Abstract
Introduction
Glioma remains incurable and a life limiting disease with an urgent need for effective therapies. Sonodynamic therapy (SDT) involves systemic delivery of non-toxic chemical agents (sonosensitizers) that accumulate in tumor cells or environment and are subsequently activated by exposure to low-frequency ultrasound to become cytotoxic agents. Herein, we discuss proposed mechanisms of action of SDT and provide recommendation for future research and clinical applications of SDT for gliomas.
Methods
Review of literature of SDT in glioma cell cultures and animal models published in Pubmed/MEDLINE before January, 2021.
Results
Different porphyrin and xanthene derivatives have proven to be effective sonosensitizers. Generation of reactive oxygen species and free radicals from water pyrolysis or sonosensitizers, or physical destabilization of cell membrane, have been identified as mechanisms of SDT leading to cell death. Numerous studies across glioma cell lines using various sonosensitizers and ultrasound parameters have documented tumoricidal effects of SDT. Studies in small animal glioma xenograft models have also consistently documented that SDT is associated with improved tumor control and longer survival of animals treated with SDT while avoiding damage of surrounding brain. There are no clinical trials completed to date regarding safety and efficacy of SDT in patients harboring gliomas, but some are beginning.
Conclusions
Pre-clinical studies cell cultures and animal models indicate that SDT is a promising treatment approach for gliomas. Further studies should define optimal sonication parameters and sonosensitizers for gliomas. Clinical trials of SDT in patients harboring gliomas and other malignant brain tumors are currently underway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma is the most common malignant primary brain tumor with an annual incidence rate of approximately 3.21 per 100,000 population [1]. Glioblastoma is a dismal disease with overall survival time of approximately 15 months [2, 3]. First-line treatment includes gross total resection and adjuvant combined chemotherapy with temozolomide and fractionated radiotherapy, followed by maintenance temozolomide [2, 4]. Unfortunately, the progression of glioblastomas is inevitable, and efficacy of the second-line treatment options is limited [5, 6]. Diffuse astrocytomas typically have slower growth rate and a more indolent course than high grade gliomas. However, low-grade tumors remain incurable and can progress to become high-grade tumors causing rapid disease progression and clinical deterioration [7, 8]. There remains an urgent need for new treatment strategies that could help to optimize prognosis of glioma patients [9, 10].
Sonodynamic therapy (SDT) involves systemic delivery of non-toxic chemical agents (sonosensitizers) that accumulate in tumor cells or environment. These agents are activated and become cytotoxic by exposure to low-intensity targeted ultrasound. Essentially, both the sensitization and ultrasound exposure are not tumoricidal by themselves; instead the cytotoxic events occur when both are combined [11]. SDT represents an emerging approach that offers the possibility of incisionless eradication of solid tumors in a site-directed manner, and this approach is becoming increasingly studied for treatment of gliomas [12, 13].
Focused ultrasound (FUS) is an emerging technology that allows controlled, spatially and temporally precise delivery of ultrasound energy to intracranial targets [14, 15]. High-intensity FUS can be used for thermal ablation and is an effective method for thalamotomy and subthalamtomy for essential tremor [16] and tremor predominant Parkinson’s disease [17]. Application of high-intensity FUS for treatment of brain tumors is limited by typically a narrow treatment envelope and long sonication time required to ablate significant tumor volume [18, 19]. At lower intensities, FUS can be applied without causing tissue damage, can be used to sonicate larger volumes and is being actively investigated for local and temporary disruption of the blood brain barrier to enhance delivery of chemotherapeutics into brain tumors [14, 20] with encouraging results in animal models and initial clinical trials [14, 18]. Therefore, the FUS can be used for SDT of intracranial gliomas with excellent spatial precision and accuracy.
In this article, we will provide comprehensive review of the concept and biological actions of SDT, review published pre-clinical studies of SDT for gliomas, and discuss potential clinical applications and future directions of SDT for gliomas.
The concept of sonodynamic therapy
Activation of a non-toxic compound by an external physical stimulus was first discovered back in 1900 s [21], by interaction of certain dyes with light, and was termed photodynamic therapy (PDT). It is worth reviewing some of the well-established concept of PDT, as some of these are shared with SDT.
PDT relies on the activation of photosensitizers by absorption of a photon of light with the appropriate wavelength. In the presence of oxygen, the excited photosensitizer forms reactive oxygen species (ROS) that can directly induce cellular damage by rapidly oxidizing cellular components. Most photosensitizers used for anti-cancer PDT operate more via creation the type II ROS rather than type I. Several compounds have received clinical approval for PDT, including tetrapyrrole structures, such as porphyrins, chlorins, bacteriochlorins and phthalocyanines. Synthetic dyes and natural products, such as hypericin, riboflavin and curcumin, have been investigated [22]. The selective nature of tumor targeting in PDT is thought to occur through tumoral accumulation via the EPR (enhanced permeability and retention) effect in tumors with leaky vasculature and defective lymphatic drainage [23].The most effective photosensitizers are typically hydrophobic compounds that accumulate in tumor cells and intercalate into membrane structures. The three main cell killing mechanisms of PDT are apoptotic, necrotic and autophagy-associated cell death that are related to photosensitizer localization in the different organelles.
Sonodynamic therapy (SDT) relies on the activation of sonosensitizers (non-toxic compounds) that upon ultrasound activation become cytotoxic by generation of ROS. Other, broader, definition of SDT have been proposed, to include non-chemical-based forms of non-thermal ultrasound therapy as well [24], with one of the most important biological effects being drug delivery [25] and direct destabilization of the plasma membrane, a mechanism known as sonoporation, that can enhance compound transport across the cell membrane [26].
In this review, we restrict SDT to the ultrasound activation of photochemical sensitizers.
Similar to PDT, SDT requires the combination of interaction of a chemical, ultrasound, and oxygen. The major advantage of SDT is the ability to provide more than ten of centimeters of penetration of ultrasound energy into soft tissues depending on the ultrasound frequency, and the possibility of delivering a tightly focused ultrasound beam for focal treatment.
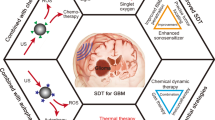
SDT started by evaluating tumor localizing porphyrins in ultrasound-induced reactions [27]. These early studies suggested that cell damage enhancement was probably mediated via single oxygen generated by activation of hematoporfirin by sonoluminescence, the generation of light by collapsing cavitation bubbles [28]. Since then, many different sonosensitizers have proven to be effective sonosensitizers, including (i) porphyrin derivatives, such as hematoporphyrin monomethyl ether (HMME), protoporphyrin IX disodium salt (PpIX), pheophorbide A, Photofrin, Photofrin II, ATX-70, ATX-S10, Ce6 and DCPH-P-Na(I); (ii) xanthene derivates, including erythosien B, rose Bengal; (iii) and inorganic sonosensitizers, such as Ti02 [29]. Several mechanisms of action of the ultrasound have since been identified (Fig. 1). In addition to direct activation of sonosensitizers by sonoluminescence and the subsequent generation of free radicals, mechanisms also include direct generation of free radicals by pyrolysis-mediated processes also taking place in the close vicinity of hot collapsing cavitation bubbles [27, 30]. Free radicals are formed via direct pyrolysis of the sonosensitizers, breaking apart the sensitizer generating free radicals that can react with other endogenous substrates to generate ROS, or by interaction with hydroxyls and hydrogen radicals formed by pyrolysis of water. Another possible mechanism of action of SDT relies on physical destabilization of the plasma membrane by the sonosensitizer, due to cell susceptibility to the mechanical action of the ultrasound, such as local shear force. While the activation process by ultrasound during SDT is generally considered to be induced by cavitation, and potentially via sonoluminescence, cell apoptosis, and pyrolysis, the exact mechanism of SDT is probably not governed by a universal phenomenon, and still needs to be analyzed based on the ultrasound treatment conditions and the formulation of a sonosensitizer [31,32,33]. Two major sonochemical products arise from ultrasonically-induced cavitation: free radicals and singlet oxygen, although the role of this later has been controversial in SDT [31]. Free radicals can induce a chain reaction of lipid peroxidation and cell damage, while singlet oxygen, once entering excited singlet state, is capable of oxidizing cellular contents. Available evidence strongly suggests a pivotal role for ROS in SDT [33] and the remaining questions mostly are concerned with the mechanism of ROS generation and which ROS are mainly responsible for mediating cytotoxic effects of SDT. Immune-modulation anti-tumor effect of SDT was also suggested [34].
Mechanisms of SDT (illustrated with 5-ALA as sonosensitizer). ROS can be produced by (1) pyrolysis of the compound (in this example ppIX) or water, (2) activation of the sonosensitizer by light produced during sonoluminescence. (3) Alternatively, compound affinity for the plasma membrane could make the cells more susceptible to mechanical stresses caused by the ultrasound. All three routes will lead to downstream signaling leading mainly to apoptosis
In Vitro studies of SDT
In vitro studies using a variety of sonosensitizers and different human and rat glioma cell lines generated evidence for the suggesting effectiveness of SDT for gliomas. SDT has been evaluated in in vitro studies using a variety of sonosensitizers, including porphyrin derivatives, such as hematoporphyrin monomethyl ether (HMME) [35, 36], and Photofrin [37,38,39], aluminum phthalocyanine disulfonate [40] and 5-aminolevulinic acid (5-ALA) [41,42,43]. Glioma cell lines used in several published studies include the C6 [35, 36, 41] and the F98 rat glioma cell lines [40], the U251 [37,38,39, 43], U105 [37], U87 [41, 43] malignant glioma cell lines and the U251Oct − 3/4 glioma stem-like cells [43]. Variable FUS parameters were utilized across studies (Table 1).
Hao et al. reported an improved apoptotic rate of HMME-SDT treated C6 rat glioma cells as compared to HMME or ultrasound alone. Ca+ 2 overload played a primary role in the apoptotic process which was associated with an increased production of ROS, decreased mitochondrial membrane potential (MMP), and increase in cyt-c [35]. In the study by Dai et al., the apoptotic effect of hematoporphyrin monomethyl ether (HMME)-SDT on C6 glioma cells was greater than HHME or ultrasound treatment alone. HMME-SDT induced apoptosis of C6 glioma cells due to increased ROS production and decreased mitochondrial membrane potential (MMP). Upregulation of caspase-9, caspase-3, and Bax expression and downregulation of Bcl-2 expression suggested a pivotal role of the mitochondrial signal pathway in the apoptotic process [36].
Hayashi et al., reported sensitivity to low-level ultrasound of both U251 and U105 human glioma cells. Photofrin enhanced ultrasound-induced cell death of the U251 cells expressing LRP/α2MR, but not in U105 cells not expressing LRP/α2MR [37]. Xu et al. reported decreased susceptibility of glioma stem-like cells to SDT than the U251 glioma cell line due to ABCG2 protein over expression causing efflux of the sonosensitizer [39]. Addition of fumitremorgin C, an ABCG2 inhibitor, resulted in a significant increase in the Photofrin-SDT mediated relative production of ROS suggesting the usefulness of fumitremorgin C in the SDT treatment of ABCG2-expressing malignant glioma cells [38].
Suehiro et al. and Sheehan et al. demonstrated greater tumoricidal effect of 5-ALA-SDT than FUS and 5-ALA alone on the U251 [43], C6 [41], U87 [41, 43] glioma cell lines and U251Oct − 3/4 glioma stem-like cells [43]. Bilmin et al. reported significant cytotoxic effects of 5-ALA-SDT on RG2 rat glioma cells [44]. Ju et al. demonstrated increased apoptosis, increased production of ROS, and loss of MMP with the addition of hyperthermia to 5-ALA-SDT in vitro. Higher levels of proteins Bax and cleaved caspase-3, 8, and 9 and lower level of bcl-2 were noted in the SDT-hyperthermia group than in the SDT alone group, the hyperthermia alone group and the control group [42]. In the study by Gonzales et al. compared to FUS alone or bleomycin alone, aluminum phthalocyanine disulfonate -SDT and bleomycin significantly inhibited growth of F98 glioma cells as three-dimensional tumor spheroids [40].
Animal models of SDT
SDT has been studied in small animal intracranial and subcutaneous glioma xenograft models (Table 2). An immunodeficient murine intracranial glioma model was used in one study [43]. The majority of studies used C6 glioma cells. Other cell lines included human glioblastoma U87 MG-Red-FLuc [42, 43, 45], U-118 MG [46] and SNB19 cells [42] ,and F98 rat malignant glioma cells [47]. The most commonly used radio-sensitizers were 5-ALA followed by sinoporphyrin. Fluorescein, hematoporphyrin monomethyl ether, Rose Bengal and iRGD modified DVDMS liposome were also tested. FUS parameters (intensity, duty cycle etc.) varied across studies and were reported inconsistently thus making challenging to make reliable comparisons between studies.
In all published studies, treatment with SDT was associated with inhibition of glioma growth and/or tumor proliferation of intracranial and subcutaneous glioma models across multiple tumor cell lines, cell lines, tested sonosentizers and FUS parameters. SDT was associated with decreased tumor growth and with longer survival of animals treated with SDT when compared to control animals [43, 45, 48,49,50]. Upregulation of apoptotic cell death mechanisms after SDT was the most widely studied mechanisms of action of SDT in in vivo glioma models. Overexpression of Terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) and upregulation of caspase-3, 8, 9, Bax and Cyto-C and downregulated expression of Bcl-2 were demonstrated with SDT [1, 3, 6, 8]. SDT was also shown to downregulate glioma angiogenesis as evident by reduction of micro-vessel density and expression of vascular endothelial growth factor (VEGF) [50].
SDT and FUS sonication of normal and peritumoral brain tissue was proven to be generally safe [47, 51, 52]. Ohmura with colleagues explored long term-effects of FUS applying 10 and 15 W/cm2, 1.04 MHz, 5-minute exposure, and found that at sonication at 15 W/cm2 caused brain lesions in all treated animals while sonication at 10 W/cm2 did not cause any lesions at 4-weeks post-sonication [52]. On the other hand, Nonanka with colleagues reported that sonication of rat brain caudoputamen region at 25 W/cm2 at 1 MHz for 5 min did not cause brain damage on histological examination independently from sonosensitizer (Rose Bengal) use. However, sonication at 110 W/cm2 at 1 MHz for 3 min induced sharply delineated coagulation necrosis in the majority of animals with greater dose of sonosensiter being associated with greater risk and size of a lesion [51].
Clinical evidence
Notwithstanding the great number of pre-clinical studies that yielded very promising results, efforts to translate this therapeutic approach into clinical practice has been limited to date. The first evidence of the effect of SDT in clinical practice has been described by Inui et al. in a case-report in which SDT was combined with GcMAF-based immunotherapy to treat a patient with terminal breast cancer (invasive ductal carcinoma, grade 3, ER+, PR+, HER2+, right axillary tumor, spinal metastases, intrapleural nodular tumor and right pleural effusion) [53]. SDT was performed using chlorin e6 and 5-ALA as sonosensitizers, and a total of 19 treatments of SDT were conducted in a three-month time span. This treatment protocol gave surprising results–the axillary tumor and intra-pleural nodular tumor disappeared completely; tumor markers were dramatically reduced; and no appreciable side effects were reported. The mechanism that is proposed to be behind the efficacy of this combined approach is the initiation of direct inflammatory necrosis inside tumors, coupled with the production of antitumor immunity via antigen-presenting cells to prevent immune escape [53]. A similar approach was used by the same research group to treat a patient with NSCLC (lung adenocarcinoma, stage 3B), using sonodynamic therapy coupled with GcMAF-based immunotherapy, TTF therapy and ozone therapy. The average survival of such patients with standard treatment protocols is only 8 months, while using this new approach no tumor growth was reported at 15 months without side effects [54].
A recent phase II clinical trial investigated the effect of DVDMS-SDT for peripheral artery disease (PAD) and demonstrated reduced plaque inflammation and improved walking performance of patients treated with SDT when compared to placebo [55]. Inflammation plays a central role in the development of atherosclerosis, and SDT is well-known for its immunomodulatory action, such as induction macrophage apoptosis, promotion of cholesterol efflux, and stabilization of atherosclerotic plaques.
As of 01/02/2021 there is one ongoing phase 0 single center, first in human, open-label study that uses ascending energy doses of SDT delivered via MRgFUS combined with intravenous 5-ALA that aims to assess safety and efficacy this approach in up to 30 patients with recurrent high-grade gliomas (NCT04559685). Eligible participants who are scheduled for tumor resection will be administered intravenous 5-ALA approximately 6–7 h prior to receiving sonodynamic therapy (SDT). Another phase 1 clinical trial has been recently approved (IRB - IRCCS C.Besta – 70/2020; 9/16/2020; 9/75) to assess the safety and feasibility of 5-ALA mediated SDT in patients with glioblastoma: 10 patients will receive SDT and the undergo a clinical and radiological follow-up for 3 weeks prior to tumor resection. In another Phase 0 single center trial (NCT04559685) ascending energy doses of SDT utilizing the MRgFUS combined with intravenous ALA (administered 6–7 h before SDT) will be tested in up to 30 patients diagnosed with recurrent high-grade gliomas who have measurable disease at recurrence defined as at least one contrast-enhancing lesion with a volume of at least 6 cm3 and ≤ 20cm3 of targeted treatment area. The authors will perform dose-escalation and time-escalation of SDT. Two other studies are focused on applying SDT for PAD (NCT03967730) and carotid atherosclerotic plaque (NCT03871725). Considering the substantial body of promising pre-clinical evidence documenting efficacy of SDT for gliomas and the potential translatability of this approach to other malignant brain tumors, clinical trials exploring safety and efficacy of SDT in glioma and other brain tumor patients are anticipated in the near future.
Future directions
To capitalize on the sonoluminescence mechanisms, novel strategies of SDT have recently been proposed, coupling sonosensitizer on the membrane of ultrasound contrast agents, shelled gas bubbles, lipid stabilized microbubble (MB), under the rationale that placing sensitizers in close proximity to MBs undergoing inertial cavitation could enhance their efficiency at generating ROS through sonoluminescence or pyrolysis-mediated processes [56]. Another potentially favorable permutation of this approach also involves attachment of a sonosensitizer to the surface oxygen-carrying MB to improve the sonodynamic effects under hypoxic conditions [57].
Because some sensitizers appear to be sensitive to both ultrasound and light, the combination of SDT with PDT has been proposed to increase therapeutic efficacy [58] and/or reduce required dose of chemicals, and benefit from deeper penetration depth and superior focusing capability in tissues compared to laser irradiation. Several preclinical studies have reported benefit of sonophotodynamic therapy (SPDT), showing stronger therapeutic effect and reduction in the required dose of chemicals, that could help protect peripheral tissue from collateral damage [29].
FUS technology is progressing rapidly. The Exablate Neuro (INSIGHTEC, Israel) platform allows spatially precise delivery of ultrasound energy to intracranial targets under MRI guidance. The NaviFUS System (NAVIFUS, Taiwan) uses pre-treatment CT/MRI images and neuro-navigation tracking system to target ultrasound energy. However, these platforms are used mostly used in the setting of BBB opening, and their value for SDT remains to be determined.
Further pre-clinical studies exploring therapeutic and immunomodulation actions of SDT are warranted to harness the full therapeutic potential of this promising approach.
Conclusions
Abundant pre-clinical studies in glioma in vitro and animal models strongly suggest that SDT is a potent and promising therapeutic approach for gliomas that can induce anti-tumor effects via activation of apoptosis, anti-tumor immune response, and via other biological mechanisms. Safety of SDT for surrounding normal brain tissues has been reliably documented in pre-clinical studies of using small animal glioma models. A fuller understanding of optimal sonication parameters as well as doses and types of sonosensitizers for gliomas is imperative. Clinical experience with SDT for gliomas remains limited, but several clinical studies of SDT for brain tumor patients are now underway.
References
Ostrom QT, Gittleman H, Truitt G et al (2018) CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neurooncology 20:iv1–iv86. https://doi.org/10.1093/neuonc/noy131
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Gilbert MR, Wang M, Aldape KD et al (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31:4085–4091. https://doi.org/10.1200/JCO.2013.49.6968
Stupp R, Brada M, van den Bent MJ et al (2014) High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 25 Suppl 3:iii93–i101. https://doi.org/10.1093/annonc/mdu050
Nam JY, de Groot JF (2017) Treatment of Glioblastoma. JOP 13:629–638. https://doi.org/10.1200/JOP.2017.025536
Bunevicius A, Sheehan JP (2021) Radiosurgery for Glioblastoma. Neurosurg Clin N Am 32:117–128. https://doi.org/10.1016/j.nec.2020.08.007
Ostrom QT, Cote DJ, Ascha M et al (2018) Adult Glioma Incidence and Survival by Race or Ethnicity in the United States From 2000 to 2014. JAMA Oncol 4:1254–1262. https://doi.org/10.1001/jamaoncol.2018.1789
Claus EB, Walsh KM, Wiencke JK et al (2015) Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus 38:E6. https://doi.org/10.3171/2014.10.FOCUS12367
Aldape K, Brindle KM, Chesler L et al (2019) Challenges to curing primary brain tumours. Nature Reviews Clinical Oncology 16:509–520. https://doi.org/10.1038/s41571-019-0177-5
Weller M, Wick W, Aldape K et al (2015) Glioma. Nat Rev Dis Primers 1:15017. https://doi.org/10.1038/nrdp.2015.17
McHale AP, Callan JF, Nomikou N et al (2016) Sonodynamic Therapy: Concept, Mechanism and Application to Cancer Treatment. Adv Exp Med Biol 880:429–450. https://doi.org/10.1007/978-3-319-22536-4_22
Son S, Kim JH, Wang X et al (2020) Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev 49:3244–3261. https://doi.org/10.1039/c9cs00648f
Bilmin K, Kujawska T, Grieb P (2019) Sonodynamic therapy for gliomas. perspectives and prospects of selective sonosensitization of glioma cells. Cells. https://doi.org/10.3390/cells8111428
Bunevicius A, McDannold NJ, Golby AJ (2020) Focused ultrasound strategies for brain tumor therapy. Oper Neurosurg (Hagerstown) 19:9–18. https://doi.org/10.1093/ons/opz374
Meng Y, Hynynen K, Lipsman N (2021) Applications of focused ultrasound in the brain: from thermoablation to drug delivery. Nat Rev Neurol 17:7–22. https://doi.org/10.1038/s41582-020-00418-z
Elias WJ, Lipsman N, Ondo WG et al (2016) A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med 375:730–739. https://doi.org/10.1056/NEJMoa1600159
Martínez-Fernández R, Máñez-Miró JU, Rodríguez-Rojas R et al (2020) Randomized trial of focused ultrasound subthalamotomy for Parkinson’s disease. N Engl J Med 383:2501–2513. https://doi.org/10.1056/NEJMoa2016311
McDannold N, Clement GT, Black P et al (2010) Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery 66:323–332. https://doi.org/10.1227/01.NEU.0000360379.95800.2F discussion 332.
Coluccia D, Fandino J, Schwyzer L et al (2014) First noninvasive thermal ablation of a brain tumor with MR-guided focused ultrasound. J Ther Ultrasound 2:17. https://doi.org/10.1186/2050-5736-2-17
Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA (2001) Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 220:640–646. https://doi.org/10.1148/radiol.2202001804
Moan J, Peng Q (2003) An outline of the hundred-year history of PDT. Anticancer Res 23:3591–3600
Abrahamse H, Hamblin MR (2016) New photosensitizers for photodynamic therapy. Biochem J 473:347–364. https://doi.org/10.1042/bj20150942
Hamblin MR, Newman EL (1994) New trends in photobiology on the mechanism of the tumour-localising effect in photodynamic therapy. J Photochem Photobiol B 23:3–8. https://doi.org/10.1016/s1011-1344(94)80018-9
Tachibana K, Feril LB, Ikeda-Dantsuji Y (2008) Sonodynamic therapy. Ultrasonics 48:253–259. https://doi.org/10.1016/j.ultras.2008.02.003
Song K-H, Harvey BK, Borden MA (2018) State-of-the-art of microbubble-assisted blood-brain barrier disruption. Theranostics 8:4393–4408. https://doi.org/10.7150/thno.26869
Lentacker I, Cock ID, Deckers R et al (2014) Understanding ultrasound induced sonoporation: definitions and underlying mechanisms. Adv Drug Deliv Rev 72:49–64. https://doi.org/10.1016/j.addr.2013.11.008
Umemura S, Yumita N, Nishigaki R, Umemura K (1990) Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn J Cancer Res 81:962–966. https://doi.org/10.1111/j.1349-7006.1990.tb02674.x
Stride EP, Coussios CC (2010) Cavitation and contrast: the use of bubbles in ultrasound imaging and therapy. Proc Inst Mech Eng 224:171–191
Yang Y, Tu J, Yang D et al (2019) Photo- and sono-dynamic therapy: a review of mechanisms and considerations for pharmacological agents used in therapy incorporating light and sound. Curr Pharm Des 25:401–412. https://doi.org/10.2174/1381612825666190123114107
MIŠÍK V, RIESZ P (2000) Free radical intermediates in sonodynamic therapy. Ann N Y Acad Sci 899:335–348. https://doi.org/10.1111/j.1749-6632.2000.tb06198.x
Rosenthal I, Sostaric JZ, Riesz P (2004) Sonodynamic therapy–a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem 11:349–363. https://doi.org/10.1016/j.ultsonch.2004.03.004
Lafond M, Yoshizawa S, Umemura S-I (2019) Sonodynamic therapy: advances and challenges in clinical translation. J Ultrasound Med 38:567–580. https://doi.org/10.1002/jum.14733
Costley D, Ewan CM, Fowley C et al (2015) Treating cancer with sonodynamic therapy: a review. Int J Hyperth 31:107–117. https://doi.org/10.3109/02656736.2014.992484
Zhang Q, Bao C, Cai X et al (2018) Sonodynamic therapy-assisted immunotherapy: a novel modality for cancer treatment. Cancer Sci 109:1330–1345. https://doi.org/10.1111/cas.13578
Hao D, Song Y, Che Z, Liu Q (2014) Calcium overload and in vitro apoptosis of the C6 glioma cells mediated by sonodynamic therapy (hematoporphyrin monomethyl ether and ultrasound). Cell Biochem Biophys 70:1445–1452. https://doi.org/10.1007/s12013-014-0081-7
Dai S, Hu S, Wu C (2009) Apoptotic effect of sonodynamic therapy mediated by hematoporphyrin monomethyl ether on C6 glioma cells in vitro. Acta Neurochir (Wien) 151:1655–1661. https://doi.org/10.1007/s00701-009-0456-5
Hayashi S, Yamamoto M, Tachibana K et al (2009) Mechanism of photofrin-enhanced ultrasound-induced human glioma cell death. Anticancer Res 29:897–905
Xu Z-Y, Wang K, Li X-Q et al (2013) The ABCG2 transporter is a key molecular determinant of the efficacy of sonodynamic therapy with Photofrin in glioma stem-like cells. Ultrasonics 53:232–238. https://doi.org/10.1016/j.ultras.2012.06.005
Xu Z-Y, Li X-Q, Chen S et al (2012) Glioma stem-like cells are less susceptible than glioma cells to sonodynamic therapy with photofrin. Technol Cancer Res Treat 11:615–623. https://doi.org/10.7785/tcrt.2012.500277
Gonzales J, Nair RK, Madsen SJ et al (2016) Focused ultrasound-mediated sonochemical internalization: an alternative to light-based therapies. J Biomed Opt 21:78002. https://doi.org/10.1117/1.JBO.21.7.078002
Sheehan K, Sheehan D, Sulaiman M et al (2020) Investigation of the tumoricidal effects of sonodynamic therapy in malignant glioblastoma brain tumors. J Neurooncol 148:9–16. https://doi.org/10.1007/s11060-020-03504-w
Ju D, Yamaguchi F, Zhan G et al (2016) Hyperthermotherapy enhances antitumor effect of 5-aminolevulinic acid-mediated sonodynamic therapy with activation of caspase-dependent apoptotic pathway in human glioma. Tumour Biol 37:10415–10426. https://doi.org/10.1007/s13277-016-4931-3
Suehiro S, Ohnishi T, Yamashita D et al (2018) Enhancement of antitumor activity by using 5-ALA-mediated sonodynamic therapy to induce apoptosis in malignant gliomas: significance of high-intensity focused ultrasound on 5-ALA-SDT in a mouse glioma model. J Neurosurg 129:1416–1428. https://doi.org/10.3171/2017.6.JNS162398
Bilmin K, Kujawska T, Secomski W et al (2016) 5-Aminolevulinic acid-mediated sonosensitization of rat RG2 glioma cells in vitro. Folia Neuropathol 54:234–240. https://doi.org/10.5114/fn.2016.62233
Pi Z, Huang Y, Shen Y et al (2019) Sonodynamic therapy on intracranial glioblastoma xenografts using sinoporphyrin sodium delivered by ultrasound with microbubbles. Ann Biomed Eng 47:549–562. https://doi.org/10.1007/s10439-018-02141-9
An Y-W, Liu H-Q, Zhou Z-Q et al (2020) Sinoporphyrin sodium is a promising sensitizer for photodynamic and sonodynamic therapy in glioma. Oncol Rep 44:1596–1604. https://doi.org/10.3892/or.2020.7695
Yoshida M, Kobayashi H, Terasaka S et al (2019) Sonodynamic therapy for malignant glioma using 220-kHz transcranial magnetic resonance imaging-guided focused ultrasound and 5-aminolevulinic acid. Ultrasound Med Biol 45:526–538. https://doi.org/10.1016/j.ultrasmedbio.2018.10.016
Wu S-K, Santos MA, Marcus SL, Hynynen K (2019) MR-guided focused ultrasound facilitates sonodynamic therapy with 5-aminolevulinic acid in a rat glioma model. Sci Rep 9:10465. https://doi.org/10.1038/s41598-019-46832-2
Sun Y, Wang H, Wang P et al (2019) Tumor targeting DVDMS-nanoliposomes for an enhanced sonodynamic therapy of gliomas. Biomater Sci 7:985–994. https://doi.org/10.1039/c8bm01187g
Song D, Yue W, Li Z et al (2014) Study of the mechanism of sonodynamic therapy in a rat glioma model. Onco Targets Ther 7:1801–1810. https://doi.org/10.2147/OTT.S52426
Nonaka M, Yamamoto M, Yoshino S et al (2009) Sonodynamic therapy consisting of focused ultrasound and a photosensitizer causes a selective antitumor effect in a rat intracranial glioma model. Anticancer Res 29:943–950
Ohmura T, Fukushima T, Shibaguchi H et al (2011) Sonodynamic therapy with 5-aminolevulinic acid and focused ultrasound for deep-seated intracranial glioma in rat. Anticancer Res 31:2527–2533
Inui T, Makita K, Miura H et al (2014) Case report: a breast cancer patient treated with GcMAF, sonodynamic therapy and hormone therapy. Anticancer Res 34:4589–4593
Inui T, Amitani H, Kubo K et al (2016) Case report: a non-small cell lung cancer patient treated with GcMAF, sonodynamic therapy and tumor treating fields. Anticancer Res 36:3767–3770
Jiang Y, Fan J, Li Y et al (2021) Rapid reduction in plaque inflammation by sonodynamic therapy inpatients with symptomatic femoropopliteal peripheral artery disease: a randomized controlled trial. Int J Cardiol 325:132–139. https://doi.org/10.1016/j.ijcard.2020.09.035
Beguin E, Shrivastava S, Dezhkunov NV et al (2019) Direct evidence of multibubble sonoluminescence using therapeutic ultrasound and microbubbles. ACS Appl Mater Interfaces 11:19913–19919. https://doi.org/10.1021/acsami.9b07084
McEwan C, Owen J, Stride E et al (2015) Oxygen carrying microbubbles for enhanced sonodynamic therapy of hypoxic tumours. J Controlled Release 203:51–56. https://doi.org/10.1016/j.jconrel.2015.02.004
Kessel D, Lo J, Jeffers R et al (1995) Modes of photodynamic vs. sonodynamic cytotoxicity. J Photochem Photobiol B 28:219–221. https://doi.org/10.1016/1011-1344(94)07111-z
Prada F, Sheybani N, Franzini A et al (2020) Fluorescein-mediated sonodynamic therapy in a rat glioma model. J Neurooncol 148:445–454. https://doi.org/10.1007/s11060-020-03536-2
Jeong E-J, Seo S-J, Ahn Y-J et al (2012) Sonodynamically induced antitumor effects of 5-aminolevulinic acid and fractionated ultrasound irradiation in an orthotopic rat glioma model. Ultrasound Med Biol 38:2143–2150. https://doi.org/10.1016/j.ultrasmedbio.2012.07.026
Acknowledgements
The authors thank Jackie Brenner for her help in the design and for the drawing of Fig. 1.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare thast they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bunevicius, A., Pikis, S., Padilla, F. et al. Sonodynamic therapy for gliomas. J Neurooncol 156, 1–10 (2022). https://doi.org/10.1007/s11060-021-03807-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03807-6