Abstract
Purpose
Dysembryoplastic neuroepithelial tumors (DNETs) are a common cause of chronic drug-resistant epilepsy and are known for their favorable surgical outcomes. Nevertheless, the seizure recurrence-free rate is not as favorable if tumorous nodules are present near the main mass. We call these small tumorous nodules in the vicinity of the main mass satellite lesions (SLs). We analyzed tumor and seizure control in the presence and following the subsequent removal of SLs.
Methods
We retrospectively reviewed the medical records, radiological data, and surgical procedures to obtain the outcomes of children who underwent resection surgery for DNET. The analyses were designed to address the associations among the demographic, tumor and seizure-related variables. A Cox proportional hazard model was used for the univariate and multivariate analyses.
Results
In total, 39 consecutive patients were included (26 males and 13 females). SLs were found in 22 patients (56%). The year-to-year analysis of patients with Engel class I was approximately 80% during the follow-up period. However, the actual seizure recurrence-free survival (RFS) rate was 82, 73 and 70% at the first, second and fifth year, respectively. The patients who initially presented with SLs had 46% seizure recurrence rates, while those without SL had 18% seizure recurrence rates.
Conclusions
As the seizure-RFS rate significantly declines over time, a more accurate seizure-free rate analysis using survival curves could be important for determining the outcome of DNET surgery. A thorough review identifying satellite lesions preoperatively and using intraoperative neuronavigation, electrocorticography (ECoG) or intraoperative ultrasonography is warranted to accomplish the wide resection of tumors with accompanying satellite lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain tumors are common causes of chronic drug-resistant epilepsy and are potentially curable by surgery [1, 2]. Dysembryoplastic neuroepithelial tumors (DNETs), gangliogliomas and pleomorphic xanthoastrocytomas are examples of such tumors [2]. These so-called long-term epilepsy-associated tumors (LEATs) are primarily located in the cerebral cortex, especially in the temporal lobe [3, 4]. Most LEATs are low-grade tumors, and surgical removal is the treatment of choice for both tumor control and seizure suppression [2].
The term DNET was introduced by Daumas-Duport et al. [5] following a review of surgically curable neuroepithelial tumors with medically intractable partial seizures. DNET is categorized as a World Health Organization (WHO) grade I tumor [6]. This tumor is frequently located in the temporal lobe and is associated with complex partial seizures that progress to medically intractable epilepsy during the first two decades of life [4].
After the first proposal, numerous articles have described DNET, and most studies have reported favorable surgical outcomes. The seizure-free rates after gross total removal (GTR) of the tumor were over 80% [7, 8]. Regarding other low-grade tumors, many studies have reported that the extent of tumor resection is the most important factor affecting both tumor and seizure control. In the neurosurgical field, the GTR of DNETs is not regarded as a difficult task, and GTR rates reach 79–100% because these tumors are primarily located in the cerebral cortex with relatively clear margins [7, 9, 10]. Furthermore, Daumas-Duport et al. [5] initially suggested that even the partial removal of DNETs provides long-term tumor control, representing an optimistic view of this disease. These findings contributed to the view that DNETs are easily curable tumors at least relatively. Nevertheless, recent studies have reported numerous DNET patients with tumor progression and recurrence [11, 12].
Based on recent studies and our cumulative experiences, in contrast to previous beliefs, DNET tumor progression may be more common because the GTR of these tumors is challenging in some patients when small tumorous nodules exist in the vicinity of the main mass. We named these nodules “satellite lesions” (SLs) because they appear separated from the main mass. We analyzed tumor and seizure control in the presence and following extirpation of SLs.
Materials and methods
We retrospectively reviewed the medical records, radiological data, surgical procedures, and outcomes of children who underwent surgery for DNET at our institution from 2000 to 2016. All tumors were histologically confirmed as DNET by an experienced neuropathologist.
All patients underwent brain MRI prior to surgery. The MRI included T2- and T1-weighted sequences with gadolinium injection. Axial, sagittal and coronal images were recorded. We divided the tumor location in the lobes into frontal, temporal, parietal and occipital. The central lobe as defined by Yasargil [13] designating the sensorimotor cortex was added to the location category if the tumor involved one or more of the precentral, postcentral or paracentral gyri because surgery for tumors in these sites is associated with a high rate of neurological deficits [14]. We further categorized the tumor locations as medial or lateral. The medial locations included the medial frontal/parietal/occipital lobes facing interhemispheric fissures and the temporal/occipital lobes medial to the collateral sulcus. SLs were defined as small dots or crescent-shaped lesions immediately adjacent to the main tumor mass surrounded by normal parenchyma (Fig. 1). The satellite lesions were typically identified as high-signal intensity in T2-weighted images and low-signal intensity on T1-weighted images without enhancement after gadolinium injection. These lesions were frequently located in the deep white matter on the medial, inner side of the tumor masses.
Satellite lesions as discrete T2 high-signal intensity nodules adjacent to main tumors and separated by a thin white matter signal. a Axial view of T2-weighted MRI. SL located on the posteromedial side of the main mass (arrow). b Axial view of T2-weighted MRI of a different patient. SL located on the medial side of the main mass adjacent to the lateral ventricle (arrow). c Axial view of T2-weighted MRI of a different patient. SL located on the medial side of the main mass. d Magnified image of c. Notably, the SL is disconnected from the main mass and separated by a thin white matter signal (arrow)
The surgical procedures were reviewed, and the extent of resection was defined according to postoperative MRI performed within 48 h after surgery. The GTR was defined as no visible residual tumor including all SLs identified on preoperative MRI. Near-total resection (NTR) indicates the complete removal of the main mass with only one or two SLs remaining. Subtotal resection (STR) indicates the presence of residual main masses (< 10% of the initial volume), and partial resection (PR) refers to more than 10% of the tumor remaining.
The surgical outcome was analyzed according to the following two different aspects: tumor progression and seizure recurrence. Tumor progression/recurrence was analyzed based on MRI during the follow-up period. The seizure outcomes were determined by a year-to-year analysis and recurrence-free survival (RFS) depending on the extent of the removal and existence of remaining SLs.
A clinical examination was performed 3 months after the initial surgery, followed by regular follow-up examinations every 6 months. The type, intensity, and frequency of seizures were recorded at each outpatient visit. The primary seizure outcome was analyzed 12 months after surgery using the Engel classification of postoperative outcome as follows: Class I, free of disabling seizures; Class II, rare disabling seizures; Class III, worthwhile improvement; and Class IV, no worthwhile improvement [15]. The same grading system was used during the annual clinical follow up when such information was available. Drug-resistant epilepsy was defined as the failure of adequate trials of two tolerated and appropriately chosen seizure medication schedules (either monotherapies or a combination) to achieve sustained seizure freedom [16].
MRI was performed every 6 months to evaluate tumor control. If no residual lesion was observed, MRI was performed yearly. A newly developed lesion at the previous tumor site including SLs was regarded as tumor progression, even without pathologic confirmation. Tumors that did not undergo secondary surgery for various reasons (e.g., slow-growing lesions or location in eloquent areas) were closely followed up.
The analyses were designed to address the associations among the demographic, tumor and seizure-related variables. These variables included gender, age at onset of seizures, duration of seizures, seizure type, seizure control after surgery, tumor location, existence of satellite lesions, extent of resection and tumor progression. SPSS Statistics version 23 (SPSS Inc., Chicago, IL, USA) was used to perform the statistical analyses. A Cox proportional hazard model was used for the univariate and multivariate analyses. The age at onset of epilepsy, gender, duration of epilepsy, location, presence of SLs, and extent of resection were included as factors assessed in the univariate and multivariate analyses. All analyses were two-sided, and a p value < 0.05 was considered significant. Factors with p < 0.1 in the univariate analyses were selected for inclusion in the multivariate analyses. Age and gender were included in the multivariate models as basic variables. A Kaplan–Meier analysis was used to estimate the cumulative percentage of tumor and seizure-free survival. The present study was approved by the institutional review board (IRB) at our institution.
Results
In total, 39 consecutive patients were included (26 males and 13 females, Table 1). The median age at the time of surgery was 10.2 years (range 3–18 years). Thirty-eight of the 39 patients presented with seizures. The median age at seizure onset was 9.6 years (range 3 months–18 years). The median follow-up duration was 92 months (range 6–155 months). Two patients were asymptomatic, and their tumors were found incidentally during trauma evaluations. Focal motor seizures ensued in one of the two asymptomatic patients after 8 years of observation. Twenty-five patients, including one who had late onset seizure, presented with focal motor seizures (64%), whereas 13 patients (33%) had focal non-motor seizures. Twenty-three patients, including one who had late onset seizures, had seizures with impaired awareness (59%), and 15 patients (39%) had seizures with awareness. The median duration of epilepsy (from seizure onset to surgery) was 13.4 months (range 0–96 months). Nineteen patients (49%) had a duration of epilepsy < 6 months, and 15 patients (39%) had a duration < 2 months. The patients received an average of 1.1 seizure medications at the time of surgery (range 0–5). Twenty-five patients of the 30 patients (83%) who were taking preoperative seizure medications ceased their seizure medications after surgery. One patient without preoperative seizure medication before surgery newly started seizure medication after surgery. Approximately half of all patients (19 patients, 49%) were considered to have intractable epilepsy prior to surgery. Seventeen of the 19 (90%) intractable epilepsy patients had their seizure medications discontinued after surgery. The mean time to discontinue seizure medication was 23 months after surgery (range 0–126 months).
Tumors located in the temporal lobe were observed in 12 patients (31%), and 27 tumors (69%) were located in extra-temporal lobes as follows: nine in the central, nine in the frontal, six in the parietal, two in the occipital and one in the insular lobe. When we divide the tumor locations into medial and lateral, 15 tumors (39%) were located in the medial location, whereas 24 tumors (62%) were located in the lateral cortexes. Among the temporal lobe tumors, six (50%) tumors were located medially.
SLs were found in 22 patients (57%). In 12 patients, only one SL was observed, but in two patients, numerous SLs were found with bubbly appearances. The mean longest SL diameter was 9.8 mm (range 1–14 mm), and the SLs were immediately adjacent to the main mass and separated by normal white matter, except for one case in which the SL was situated 3 mm from the main mass. The SLs appeared as high-signal intensity lesions on the T2-weighted and low-intensity lesions on the T1-weighted images. None of the SLs was enhanced by gadolinium. The separation from the main mass was confirmed on three planar images in all patients. GTR was achieved in approximately half of the patients (20 patients, 51%). Eight patients (21%) underwent NTR, and the other 11 patients underwent STR (28%). However, GTR was achieved in seven of the 22 cases (32%) with SLs. In contrast, GTR was possible in 12 cases (71%) of tumors without SLs. Except for one case (7%) in which a part of the main mass remained while the SL was removed, the SLs were residual lesions of all non-GTR tumors with accompanying SLs. The relationship between the extent of resection and tumor location showed that the medial location had a lower rate of GTR (27%) than the non-medial locations (63%).
One tumor progression occurred after GTR. However, 13 of the 19 (68%) not completely resected tumors subsequently progressed. The median tumor-progression free survival (PFS) was 54 months. The actual overall tumor-PFS declined to 92, 83, 63 and 55% at the first, second, fifth and tenth year after surgery, respectively (Fig. 2). The presence of SLs and achievement of GTR were the most significant factors (p < 0.1) associated with tumor progression based on the univariate analysis. According to the multivariate analysis, age at seizure onset and achievement of GTR were associated with an increased risk of tumor progression (Table 2).
Tumor progression-free survival (Tumor PFS) in relation to the extent of resection. In each plot, the X-axis represents time in years, and the Y-axis represents the proportion. a Actual tumor PFS steadily declines to 92, 83 and 63% in the first, second and fifth year after surgery, respectively. b There is a significant difference in the tumor PFS between the gross total resection (GTR) and the non-GTR groups
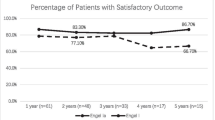
At the first year, 33 patients (85%) had Engel Class I outcomes. Thirteen patients were followed up to 10 years; 11 patients (85%) were Engel Class I. The year-to-year analysis of the patients with Engel class I was approximately 80% as previously reports in other studies [7]. In contrast, the actual seizure-RFS was 82, 73, 70 and 60% at the first, second, fifth year and tenth year, respectively (Fig. 3). The presence of SLs was the only factor with p < 0.05 in the multivariate analysis. The patients who initially presented with SLs had a 46% seizure recurrence, and those without SLs had 18% seizure recurrence. In total, 53% of the tumors located in the medial side showed seizure recurrence, while 21% of the tumors located in the non-medial side showed seizure recurrence (Table 3).
Seizure outcomes among the patients. In B, C, and D, the X-axis represents time in years, and the Y-axis represents the proportion. A Year-to-year analysis of patients by Engel classification as follows: light blue, class I; gray, class II; blue, class III; and dark blue, class IV. The y-axis represents the percentage of Engel class I outcomes, and the x-axis represents the postoperative years. However, the actual seizure recurrence-free survival (seizure RFS) declines over time. B Seizure RFS declines steeply during the first two postoperative years and then stabilizes. C Seizure RFS significantly differs between patients with satellite lesions (SLs) and patients without SLs. D Seizure RFS also differs between GTR and non-GTR group. However, it did not reach statistical significance (p = 0.054)
Complications developed in 11 patients (28%), including five patients with hemiparesis, two patients with hemianopsia, two patients with quadrantanopsia, one patient with hemianopsia and hemiparesis, and one patient with brain abscess. Regarding the location, three of six temporo-medial located tumors (50%) developed complications after resection. In addition, complications occurred in four of nine central lobe located tumors (44%) after surgery. In five patients, hemiparesis was transient and fully improved over time. In one patient, weakness of hand grasping power was permanent. Two patients had postoperative quadrantanopsia, which was not disabling and was not recognized until postoperative ophthalmologic examination. The patient with brain abscess underwent surgical aspiration and antibiotics therapy. The patient was cured without neurologic deficits.
Discussion
Tumor progression
In contrast to common beliefs, previous papers have also reported the aggressive course of the tumor, including rapid growth, progression and malignant transformation of DNET [17,18,19,20]. Furthermore, a recent study reported a GTR rate of only 46%, which is similar to our analysis (51%) [8]. In our study, the tumor-PFS gradually decreased over 10 years, resulting in a plateau at 55%.
Numerous studies suggest that GTR is one of the most important factors for tumor control, and our data support this notion [21,22,23,24,25]. However, the achievement of GTR is not always an easy task. The presence of SLs, central lobe involvement, and a medial location negatively affected the extent of resection. DNETs are located in temporo-medial structures in 46% of patients [7]. In total, 44% of patients with tumors in central locations and half of the patients with tumors in temporo-medial locations developed complications, such as hemianopsia or hemiplegia, in our study, and achieving GTR in tumors located in such areas is challenging for neurosurgeons. Quaddoumi et al. [26] also reported 67% visual field defects (quadrantanopia or hemianopsia) after DNET surgery.
The presence of SLs was significantly associated with a low likelihood of GTR. In most patients without GTR, the SL was the portion left postoperatively. Therefore, the extirpation of SLs is crucial because GTR is the most important variable affecting long-term tumor control. Unfortunately, SLs were found in 57% of DNETs on preoperative MRI, and all SLs were located in the medial direction of the main mass. The SLs separated from the main mass on MRI and intraoperative findings support this notion. In addition, SLs are usually obscured in the surgical field by thin layers of normal white matter. To achieve GTR, breaching the white matter is required. Exploring and removing SLs carries the risk of compromising important white matter tracts, such as the corticospinal tracts and optic radiations. Therefore, it is important to carefully evaluate preoperative imaging to detect SLs and adjacent important white matter tracts.
In our study, the multivariate analysis revealed that a younger age at symptom onset and incomplete resection were significant variables for tumor control. A younger age was also associated with worse tumor control of gangliogliomas [27]. In pediatric brain tumors, a younger age onset is generally disadvantageous in terms of recurrence for a given pathological entity, reflecting different tumor biologies, genetic backgrounds, and limitations of treatment in younger children.
Notably, not all patients with tumor progression had seizure recurrence or required urgent reoperation. The growth of residual/recurred tumor was highly variable, and treatment was tailored according to the individual patients’ symptoms and tumor growth rates.
Seizure recurrence
In contrast to the belief that DNETs are accompanied by chronic epilepsy, less than half of our patients (19 patients, 49%) suffered from intractable epilepsy. This finding may be attributable to the current trend of early MRI at the first or second seizure. If our patients had been on seizure medications for a long time without MRI, many of them could be defined as having chronic drug-resistant epilepsy. Therefore, in current practice, DNET may be an epilepsy-associated tumor (EAT) and not a LEAT in the traditional concept.
Our analysis showed a lower proportion of temporal locations than previous studies. The temporal lobe is the most common site of origin of DNETs, accounting for approximately half of all cases [8]. The higher proportion of extra-temporal locations in our study may be related to the lower representation of drug-resistant epilepsy. Notably, central lobe involvement was observed in a high proportion of our cohort. A central lobe location renders GTR a difficult task because of the risk of permanent motor deficits [28].
In the present study, the seizure-free rate at the first year postoperatively was 85%, and this rate was maintained over a 10-year period, which is consistent with the results of other studies [7, 24]. However, the actual seizure-RFS declined rather steeply to 73% until 2 years postoperatively and gradually declined thereafter. The higher apparent proportion of Engel class I patients reflects the fact that some patients experiencing seizure recurrence became seizure-free by reoperation or restarting seizure medications. Therefore, the seizure RFS displayed in the survival plots reflects the patient burdens (seizure recurrence and retreatment) more accurately than the year-to-year analysis. The seizure RFS was 60% 10 years after surgery, suggesting that the seizure outcome of DNET may not be as favorable as previously thought.
In most studies investigating DNET, GTR is considered a major prognostic factor [2, 22, 29, 30]. In one study, GTR led to seizure freedom in 87% of cases, whereas STR yielded only 55% of seizure-free patients [31]. Interestingly, in our study, the multivariate analysis revealed that the presence of SLs was the most significant variable affecting the seizure outcome, surpassing the effect of GTR. It is intriguing that seizure recurrence occurred despite the complete removal of the tumor (and no tumor recurrence). Therefore, GTR is crucial for tumor control; however, for seizure control, more than GTR is required. This result suggests that the presence of SLs is also associated with epileptogenicity.
Focal cortical dysplasia (FCD) is frequently reported as an associated pathology in DNET and is noted in 54% of cases in larger series [32], and the optimal surgical strategy for DNET between lesionectomy and extended cortical resection has long been controversial [33, 34]. Hence, the correlation among the radiological features of DNET, FCD and surgical outcome has been examined. Chassoux et al. [23]. proposed MRI-based three-tier classification of DNET and reported that a dysplasia-like lesion with T1 iso-/hypo-intensity and gray-white matter blurring was associated with the presence of FCD and poor seizure outcome. Furthermore, Palmini et al. [35] posed the possibility of ictal-onset zones that are apparently more extensive than the tumor in DNET. These analyses are mainly related to MRI signals and the characteristics of the tumor margins; however, the concept of SLs was not considered in these studies. If the presence of SLs reflects more extensive abnormalities surrounding the DNET, extended resection using invasive studies, electrocorticograms or intraoperative ultrasonography may be helpful for seizure control.
The multifocal development of tumors in the region of cortical dysgenesis may underlie DNET pathogenesis. Recent genetic analyses revealed that somatic alterations of the fibroblast growth factor receptor 1 (FGFR1) gene is a recurrent event in the pathogenesis of DNET [36, 37]. FGFR1 is an upstream receptor tyrosine kinase regulating the RAS-RAF-MAPK signaling, the canonical pathway in pediatric low-grade gliomas [38]. Interestingly, recent researches indicated that somatic alterations of PI3K-AKT-mTOR pathway, another downstream cascade of FGFR1, underlie FCD and hemimegalencephaly [39, 40]. Therefore, further studies exploring molecular data from DNET patients could unravel the pathogenesis of DNET, SL, and FCD.
Conclusion
A substantial portion of DNET patients present with SLs, and SLs are major obstacles to achieving GTR. Complete resection, including of both the main mass and SLs, is most important for tumor control, and > 90% of patients with GTR attain long-term tumor PFS. Nevertheless, seizure control is more strongly affected by the presence of SLs than by GTR. This result suggests that SLs may be a separate tumor arising from the background of cortical dysgenesis.
Data availability
The datasets of the current study are available from the corresponding author on request.
References
Blumcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, Pfafflin M, Elger C, Widman G, Schramm J, Becker A, Braun KP, Leijten F, Baayen JC, Aronica E, Chassoux F, Hamer H, Stefan H, Rossler K, Thom M, Walker MC, Sisodiya SM, Duncan JS, McEvoy AW, Pieper T, Holthausen H, Kudernatsch M, Meencke HJ, Kahane P, Schulze-Bonhage A, Zentner J, Heiland DH, Urbach H, Steinhoff BJ, Bast T, Tassi L, Lo Russo G, Ozkara C, Oz B, Krsek P, Vogelgesang S, Runge U, Lerche H, Weber Y, Honavar M, Pimentel J, Arzimanoglou A, Ulate-Campos A, Noachtar S, Hartl E, Schijns O, Guerrini R, Barba C, Jacques TS, Cross JH, Feucht M, Muhlebner A, Grunwald T, Trinka E, Winkler PA, Gil-Nagel A, Toledano Delgado R, Mayer T, Lutz M, Zountsas B, Garganis K, Rosenow F, Hermsen A, von Oertzen TJ, Diepgen TL, Avanzini G, Consortium E (2017) Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med 377(17):1648–1656. https://doi.org/10.1056/nejmoa1703784
Luyken C, Blumcke I, Fimmers R, Urbach H, Elger CE, Wiestler OD, Schramm J (2003) The spectrum of long-term epilepsy-associated tumors: long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia 44(6):822–830
Fried I, Kim JH, Spencer DD (1994) Limbic and neocortical gliomas associated with intractable seizures: a distinct clinicopathological group. Neurosurgery 34(5):815–824
Maria T, Ingmar B, Eleonora A (2012) Long-term epilepsy-associated tumors. Brain Pathol 22(3):350–379. https://doi.org/10.1111/j.1750-3639.2012.00582.x
Daumas-Duport C, Scheithauer BW, Chodkiewicz J-P, Laws JER, Vedrenne C (1988) Dysembryoplastic neuroepithelial tumor: a surgically curable tumor of young patients with intractable partial seizuresreport of thirty-nine cases. Neurosurgery 23(5):545–556. https://doi.org/10.1227/00006123-198811000-00002
Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820
Bonney PA, Boettcher LB, Conner AK, Glenn CA, Briggs RG, Santucci JA, Bellew MR, Battiste JD, Sughrue ME (2016) Review of seizure outcomes after surgical resection of dysembryoplastic neuroepithelial tumors. J Neurooncol 126(1):1–10. https://doi.org/10.1007/s11060-015-1961-4
Nguyen HS, Doan N, Gelsomino M, Shabani S (2017) Dysembryoplastic neuroectodermal tumor: an analysis from the Surveillance, Epidemiology, and End Results Program, 2004–2013. World Neurosurgery 103:380–385. https://doi.org/10.1016/j.wneu.2017.04.093
Zhang J-g Hu, W-z Zhao R-j, L-f Kong (2014) Dysembryoplastic neuroepithelial tumor: a clinical, neuroradiological, and pathological study of 15 cases. J Child Neurol 29(11):1441–1447. https://doi.org/10.1177/0883073813490831
Santos MV, de Oliveira RS, Machado HR (2014) Approach to cortical dysplasia associated with glial and glioneuronal tumors (FCD type IIIb). Child’s Nervous System 30(11):1869–1874. https://doi.org/10.1007/s00381-014-2519-z
Daghistani R, Miller E, Kulkarni AV, Widjaja E (2013) Atypical characteristics and behavior of dysembryoplastic neuroepithelial tumors. Neuroradiology 55(2):217–224. https://doi.org/10.1007/s00234-013-1135-z
Maher CO, White JB, Scheithauer BW, Raffel C (2008) Recurrence of dysembryoplastic neuroepithelial tumor following resection. Pediatr Neurosurg 44(4):333–336
Yasargil MG (1984) Microneurosurgery: operative treatment of CNS tumors 4A. Thieme Stratton, New York
Kim YH, Kim JS, Lee SK, Chung CK (2017) Neurologic outcome after resection of parietal lobe including primary somatosensory cortex: implications of additional resection of posterior parietal cortex. World Neurosurg 106:884–890. https://doi.org/10.1016/j.wneu.2017.07.066
Engel J Jr (1993) Outcome with respect to epileptic seizures. In: Engel J Jr (ed) Surgical treatment of the epilepsies. Raven Press, New York, pp 609–621
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J (2010) Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE commission on therapeutic strategies. Epilepsia 51(6):1069–1077
Sampetrean O, Maehara T, Arai N, Nemoto T (2006) Rapidly growing dysembryoplastic neuroepithelial tumorcase report. Neurosurgery 59(6):E1337–E1338. https://doi.org/10.1227/01.NEU.0000245621.62721.79
Chao L, Tao XB, Jun YK, Xia HH, Wan WK, Tao QS (2013) Recurrence and histological evolution of dysembryoplastic neuroepithelial tumor: a case report and review of the literature. Oncol Lett 6(4):907–914
Nadi M, Ahmad T, Huang A, Hawkins C, Bouffet E, Kulkarni AV (2016) Atypical teratoid rhabdoid tumor diagnosis after partial resection of dysembryoplastic neuroepithelial tumor: case report and review of the literature. Pediatr Neurosurg 51(4):191–198
Ray WZ, Blackburn SL, Casavilca-Zambrano S, Barrionuevo C, Orrego JE, Heinicke H, Dowling JL, Perry A (2009) Clinicopathologic features of recurrent dysembryoplastic neuroepithelial tumor and rare malignant transformation: a report of 5 cases and review of the literature. J Neurooncol 94(2):283. https://doi.org/10.1007/s11060-009-9849-9
Stokland T, Liu J-F, Ironside JW, Ellison DW, Taylor R, Robinson KJ, Picton SV, Walker DA (2010) A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702). Neuro-Oncology 12(12):1257–1268. https://doi.org/10.1093/neuonc/noq092
Nolan M, Sakuta R, Chuang N, Otsubo H, Rutka J, Or Snead, Hawkins C, Weiss S (2004) Dysembryoplastic neuroepithelial tumors in childhood long-term outcome and prognostic features. Neurology 62(12):2270–2276
Chassoux F, Rodrigo S, Mellerio C, Landre E, Miquel C, Turak B, Laschet J, Meder JF, Roux FX, Daumas-Duport C, Devaux B (2012) Dysembryoplastic neuroepithelial tumors: an MRI-based scheme for epilepsy surgery. Neurology 79(16):1699–1707. https://doi.org/10.1212/WNL.0b013e31826e9aa9
Chang EF, Christie C, Sullivan JE, Garcia PA, Tihan T, Gupta N, Berger MS, Barbaro NM (2010) Seizure control outcomes after resection of dysembryoplastic neuroepithelial tumor in 50 patients. J Neurosurg Pediatr 5(1):123–130
Sakuta R, Otsubo H, Nolan MA, Weiss SK, Hawkins C, Rutka JT, Chuang NA, Chuang SH, Carter Snead O (2004) Recurrent intractable seizures in children with cortical dysplasia adjacent to dysembryoplastic neuroepithelial tumor. J Child Neurol 19(3):377–384
Qaddoumi I, Ellison DW, Morris EB, Broniscer A, Boop F, Merchant T, Palmer SL, Gajjar A (2010) Dysembryoplastic neuroepithelial tumors and cognitive outcome. Cancer 116(23):5461–5469. https://doi.org/10.1002/cncr.25528
Haydon DH, Dahiya S, Smyth MD, Limbrick DD, Leonard JR (2014) Greater extent of resection improves ganglioglioma recurrence-free survival in children: a volumetric analysis. Neurosurgery 75(1):37–42. https://doi.org/10.1227/neu.0000000000000349
Ranger A, Diosy D (2015) Seizures in children with dysembryoplastic neuroepithelial tumors of the brain—a review of surgical outcomes across several studies. Child’s Nervous System 31(6):847–855. https://doi.org/10.1007/s00381-015-2675-9
Bilginer B, Yalnızoglu D, Soylemezoglu F, Turanlı G, Cila A, Topçu M, Akalan N (2008) Surgery for epilepsy in children with dysembryoplastic neuroepithelial tumor: clinical spectrum, seizure outcome, neuroradiology, and pathology. Child’s Nervous System 25(4):485. https://doi.org/10.1007/s00381-008-0762-x
Francine C, Catherine D-D (2013) Dysembryoplastic neuroepithelial tumors: where are we now? Epilepsia 54(s9):129–134. https://doi.org/10.1111/epi.12457
Englot DJ, Berger MS, Barbaro NM, Chang EF (2012) Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia 53(1):51–57. https://doi.org/10.1111/j.1528-1167.2011.03269.x
Thom M, Toma A, An S, Martinian L, Hadjivassiliou G, Ratilal B, Dean A, McEvoy A, Sisodiya SM, Brandner S (2011) One hundred and one dysembryoplastic neuroepithelial tumors: an adult epilepsy series with immunohistochemical, molecular genetic, and clinical correlations and a review of the literature. J Neuropathol Exp Neurol 70(10):859–878. https://doi.org/10.1097/NEN.0b013e3182302475
Giulioni M, Rubboli G, Marucci G, Martinoni M, Volpi L, Michelucci R, Marliani AF, Bisulli F, Tinuper P, Castana L, Sartori I, Calbucci F (2009) Seizure outcome of epilepsy surgery in focal epilepsies associated with temporomesial glioneuronal tumors: lesionectomy compared with tailored resection. J Neurosurg 111(6):1275–1282. https://doi.org/10.3171/2009.3.Jns081350
Nitin T, Yoshua E (2013) Resection strategies in tumoral epilepsy: is a lesionectomy enough? Epilepsia 54(s9):72–78. https://doi.org/10.1111/epi.12448
André P, Eliseu P, Duval SV (2013) Developmental tumors and adjacent cortical dysplasia: single or dual pathology? Epilepsia 54(s9):18–24. https://doi.org/10.1111/epi.12438
Rivera B, Gayden T, Carrot-Zhang J, Nadaf J, Boshari T, Faury D, Zeinieh M, Blanc R, Burk DL, Fahiminiya S, Bareke E, Schuller U, Monoranu CM, Strater R, Kerl K, Niederstadt T, Kurlemann G, Ellezam B, Michalak Z, Thom M, Lockhart PJ, Leventer RJ, Ohm M, MacGregor D, Jones D, Karamchandani J, Greenwood CM, Berghuis AM, Bens S, Siebert R, Zakrzewska M, Liberski PP, Zakrzewski K, Sisodiya SM, Paulus W, Albrecht S, Hasselblatt M, Jabado N, Foulkes WD, Majewski J (2016) Germline and somatic FGFR1 abnormalities in dysembryoplastic neuroepithelial tumors. Acta Neuropathol 131(6):847–863. https://doi.org/10.1007/s00401-016-1549-x
Qaddoumi I, Orisme W, Wen J, Santiago T, Gupta K, Dalton JD, Tang B, Haupfear K, Punchihewa C, Easton J (2016) Genetic alterations in uncommon low-grade neuroepithelial tumors: BRAF, FGFR1, and MYB mutations occur at high frequency and align with morphology. Acta Neuropathol 131(6):833–845
Blümcke I, Aronica E, Becker A, Capper D, Coras R, Honavar M, Jacques TS, Kobow K, Miyata H, Mühlebner A (2016) Low-grade epilepsy-associated neuroepithelial tumours—the 2016 WHO classification. Nat Rev Neurol 12(12):732
Lee JH, Huynh M, Silhavy JL, Kim S, Dixon-Salazar T, Heiberg A, Scott E, Bafna V, Hill KJ, Collazo A (2012) De novo somatic mutations in components of the PI3 K-AKT3-mTOR pathway cause hemimegalencephaly. Nat Genet 44(8):941
Lim JS, W-i Kim, Kang H-C, Kim SH, Park AH, Park EK, Cho Y-W, Kim S, Kim HM, Kim JA (2015) Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat Med 21(4):395
Acknowledgements
We thank our patients and their families for their kind contribution to the research.
Author information
Authors and Affiliations
Contributions
JY, SKK, KCW, JHP contributed to the design, data analysis, manuscript drafting and editing of the study. KJK, JHC, BCL, SHP contributed to acquisition and analysis of data. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Ethical approval
This study was approved by the Institutional Review Board (IRB) of the Seoul National University Hospital (Approval No. H-1807-067-958). Obtaining Informed consent was waived by the IRB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, J., Kim, SK., Kim, K.J. et al. Satellite lesions of DNET: implications for seizure and tumor control after resection. J Neurooncol 143, 437–445 (2019). https://doi.org/10.1007/s11060-019-03174-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03174-3