Abstract
Background
Previous series have demonstrated CNS activity for immune checkpoint inhibitors, yet no prior data exists regarding whether this activity can improve outcomes of stereotactic radiosurgery.
Methods
In this single institution retrospective series, the clinical outcomes of 80 consecutive lung cancer patients treated with concurrent immune checkpoint inhibitors and stereotactic radiosurgery were compared to 235 in the historical control cohort in which patients were treated prior to immune checkpoint inhibition being standard upfront therapy. Overall survival was estimated using the Kaplan Meier method. Cumulative incidence of local progression was estimated using a competing risk model.
Results
Median overall survival time was improved in patients receiving upfront immunotherapy compared to the historical control group (40 months vs 8 months, p < 0.001). Factors affected overall survival include concurrent immunotherapy (HR 0.23, p < 0.0001) and KPS (HR 0.97, p = 0.0001). Cumulative incidence of local failure in the historical control group was 10% at 1 year, compared to 1.1% at 1 year in the concurrent immunotherapy group (p = 0.025). Factors affected local control included use of concurrent immunotherapy (HR 0.09, p = 0.012), and lowest margin dose delivered to a metastasis (HR 0.8, p = 0.0018).
Conclusion
Local control and overall survival were both improved in patients receiving concurrent immune checkpoint inhibitors with radiosurgery compared to historical controls. While these data remain to be validated, they suggest that brain metastasis patients may benefit from concurrent use of immunotherapy with SRS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The increasing use of immunotherapy in the metastatic cancer population has led to a larger number of patients receiving the combination of immunotherapy and stereotactic radiosurgery (SRS) for brain metastases. It is estimated that 44% of patients with metastatic cancer are eligible to receive immune checkpoint inhibitor therapy [1]. Moreover, over 25% of the 180,000 brain metastasis patients in the US every year are treated with SRS [2, 3]. Non-small cell lung cancer (NSCLC) patients represent 50% of patients with brain metastases [4]. Moreover, immunotherapy has moved into the upfront setting for standard treatment of newly diagnosed metastatic disease for NSCLC [5]. The combination of SRS with immune checkpoint inhibitors has recently been found to be associated with improved survival outcomes [6], lower incidence of new brain metastases [7], decreased need for salvage treatments [8], but also an increased toxicity associated with treatment [9]. Because of the evolution in patient outcomes due to this combination, it is imperative that studies continue to assess the entire spectrum of clinical outcomes to determine whether the combination of SRS and immunotherapy may change outcomes from what has classically been seen in clinical practice.
One aspect of immune checkpoint inhibitors treatment that has garnered a significant amount of interest is their ability to independently lead to a response from brain metastases. A recent phase II study of patients with brain metastases from melanoma treated with combined ipilimumab and nivolumab without any radiotherapy demonstrated a response rate of 56% [10]. Another study which included both melanoma and non-small cell lung cancer patients with non-treated brain metastases demonstrated a 22% and 33% response rate for melanoma and lung cancer brain metastases, respectively [11]. While the response rates from immune checkpoint inhibitors are inferior to those seen with SRS, the main goal of immunotherapy in NSCLC patients is to treat systemic disease, while SRS is utilized to control intracranial disease.
The question that arises given the concurrent use of immune checkpoint inhibitors with SRS is whether such concurrent use may improve the efficacy of SRS. Several series have previously been published showing that the use of systemic therapy such as small molecule targeted agents [12, 13] as well as cytotoxic chemotherapy [14] can improve the local efficacy of SRS. While the efficacy of SRS for the NSCLC brain metastasis population is good [15], there are subpopulations that are at higher rate of local progression after SRS including those with resection cavities [16] and larger tumors [17]. Given the previous published activity of immune checkpoint inhibitors given as monotherapy and the ability of other classes of systemic agents to improve the local efficacy of SRS, we attempted to assess the ability of immune checkpoint inhibitors to improve SRS local control in NSCLC brain metastasis population. To this end, we conducted the present study to assess the local control outcomes of SRS in conjunction with concurrent immune checkpoint inhibition compared to a historical control population of NSCLC patients who were treated in the era prior to upfront immunotherapy use.
Methods
Data acquisition
This study was approved by the Wake Forest School of Medicine institutional review board. Patients who received immune checkpoint inhibitors concurrently (within 30 days either before or after) with SRS were identified within our institutional prospective radiosurgery database. PD-1 inhibitors and PD-L1 inhibitors were considered to be immune checkpoint inhibitors. A historical control population was identified as patients treated with the same histologies for which immune checkpoint inhibition is used, but that was treated in the era prior to immune checkpoint inhibitors becoming standard of care upfront therapy. Patients were included in the study if they would be eligible for an upfront immunotherapy-based regimen according to the modern standard of care (as such, patients with targetable mutations who would be treated with upfront targeted agents and not immunotherapy would be ineligible). Patients were excluded from both cohorts of the study if they had previously received whole brain radiotherapy or previous immune checkpoint inhibition that was more than 1 month from SRS. Clinical and imaging outcomes were determined via the electronic medical records.
Stereotactic radiosurgery
SRS was performed on the Gamma Knife Perfexion Unit (Elekta AB, Stockholm, Sweden). Patients were treated with a headframe fixation. Patients underwent same day MRI on a 3 Tesla scanner (GE Healthcare, Chicago, USA). Treatment planning was performed on the GammaPlan Treatment Planning System. Dosing of metastases was done using the guidelines from the RTOG 90-05 study [18] (Figs. 1 and 2).
Patient follow-up and response assessment
Patients underwent a follow-up MRI of the brain approximately 6–8 weeks after SRS, and then every 3 months thereafter for approximately 24 months. After that, follow-up MRI’s were spaced out to every 4–6 months so long as there was no evidence of tumor progression.
Local progression was determined as a pathologically proven recurrence of cancer within the prior SRS treatment volume or by imaging criteria previously described [19]. If pathological confirmation was not available, patients with suspected treatment failure were followed with serial imaging and treated conservatively with steroid therapy if symptoms were present. Imaging recurrence was determined by a 25% increase in the size of the lesion, or serial increases in the size of enhancement with corresponding increases in perfusion on perfusion weighted imaging sequences. In patients experiencing post-SRS symptoms, dexamethasone was generally prescribed 4 mg twice daily for mild symptoms, while 4 mg three times daily was prescribed for more significant symptoms or neurologic dysfunction.
Statistics
Descriptive statistics (means, standard deviations for continuous measures and counts/percentages for categorical variables) were calculated for all participants in each group. Continuous variables were compared between the two groups using 2-sample t-tests and categorical measures were compared using Chi-square tests. Next, overall survival was estimated using the Kaplan–Meier approach and the groups were compared using Log-Rank tests. Cox proportional hazards regression models were fit to identify factors that affected overall survival. First a model was fit that included age, gender, race, KPS, extent of extracranial disease, lowest marginal dose delivered and number of metastases treated at SRS. Next, a stepwise selection process was used to re-fit the multivariate model including only variables that were significant at p = 0.05. Cumulative incidence of local progression was estimated using Fine and Gray’s methodology [20]. A competing risk model was developed to estimate the single variable subdistribution hazard ratio associated with predictors of local recurrence. Using this approach, we examined the impact of the covariates listed above in predicting local recurrence. In order to account for potential imbalances in baseline characteristics, we performed a subsequent sensitivity analysis to examine overall survival and the competing risk model for local recurrence using a propensity score adjusted approach [21]. In these analyses, we fit the propensity score for the conditional probability of being in the experimental group by including age, gender, number of metastases treated at SRS, lowest marginal dose delivered, KPS, extent of extracranial disease, and craniotomy (yes/no) as covariates. We then assessed balance between groups after adjusting for propensity score quintile (Table 1), and subsequently fit the overall survival and competing risk models adjusting for propensity score quintile to allow for group comparisons to be made accounting for the non-randomized nature of this data. Statistics were performed using SAS v9.4 software.
Results
Patient characteristics
A total of 80 NSCLC patients in the immunotherapy cohort who were treated with SRS between 10/16/2014 and 9/6/2019. These patients received immune checkpoint inhibitors within 30 days of having SRS for brain metastasis (n = 80). A total of 235 patients in the historical control group between 2/7/2003 and 3/1/2017 who were treated with SRS without immunotherapy for brain metastases from NSCLC. A summary of patient characteristics is located in Table 1. Group comparisons after propensity score quintile adjustment are also shown in Table 1.
Survival and local control
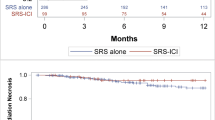
Kaplan Meier method was used to estimate overall survival in both the historical control group and the cohort receiving upfront immunotherapy concurrently with SRS. Median survival for the historical control group was 8 months. Overall survival at 6, 12 and 24 months for the historical control group was 57%, 36% and 14%, respectively. Median survival for the concurrent immunotherapy group was 40 months (which was statistically superior to the historical control arm, log rank p < 0.001). Overall survival at 6, 12 and 24 months for the concurrent immunotherapy group was 83%, 78% and 59%, respectively. These results were not changed after adjustment for propensity score quintile (p < 0.001 for group comparison based on Log-Rank statistic).
Fine Gray method was used to perform a competing risks analysis to account for the competing risk of death in determination of the local control. Cumulative incidence of local failure at 6, 12 and 24 months for the historical control group was 5.7%, 10.0% and 15.7%, respectively. Cumulative incidence of local failure at 6, 12 and 24 months for the concurrent immunotherapy group was 0.6%, 1.1% and 1.7%, respectively (p = 0.025, overall). After propensity score quintile adjustment these results remained consistent (p = 0.028, overall adjusted).
Factors affecting overall survival and local control
Results of multivariate analyses are summarized in Table 2. Multivariate cox proportional hazard regression identified factors that affected overall survival. Factors that affected overall survival included use of concurrent immunotherapy (HR 0.285, 95% CI 0.19–0.43 (p < 0.0001), extent of extracranial disease: oligometastatic (HR 1.37, 95% CI 1.01–1.85), widespread (HR 1.86, 95% CI 1.24–2.81), p = 0.005) and, KPS (HR 0.97, 0.0001).
Multivariate competing risks analysis identified increased hazard of local failure. Factors that affected local control included use of concurrent immunotherapy (sHR 0.12, 95% CI 0.011–0.568 p = 0.012), older age (sHR 0.97, 95% CI 0.95–0.996 per year, p = 0.023) and lowest margin dose delivered to a metastasis (sHR 0.798, 95% CI 0.69–0.92, p = 0.0018).
Toxicity
A Fisher exact test was performed to assess the difference in risk of grade 3 adverse radiation effect. The risk of grade 3 adverse radiation effect was 3% in patients receiving immunotherapy and 1% in patients not receiving immunotherapy (p = 0.15).
Discussion
The mechanism by which immune checkpoint inhibitors may affect the local control of radiosurgery is likely due to immune cell infiltration into tumors [22]. At this time, it is not entirely clear why some tumors of the same histology will respond well to immunotherapy, while others do not. Some of this variability is likely due to the differential expression of the PD-L1 antigen on tumor cells [23]. However, it is unlikely that this effect can be completely explained by PD-L1 expression as some PD-L1 negative tumors may still respond to immune checkpoint inhibitors [24]. While immune cell trafficking into the central nervous system is generally rare, the immune system has developed a means for crossing of the blood brain barrier, and this process can seemingly be upregulated in disease states such as cancer and inflammation [25, 26].
The present series represents a significant advancement in the literature for patients with brain metastases. The local control rate for the cohort treated with concurrent immunotherapy represents among the best previously reported in the scientific literature for SRS. If these results can be validated by an independent dataset, they could suggest that immunotherapy should be used concurrently with SRS, particularly in the setting where local control from SRS may be suboptimal (e.g. larger lesions). In the future Immune checkpoint inhibition alone may represent an option to assess in clinical trials both in the definitive and adjuvant setting for brain metastases. For example, patients with numerous lesions may best be an ideal population to asses in an attempt to avoid or postpone WBRT.
As the use of SRS has expanded greatly over the past decade, several additional clinical scenarios have arisen in which SRS alone is potentially inadequate for treatment of CNS disease. Patients with leptomeningeal disease generally have suboptimal results when treated with SRS alone [27]. This is due to the likelihood that SRS insufficiently targets disease given a diffuse disseminated pattern of spread in which the abnormality seen on MRI underestimates the extent of disease. Moreover, adjuvant treatment of resection cavities after surgical removal of brain metastases yields a moderate risk of treatment failure adjacent to the SRS field, particularly when the cavity size is greater than 3 cm [28]. Again, targeting may be insufficient in these cases as identifying the region in which tumor will recur, particularly when up against the volume constraints of large cavities, can be challenging. These clinical scenarios represent ones in which an adjuvant therapy could improve the therapeutic ratio of SRS by potentially having activity against microscopically occult disease that may have been insufficiently targeted and treated by SRS.
Larger volume lesions have a higher rate of local progression after SRS alone [18], and they also have a higher rate of radiation necrosis when immune checkpoint inhibitors are used concurrently [9]. Clinical trials are presently being developed to determine if hypofractionated radiotherapy may be able to decrease the likelihood of radiation necrosis when immunotherapy is being used concurrently with treatment of brain metastases [9]. Another option, given the biologic activity seen with the usage of concurrent immune checkpoint inhibitors, is to de-escalate the biologic doses of radiation delivered to brain metastases. This paradigm of dose de-escalation has been used successfully to mitigate toxicity in other cancer subtypes such as HPV positive head and neck cancers [29]. Elucidation of biomarkers for brain metastasis immunotherapy response may first be necessary for such an approach, but this may be on the horizon [30].
There are several limitations of the present study. As a retrospective review, its result are limited to hypothesis generation and are subject to patient selection biases. However, in order to address the selection bias limitation we used propensity score methods to adjust for baseline imbalances, and examined the main findings of the paper adjusting for propensity score quintiles. It should be noted that the propensity score adjustment was able to balance all baseline covariates except for the number of metastases at SRS which remained unbalanced between groups even after propensity score adjustment. However, this measure was imbalanced in favor of the control group (i.e. fewer metastases in the control group when compared to the experimental group after adjustment 2.6 (control) vs 3.5 (experimental) which would suggest that the estimates from our survival models would be conservative. In addition, the cohort receiving immunotherapy experienced very few local failure events. It is unclear if with greater amount of follow-up, there would be late local failures that would ultimately develop. Moreover, the lack of long term follow-up also affected the ability to assess for differences in late toxicities, as it is known that radiation necrosis events can be seen several years after SRS [31]. The toxicity difference seen in the present study was nearly statistically significant, and with a greater follow-up time, it may ultimately show a difference as has been seen in other series assessing toxicity of immunotherapy and SRS. In addition, patients were scanned using multiple MRI protocols and scanners over the course of the study (including with multiple magnet strength scanners and varying relaxivity of gadolinium agents). These differences may have affected the timing and sensitivity to early local failures. Moreover, some patients were determined to have local failure based on imaging alone. While multiple scans were generally used to determine a local failure if pathology was not available, this technique is imperfect. In spite of the limitations, the present series strongly suggests that there is a synergy between SRS and immune checkpoint inhibitors in the local treatment of brain metastases. While these data remain to be validated prospectively, they may have clinical utility for patients with higher risk of local failure from SRS alone.
References
Haslam A, Gill J, Prasad V (2020) Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open 3:e200423
Kann BH, Park HS, Johnson SB et al (2017) Radiosurgery for brain metastases: changing practice patterns and disparities in the United States. J Natl Compr Cancer Netw 15:1494–1502
Ellis TL, Neal MT, Chan MD (2012) The role of surgery, radiosurgery and whole brain radiation therapy in the management of patients with metastatic brain tumors. Int J Surg Oncol 2012:952345
Haughton ME, Chan MD, Watabe K et al (2016) Treatment of brain metastases of lung cancer in the era of precision medicine. Front Biosci 8:219–232
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092
Lanier CM, Hughes R, Ahmed T et al (2019) Immunotherapy is associated with improved survival and decreased neurologic death after SRS for brain metastases from lung and melanoma primaries. Neurooncol Pract 6:402–409
LeCompte MC, Hughes RT, Farris M et al (2020) Impact of brain metastasis velocity on neurologic death for brain metastasis patients experiencing distant brain failure after initial stereotactic radiosurgery. J Neurooncol 146:285–292
McTyre E, Ayala-Peacock D, Contessa J et al (2018) Multi-institutional competing risks analysis of distant brain failure and salvage patterns after upfront radiosurgery without whole brain radiotherapy for brain metastasis. Ann Oncol 29:497–503
Helis CA, Hughes RT, Glenn CW et al (2020) Predictors of adverse radiation effect in brain metastasis patients treated with stereotactic radiosurgery and immune checkpoint inhibitor therapy. Int J Radiat Oncol Biol Phys 108:295–303
Tawbi HA, Forsyth PA, Algazi A et al (2018) Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 379:722–730
Goldberg SB, Schalper KA, Gettinger SN et al (2020) Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol 21:655–663
Cochran DC, Chan MD, Aklilu M et al (2012) The effect of targeted agents on outcomes in patients with brain metastases from renal cell carcinoma treated with gamma knife surgery. J Neurosurg 116:978–983
Johnson AG, Ruiz J, Hughes R et al (2015) Impact of systemic targeted agents on the clinical outcomes of patients with brain metastases. Oncotarget 6:18945–18955
Harris S, Chan MD, Lovato JF et al (2012) Gamma knife stereotactic radiosurgery as salvage therapy after failure of whole-brain radiotherapy in patients with small-cell lung cancer. Int J Radiat Oncol Biol Phys 83:e53–e59
Hughes RT, Masters AH, McTyre ER et al (2019) Initial SRS for patients with 5 to 15 brain metastases: results of a multi-institutional experience. Int J Radiat Oncol Biol Phys 104:1091–1098
Jensen CA, Chan MD, McCoy TP et al (2011) Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. J Neurosurg 114:1585–1591
Dohm AE, Hughes R, Wheless W et al (2018) Surgical resection and postoperative radiosurgery versus staged radiosurgery for large brain metastases. J Neurooncol 140:749–756
Shaw E, Scott C, Souhami L et al (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys 47:291–298
Ayala-Peacock DN, Peiffer AM, Lucas JT et al (2014) A nomogram for predicting distant brain failure in patients treated with gamma knife stereotactic radiosurgery without whole brain radiotherapy. Neuro Oncol 16:1283–1288
Fine JP, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
D’Agostino RB Jr (1998) Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17(19):2265–2281. https://doi.org/10.1002/(sici)1097-0258(19981015)17:19%3c2265::aid-sim918%3e3.0.co;2-b
Soike MH, Logue J, Qasem S et al (2019) CD138 plasma cells may predict brain metastasis recurrence following resection and stereotactic radiosurgery. Sci Rep 9:14385
Wolchok JD, Kluger H, Callahan MK et al (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122–133
Kefford R, Ribas A, Hamid O et al (2014) Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol 32:3005–3005
Engelhardt B, Coisne C (2011) Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids Barriers CNS 8:4
Greter M, Heppner FL, Lemos MP et al (2005) Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med 11:328–334
Wang EC, Huang AJ, Huang KE et al (2017) Leptomeningeal failure in patients with breast cancer receiving stereotactic radiosurgery for brain metastases. J Clin Neurosci 43:6–10
Brown PD, Ballman KV, Cerhan JH et al (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1049–1060
Gabani P, Lin AJ, Barnes J et al (2019) Radiation therapy dose de-escalation compared to standard dose radiation therapy in definitive treatment of HPV-positive oropharyngeal squamous cell carcinoma. Radiother Oncol 134:81–88
Su J, Song Q, Qasem S et al (2020) Multi-omics analysis of brain metastasis outcomes following craniotomy. Front Oncol 10:615472
Minniti G, Clarke E, Lanzetta G et al (2011) Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol 6:48
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdulhaleem, M., Johnston, H., D’Agostino, R. et al. Local control outcomes for combination of stereotactic radiosurgery and immunotherapy for non-small cell lung cancer brain metastases. J Neurooncol 157, 101–107 (2022). https://doi.org/10.1007/s11060-022-03951-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-03951-7