151. https://doi.org/10.1016/j.eplepsyres.2016.08.028
Kim S-K, Wang K-C, Hwang Y-S et al (2001) Intractable epilepsy associated with brain tumors in children: surgical modality and outcome. Child’s Nerv Syst 17:445–452. https://doi.org/10.1007/s003810000431
Lee J, Lee BL, Joo EY et al (2009) Dysembryoplastic neuroepithelial tumors in pediatric patients. Brain Develop 31:671–681. https://doi.org/10.1016/j.braindev.2008.10.002
Nagarajan L, Lee M, Palumbo L et al (2015) Seizure outcomes in children with epilepsy after resective brain surgery. Eur J Pediatr Neurol 19:577–583. https://doi.org/10.1016/j.ejpn.2015.05.006
Ogiwara H, Nordli DR, DiPatri AJ et al (2010) Pediatric epileptogenic gangliogliomas: seizure outcome and surgical results: clinical article. PED 5:271–276. https://doi.org/10.3171/2009.10.PEDS09372
Yang J, Kim S-K, Kim KJ et al (2019) Satellite lesions of DNET: implications for seizure and tumor control after resection. J Neurooncol 143:437–445. https://doi.org/10.1007/s11060-019-03174-3
Dagar A, Chandra PS, Chaudhary K et al (2011) Epilepsy surgery in a Pediatric Population: a retrospective study of 129 children from a Tertiary Care Hospital in a developing country along with Assessment of Quality of Life. Pediatr Neurosurg 47:186–193. https://doi.org/10.1159/000334257
Téllez-Zenteno JF, Ronquillo LH, Moien-Afshari F, Wiebe S (2010) Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res 89:310–318. https://doi.org/10.1016/j.eplepsyres.2010.02.007
Dwivedi R, Ramanujam B, Chandra PS et al (2017) Surgery for drug-resistant Epilepsy in Children. N Engl J Med 377:1639–1647. https://doi.org/10.1056/NEJMoa1615335
Widjaja E, Jain P, Demoe L et al (2020) Seizure outcome of pediatric epilepsy surgery: systematic review and meta-analyses. Neurology 94:311–321. https://doi.org/10.1212/WNL.0000000000008966
Rana A, Musto AE (2018) The role of inflammation in the development of epilepsy. J Neuroinflammation 15:144. https://doi.org/10.1186/s12974-018-1192-7
Ruda R, Bello L, Duffau H, Soffietti R (2012) Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neurooncology 14:iv55–iv64. https://doi.org/10.1093/neuonc/nos199
Roth J, Bercovich O, Roach A et al (2020) Seizures following surgery for supratentorial extratemporal low-grade tumors in children: a multicenter retrospective study. J Neurosurgery: Pediatr 26:27–33. https://doi.org/10.3171/2020.2.PEDS19673
Arocho-Quinones EV, Lew SM, Handler MH et al (2020) Magnetic resonance–guided stereotactic laser ablation therapy for the treatment of pediatric brain tumors: a multiinstitutional retrospective study. J Neurosurgery: Pediatr 26:13–21. https://doi.org/10.3171/2020.1.PEDS19496
Curry DJ, Gowda A, McNichols RJ, Wilfong AA (2012) MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav 24:408–414. https://doi.org/10.1016/j.yebeh.2012.04.135
Hagemann A, Bien CG, Kalbhenn T et al (2022) Epilepsy surgery in Extratemporal vs temporal lobe Epilepsy: changes in Surgical volumes and seizure outcome between 1990 and 2017. Neurology 98:e1902–e1912. https://doi.org/10.1212/WNL.0000000000200194
West S, Nolan SJ, Cotton J et al (2015) Surgery for epilepsy. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD010541.pub2
Giulioni M (2014) Epilepsy associated tumors: review article. WJCC 2:623. https://doi.org/10.12998/wjcc.v2.i11.623
Gadot R, Khan AB, Patel R et al (2022) Predictors of postoperative seizure outcome in supratentorial meningioma. J Neurosurg 137:515–524. https://doi.org/10.3171/2021.9.JNS211738
Barba C, Cossu M, Guerrini R et al (2021) Temporal lobe epilepsy surgery in children and adults: a multicenter study. Epilepsia 62:128–142. https://doi.org/10.1111/epi.16772
Paolicchi JM, Jayakar P, Dean P et al (2000) Predictors of outcome in pediatric epilepsy surgery. Neurology 54:642–642. https://doi.org/10.1212/WNL.54.3.642
Jenny B, Smoll N, El Hassani Y et al (2016) Pediatric epilepsy surgery: could age be a predictor of outcomes? PED 18:235–241. https://doi.org/10.3171/2015.10.PEDS14413
Collins KL, Pollack IF (2020) Pediatric Low-Grade Gliomas. Cancers 12:1152. https://doi.org/10.3390/cancers12051152
Broniscer A, Baker SJ, West AN et al (2007) Clinical and molecular characteristics of Malignant Transformation of Low-Grade Glioma in Children. JCO 25:682–689. https://doi.org/10.1200/JCO.2006.06.8213
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, led by F.M., N.G. and H.W. The literature search and data extraction was performed by P.A., H.D., and A.S. Statistical analysis and figure preparation was performed by K.K. Manuscript design was led by K.K., P.A., H.S. and A.B.K. The final draft of the manuscript was written by K.K.; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This is a retrospective review of previously published data with no ethical approval required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.



Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katlowitz, K.A., Athukuri, P., Sharma, H. et al. Seizure outcomes after resection of primary brain tumors in pediatric patients: a systematic review and meta-analysis. J Neurooncol 164, 525–533 (2023). https://doi.org/10.1007/s11060-023-04446-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04446-9