Abstract
Introduction
Stereotactic radiosurgery (SRS) is an emerging treatment for patients with multiple brain metastases (BM). The present work compares the SRS of multiple brain metastases with whole-brain radiotherapy (WBRT).
Methods
We performed a matched-pair analysis for 128 patients with multiple BM treated with either SRS or WBRT over a 5-year period. Patients were matched pairwise for seven potential prognostic factors. A mixed Cox Proportional Hazards model with univariate and multivariate analysis was fitted for overall survival (OS). Distant intracranial progression-free survival (icPFS) and local control were assessed using a Fine and Gray subdistribution hazard model and considering death as competing event.
Results
Patients undergoing SRS had a median of 4 BM (range 3–16). 1-year local control of individual BM following SRS was 91.7%. Median OS in the SRS subgroup was 15.7 months (IQR 9.7–36.4) versus 8.0 months (interquartile range, IQR 3.8–18.0) in the WBRT subgroup (HR 2.25, 95% CI [1.5; 3.5], p < 0.001). Median icPFS was 8.6 (IQR 3.4–18.0) versus 22.4 (IQR 5.6–28.6) months, respectively (HR for WBRT 0.41, 95% CI [0.24; 0.71], p = 0.001). Following SRS, synchronous BM diagnosis (HR 2.51, 95% CI [1.30; 4.70], p = 0.004), higher initial number of BM (HR 1.21, 95% CI [1.10; 1.40], p = 0.002) and lung cancer histology (HR 2.05, 95% CI [1.10; 3.80], p = 0.024) negatively impacted survival. Excellent clinical performance (KPI 90%) was a positive prognosticator (HR 0.38, 95% CI [0.20; 0.72], p = 0.003), as was extracerebral tumor control (HR 0.48, 95% CI [0.24; 0.97], p = 0.040). Higher initial (HR 1.19, 95% CI [1.00; 1.40], p < 0.013) and total number of BM (HR 1.23, 95% CI [1.10; 1.40], p < 0.001) were prognostic for shorter icPFS.
Conclusion
This is the first matched-pair analysis to compare SRS alone versus WBRT alone for multiple BM. OS was prolonged in the SRS subgroup and generally favorable in the entire cohort. Our results suggest SRS as a feasible and effective treatment for patients with multiple BM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stereotactic radiosurgery (SRS) is the recommended treatment for patients with limited brain metastases, according to current guidelines [1, 2]. For patients with multiple brain metastases (BM), whole-brain radiotherapy (WBRT) is recommended. Recently, however, a large prospective observational trial by Yamamoto et al. could demonstrate overall survival to be non-inferior for patients with 5–10 BM, compared to patients with 2–4 BM when all lesions are treated with SRS instead of WBRT. Furthermore, their study showed that for the majority of patients who developed distant intracerebral recurrences after SRS, repeated SRS of new lesions was adequate salvage treatment [3].
The significant neurotoxicity and negative impact on quality of life associated with WBRT have been conclusively demonstrated in several phase-3 trials [4,5,6]. Thus, a strong rationale exists for the use of SRS over WBRT, where feasible and indicated.
Recent technical developments have facilitated the stereotactic treatment of multiple BM. Today, several different systems exist that allow high-dose conformity and steep dose gradients, combined with relatively time-efficient treatment and precise treatment application: Robotic radiosurgery with continuous motion tracking is the approach employed by the CyberKnife system (Accuray Inc., Sunnyvale California), while the Gamma Knife (Elekta, Stockholm, Sweden) was first in employing rigid fixation of the head within a stereotactic frame [7, 8]. Both methods have been well established for more than two decades, while more recently introduced systems are based on the use of single-isocenter volumetric modulated arc therapy (VMAT). Those systems provide enhanced treatment efficiency and are more widely available, while making some compromises in terms of dose conformity [9,10,11].
At our center, patients with up to ten simultaneous brain metastases have been treated with robotic SRS or hypofractionated stereotactic radiotherapy (HFSRT) from the beginning of 2016. To date, no prospective randomized trial exists for the comparison of SRS and WBRT in this setting. Retrospective analyses are prone to selection bias and a matched-pair design is one methodology capable of decreasing this risk [12]. The present work is the first to compare the SRS of multiple brain metastases with WBRT, performing a matched-pair analysis for a total of 128 patients treated at our institution.
Patients and methods
128 patients who received either SRS/HFSRT or WBRT for the initial treatment of multiple BM between 2015 and 2019 were included in this analysis. The SRS subgroup consisted of 64 patients with 3 to 16 BM per patient (maximum 10 BM per treatment session) and a total of 313 BM treated with CyberKnife SRS/HFSRT. The WBRT subgroup was identified from a cohort of 988 patients who received WBRT by individually matching patients 1:1 for seven potential prognostic factors. Patients were required to match for the following characteristics: age at radiotherapy, primary tumor, interval between initial diagnosis and diagnosis of BM, the presence of extracranial metastases, Karnofsky Performance Score index (KPI), initial number of BM and recursive partitioning analysis (RPA) class. Here, the seven abovementioned characteristics, deemed to have the highest prognostic value, yielded the best compromise between exact matching, while maintaining an adequate sample size for analysis.
Patient and treatment data was extracted from a clinical database maintained at our institution and from medical and official records [13]. All reviews were performed following institutional guidelines and the Declaration of Helsinki of 1975 in its most recent version. Ethics approval for the study and a waiver of written informed consent was granted by the Heidelberg University ethics committee on April 12th, 2018 (#S-172/2018). Patient confidentiality was maintained by anonymizing patient data to remove any identifying information.
Patient and treatment characteristics
Median patient age at the beginning of radiotherapy (RT) was 60 years and the most common histology was non-small cell lung cancer (NSCLC), followed by breast cancer. Detailed patient characteristics are illustrated in Table 1 for the entire cohort and in Table 2 regarding the SRS and WBRT subgroups.
Treatment indication was discussed interdisciplinarily in the context of our institution’s comprehensive cancer center. Regarding treatment allocation, it must be noted that the CyberKnife M6 was introduced to our center in early 2016, providing the necessary technical requirements for the SRS of multiple BM. An institutional paradigm shift followed, so that from that timepoint on, SRS was more frequently used instead of WBRT for the treatment of multiple BM. Whenever feasible, it was aspired to treat distant intracerebral recurrences after SRS with repeated SRS so that 50% of the patients in the SRS subgroup (n = 32) received more than one treatment course.
For all cranial RT, an individual thermoplastic head fixation mask was fitted for each patient. Treatment planning for SRS was based on high-resolution computed tomography (CT) and magnetic resonance imaging (MRI). Standardized imaging protocols were used for all patients, complying to the following specifications: CT scan was acquired with 1 mm slice thickness. MRI contained a contrast-enhanced, T1-weighted, three-dimensional sequence with multiplanar reconstruction and a slice thickness of ≤ 1 mm. The MRI was thoroughly co-registered and served as basis for target and organs at risk (OAR) delineation. Gross tumor volume (GTV) consisted of all contrasted tissue in the T1-weighted MRI. A safety margin of 1 mm was added to the GTV by isotropic expansion to create the planning target volume (PTV). Dose prescription was done according to metastasis size and in compliance with current guidelines [14, 15]. The most commonly prescribed margin doses were 20 or 18 Gy to the 70% isodose, covering at least 98% of the PTV with a conformity index (CI) of < 1.1 as a planning objective. Lesions exceeding a maximum diameter of 3 cm were treated with HFSRT, most commonly 6 × 5 Gy to the 70% isodose. Treatment planning for CyberKnife was done in Accuray’s Multiplan v5.3 and subsequent versions. Treatment was delivered using the CyberKnife M6 (Accuray Inc., Sunnyvale California). Details regarding SRS/HFSRT characteristics are illustrated in Table 1.
Treatment planning for WBRT was done in Oncentra External Beam v4.5 (Elekta, Stockholm Sweden) using a 3 mm computed tomography (CT). The prescribed dose for WBRT was 30 Gy in 10 fractions. In 6 selected cases (9.3%), an additional dose of 9 Gy in 3 fractions to large brain metastases was applied after three-dimensional conformal (3DCRT) treatment planning. Treatment was delivered at a linear accelerator using two laterally opposing fields for WBRT and multi-field technique for 3DCRT, as has been previously described [16, 17].
Statistical analysis
For baseline analyses, descriptive statistics are used, continuous variables are given as means (standard deviation, SD) and/or median (interquartile range (IQR) and range, as appropriate) and categorical variables as absolute and relative frequencies. The quantiles of the follow-up time were calculated using the reverse Kaplan–Meier method [18]. Overall survival was calculated from the beginning of RT to the date of death or last follow-up. Intracranial progression-free survival in the distant brain (icPFS) and local control (LC) were calculated from the beginning of RT to last imaging follow-up or confirmed progression. Overall survival was investigated using the method of Kaplan–Meier. To identify prognostic factors on overall survival, univariate and multivariate mixed Cox Proportional regression models were used. The matching ID was included as random effect to account for the matching procedure, the prognostic factors were included as fixed effects. To identify prognostic factors on LC and icPFS, Fine and Gray subdistribution hazard models with univariate and multivariate analysis were fitted for the cumulative incidence of progression, considering death as competing event. Variables with statistical significance in univariate analysis and those of special clinical interest were considered in multivariate regression. Since this was a retrospective exploratory data analysis, p-values are of descriptive nature. A descriptive p-value of < 0.05 was considered as statistically significant. Statistical analyses were performed with the software R Version 3.5.1.
Results
Survival and prognostic factors
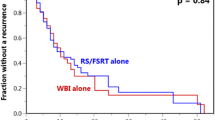
Median follow-up time for overall survival (OS), as estimated by the reverse Kaplan–Meier method (KM), was 35.8 months (IQR 24.6.7–41.3) for the entire cohort. At the time of this analysis, 120 patients had died, and 8 patients were still alive, corresponding to 0.49 (KM estimate; CI 0.41–0.59) survival probability at 12 months and 0.23 (KM estimate; CI 0.17–0.33) at 24 months. Survival significantly differed between the SRS and WBRT subgroups with a median OS of 15.7 months (IQR 9.7–36.4) in the SRS subgroup and 8.0 months (IQR 3.8–18.0) in the WBRT subgroup (hazard ratio (HR) for WBRT 2.25, 95% CI [1.5; 3.5], p < 0.001). Survival curves stratified by treatment modality are displayed in Fig. 1a.
Overall survival (a), distant intracranial progression-free survival (b) and local control (c) for 128 patients with multiple brain metastases treated with either stereotactic radiosurgery (SRS) or whole-brain radiotherapy (WBRT); overall survival was analyzed using the method of Kaplan–Meier, whereas for local and intracranial control, the cumulative incidence of failure is displayed, considering death as competing event
Univariate and multivariate analysis were performed on both subgroups, as well as the entire cohort to assess potential prognostic factors. This was done independently for the endpoints of OS, as well as LC and icPFS. Covariates tested for prognostic significance were age, gender, timepoint of diagnosis of BM (within or later than three months after primary diagnosis), extracerebral tumor control, KPI, primary histology, initial number of BM, RPA class, and Graded Prognostic Assessment (GPA) score. In the SRS subgroup, where detailed information for every lesion was available, additionally the total number and volume of treated BM were included as covariates. Results of univariate analysis for the endpoint of OS are displayed in Table 3, showing hazard ratios and p-values only for significant covariates, and in Supplementary Tables 1–3, detailing all covariates. Prognosticators of OS differed between subgroups: In the SRS subgroup, synchronous BM diagnosis (HR 2.51, 95% CI [1.30; 4.70], p = 0.004), a higher initial number of BM (HR 1.21, 95% CI [1.10; 1.40], p = 0.002) and lung cancer histology (HR 2.05, 95% CI [1.10; 3.80], p = 0.024) negatively impacted survival, whereas excellent clinical performance (KPI 90%) was a positive prognosticator (HR 0.38, 95% CI [0.20; 0.72], p = 0.003), as was extracerebral tumor control (HR 0.48, 95% CI [0.24; 0.97], p = 0.040). In multivariate analysis, a higher initial number of BM stayed prognostic of inferior OS (HR 1.24, 95% CI [1.08; 1.42], p = 0.002). RPA score and GPA class showed no prognostic accuracy in this subgroup. For the WBRT subgroup, the univariate analysis yielded higher age (HR 1.03, 95% CI [1.00; 1.10], p = 0.043) and male gender (HR 2.01, 95% CI [1.20; 3.40], p = 0.011) as negative prognosticators for OS. RPA class 1 was a positive prognosticator for OS (HR 0.41, 95% CI [0.17; 0.96], p = 0.040. Delivery of a boost to the largest metastases seemed to be a positive prognostic factor in univariate (HR 0.36, 95% CI [0.13; 1.00], p = 0.053), though not in multivariate analysis. Analyzing the entire combined cohort, treatment modality (SRS vs. WBRT) was the strongest prognosticator for OS with inferior outcome for WBRT (HR 2.25, 95% CI [1.5; 3.5], p < 0.001). Additional prognosticators for inferior OS were higher age at radiotherapy (HR 1.02, 95% CI [1.00; 1.02], p < 0.047) and RPA class 3 (HR 2.43, 95% CI [1.10; 5.50], p < 0.033). Excellent clinical performance with a KPI of ≥ 90% was prognostic of superior OS (HR 0.57, 95% CI [0.37; 0.88], p < 0.010). In multivariate analysis, RT modality (HR 2.16, 95% CI [1.30; 3.50], p < 0.033) and RPA class 3 (HR 2.75, 95% CI [1.10; 6.80], p < 0.028) stayed prognostic of inferior OS. The timepoint of first RT, which was included into analysis as a surrogate for differences in systemic treatment over time, did not relevantly influence OS in the SRS and WBRT subgroups or in the combined cohort.
Intracranial progression-free survival
Median follow-up time for icPFS in the distant brain, as estimated by the reverse Kaplan–Meier method, was 13.8 months (IQR 8.4–19.6) for the entire cohort. icPFS differed significantly between the SRS and WBRT subgroups with a median icPFS of 8.6 (IQR 3.4–18.0) months in the SRS subcohort and 22.4 (IQR 5.6–28.6) months in the WBRT subcohort (HR for WBRT 0.41, 95% CI [0.24; 0.71], p = 0.001). Results of the univariate and multivariate Fine and Gray subdistribution hazard model (death as competing event) for the endpoint of icPFS are displayed in Table 4 and Supplementary Tables 4–6, as well as Fig. 1b. In univariate analysis of the SRS subcohort, a higher total number of metastases was significantly associated with shorter icPFS (HR 1.24, 95% CI [1.10; 1.40], p < 0.001). A higher initial number (HR 3.02, 95% CI [1.40; 6.70], p = 0.007) and total number of metastases (HR 1.38, 95% CI [1.20; 1.60], p < 0.001) were independently prognostic for shorter icPFS in multivariate analysis. The effect of a higher number of metastases on shorter icPFS was similarly observed in the WBRT cohort (HR 3.48, 95% CI [1.30; 9.00], p = 0.010), as well as in the analysis of the combined cohort (HR 2.05, 95% CI [1.10; 3.70], p = 0.016) (detailed results displayed in Table 4). 6 patients (9.3%) in the WBRT subgroup received SRS as salvage therapy for newly occurring BM. 32 patients (50%) in the SRS subgroup received repeated radiosurgery for new BM; four patients from the SRS subgroup (6.3%) ultimately received salvage WBRT. The delivery of salvage therapy for new BM showed no statistically significant effect on OS in either subgroup, nor in the combined cohort (Supplementary Tables 1–3).
Local control
Local control was defined as stable or regressive contrast enhancement for all lesions visible prior to SRS or WBRT. Multivariate analysis revealed urologic histology to be associated with inferior LC in the SRS subgroup (HR 4.76, 95% CI [1.20; 18.00], p = 0.024). A higher initial number of BM was associated with inferior LC in the WBRT subgroup (HR 8.45, 95% CI [1.70; 43.00], p = 0.010) and in the analysis of the combined cohort (HR 4.21, 95% CI [1.50; 12.00], p = 0.007). Detailed results of the univariate and multivariate Fine and Gray subdistribution hazard model (death as competing risk) for the endpoint of LC are displayed in Table 5 and Supplementary Tables 7–9, as well as Fig. 1c.
For individual lesions treated with SRS, LC was 91.7% after 12 months. 26 of 313 treated lesions developed progressive contrast enhancement. In 8 cases, tumor progression was diagnosed on the basis of radiological workup and overall clinical evaluation; in the rest of the cases, radiation induced changes (RIC) were suspected. In 12 of the cases with suspected RIC, patients had received targeted systemic therapy with either pembrolizumab or afatinib during or following SRS. Neurosurgical resection was performed in two of those cases where diagnosis was uncertain, with pathology confirming the suspected radionecrosis and finding no vital tumor cells in the sample. Four additional cases were confirmed clinically, as contrast enhancement regressed again following treatment with dexamethasone and bevacizumab in analogy to the findings of Levin et al. [19].
Discussion
A growing body of literature has recently been published, reporting on patients with multiple BM treated with SRS. The largest cohort has been described by Yamamoto et al. in a prospective observational study (JLGK0901) and included 1194 patients with 1–10 BM [3, 20, 21]. Survival was found not to differ significantly between patients with 2–4 and 5–10 BM, yielding a median OS of 10.8 months for those patients. Survival rates of 7.5 to 10.5 months have been reported in other, mainly retrospective analyses [22,23,24,25]. Highly favorable survival rates exceeding 21 months (median OS not reached) have been reported in a carefully selected cohort of patients treated with repeated SRS [26]. At 15.7 months, the median OS found in the SRS subgroup of our analysis compares favorably to the figures reported in recent literature and surpasses the survival rates reported by Yamamoto et al. for the prognostically superior group with only one BM (median OS 13.9 months). Baseline characteristics in our analysis were comparable to those in the JLGK0901 cohort, regarding cumulative BM volume per patient in particular, which was described as significant to OS [3, 27]. Other factors prognostic for OS included KPI, sex and especially extracerebral tumor control [3, 27]. The latter characteristics were confirmed as significant prognosticators in our analysis for the SRS subgroup and improved extracranial tumor control with the use of novel systemic agents probably contributing to the improved OS found in this analysis.
The OS rates reported in the four phase III trials comparing WBRT alone with WBRT + SRS for patients with 1–3 BM ranged between 6.5 and 10.4 months with a notable trend towards improved OS in the more recent trials [4, 5, 28, 29]. Since OS in those trials did not significantly differ between WBRT and WBRT + SRS, survival in the WBRT subcohort of the present analysis at 8.0 months falls within the expected range based on available data.
We found OS to be significantly longer for patients receiving SRS compared to WBRT. Although patients in this analysis were matched pairwise for seven potential prognostic factors, a residual selection bias cannot be eliminated as one possible reason for this difference. Typically, patients with a more favorable prognosis would be more likely selected to receive SRS instead of WBRT, whenever feasible. In clinical routine, this decision is based on the overall case constellation, considering established prognostic factors such as KPI and RPA / GPA scores, but also other aspects not herein represented, such as the extent of systemic tumor burden, comorbidities and the availability of systemic treatment options. The past years have seen notable improvements in OS for different histologies due to the advent of immunotherapy and similar targeted therapies [30,31,32,33,34]. Consequently, we hypothesized that an earlier timepoint of BM diagnosis could possibly have negatively influenced the systemic treatment patients received and indirectly overall prognosis. To better account for the influence of systemic treatment, on which comprehensive information was not available, we included the timepoint of treatment in our OS analysis as a surrogate parameter. Here, no relevant effect was observed, leading to the conclusion that in our analysis, the effect on OS of differing systemic therapies over time was less pronounced than originally assumed.
The factors chosen for pairwise matching have previously been prognostic for OS in prospective clinical trials and have been employed in several matched-pair analyses for patients with BM [12, 35,36,37,38]. However, those analyses have focused on patients receiving WBRT. It has been discussed that for patient collectives receiving SRS, established prognostic scores such as RPA and GPA might not be valid [39, 40]. Badakhshi et al. recently analyzed the validity of five established prognostic scores on a collective of 80 melanoma patients with BM treated with SRS and found that the prognostic accuracy was not ideal for this particular constellation [40]. Those results are plausible, particularly in the light of melanoma being among the entities for which overall prognosis was most decisively influenced by the introduction of immunotherapy [41]. Similarly promising early results have been shown regarding the CNS activity of Pembrolizumab in NSCLC with prolonged response of BM and improved OS and prospective trials on the subject are ongoing [42]. Melanoma and NSCLC patients made up for 65% of the patients in our analysis and in agreement with the data discussed above, we found low prognostic accuracy for the established RPA and GPA scores.
For patients receiving WBRT, the CNS spread is typically decisive for overall prognosis [28, 43, 44]. One reason lies in the dose of WBRT, which is limited by normal tissue tolerance and generally insufficient for lasting tumor control [12, 28]. In the case of intracerebral failure, which frequently occurs, options for salvage therapy are limited. On the contrary, doses applied during SRS are locally ablative and provide excellent local control of the irradiated lesion of around 90% after 12 months, as confirmed in our analysis [14, 45]. Additionally, subsequent to SRS, patients included in our analysis were typically followed up rigorously, including high-resolution MRI. Accordingly, the occurrence of new BM would receive timely salvage therapy, if feasible by repeated SRS. The consequence is a reduction of the impact of CNS spread on survival for SRS patients, since this CNS spread can be controlled by a combination of locally ablative doses and an effective means of salvaging newly occurring BM.
Median distant intracranial progression-free survival in the current analysis was 8.6 months following SRS and 22.4 months following WBRT, differing significantly between those subgroups. This finding is in agreement with previously published studies that have found the addition of WBRT to SRS to significantly improve intracranial progression-free survival, compared to SRS alone, though not overall survival [4, 6, 12, 28]. However, a randomized comparison of WBRT alone vs. SRS alone for patients with multiple BM, has not yet been done. We found a higher number of BM to be associated with inferior icPFS in the SRS and WBRT subgroups, as well as in the analysis of the combined cohort. Recently, Farris et al. proposed the brain metastasis velocity score (BMV) as a new metric to quantify the relationship between early distant brain failure and survival and similarly found a higher number of BM to be associated with a shorter interval to distant intracranial failure [46]. The BMV was subsequently validated by Yamamoto et al. in a cohort of 833 patients treated with repeated SRS [47].
Intracerebral failure following WBRT was predominantly local in our analysis (progression of pre-existing BM) and not due to new BM, suggesting that the dose of WBRT is insufficient to lastingly control macroscopic BM. Previous trials attempted to deliver a more adequate dose by combining SRS and WBRT or adding a stereotactic boost to minimize the risk of local failure, which was not done in the WBRT subgroup of the current analysis [4, 6, 12, 28]. To assess the effect of local dose escalation in the context of WBRT, we examined the patients who received a boost of 3 × 3 Gy to larger BM. We found that the delivery of a boost positively affected OS in the WBRT subgroup in univariate analysis, though not in multivariate analysis. An effect on LC or icPFS could not be detected, since here again the number of BM seemed to play a more dominant role, as discussed above.
When assessing LC following SRS on the basis of MR imaging, caution for diagnostic uncertainties has to be applied in the interpretation of results. Available literature suggests that in up to 70% of the cases where progressive contrast enhancement is detected in MRI, it is possibly rather due to radiation-induced changes/radionecrosis, than due to real tumor progression [48,49,50]. To date, no reliable radiologic or clinical criteria exist to differentiate between necrosis and tumor progression, although proposed methods include measuring the T1/T2 ratio on MRI, employing positron emission tomography (PET) or radiomics analyses [49, 51,52,53]. On the basis of individual risk–benefit assessment, neurosurgical resection—and thus pathologic confirmation—is only performed in a small minority of cases, usually not exceeding 30% [48, 54]. In our analysis, the majority of cases (70%) with progressive contrast enhancement were attributed to radiation-induced changes and in 2 cases pathologic confirmation was obtained. Four additional cases were confirmed clinically, as contrast enhancement regressed again following treatment with dexamethasone and bevacizumab in analogy to the findings of Levin et al. (19).
Limitations of this analysis include its retrospective design with inherent selection bias, as well as the relatively small number of patients. Though a matched-pair design was chosen to minimize selection bias, as well as imbalances between treatment groups, a residual bias possibly remains, as was discussed in detail. A further limitation is the unavailability of detailed information regarding systemic treatments received concurrent to and following radiotherapy. With modern substances rapidly gaining relevance to the prognosis of BM, future analyses will have to be adjusted for this potential confounder.
Our analysis is strengthened by its rigorous matching of patients for prognostic factors to minimize bias. The use of a matched-pair design allowed for the consideration of seven prognostic factors, resulting in a higher number of matching variables than would have been possible e.g. with the use of a propensity score with respect to underlying sample size. Due to the high overall mortality in patient collectives with BM, accuracy of the assessment of local control endpoints is often limited in comparable analyses in literature. By considering death as a competing event in our analysis, we have adequately addressed this caveat. Lastly, separate assessment of local and distant intracranial control allowed us to recognize differences in the primary pattern of failure following SRS or WBRT, as discussed above.
Conclusion
To the best of our knowledge and in the absence of a randomized trial, this is the first analysis to systematically compare SRS alone versus WBRT alone for multiple BM with means of matched-pair analysis. Irrespective of a possible residual selection bias, patient survival was prolonged in the SRS subgroup and generally favorable in the entire cohort. Prognostic factors for OS included the initial number of metastases, clinical performance, extracerebral tumor control, age and RPA class. WBRT prolonged distant brain control, though not improving survival. Intracranial failure was seen predominantly in irradiated lesions following WBRT and in the distant brain following SRS. Our results suggest SRS to be a feasible and effective treatment for patients with multiple BM.
References
Weller M (2015) Leitlinien für Diagnostik und Therapie in der Neurologie: Hirnmetastasen und Meningeosis neoplastica. DGN 30:1–42
National Comprehensive Cancer Network (2016) Central nervous system cancers V1.2019, p 123. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf
Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S et al (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15:387–395. https://doi.org/10.1016/S1470-2045(14)70061-0
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH et al (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10:1037–1044. https://doi.org/10.1016/S1470-2045(09)70263-3
Aoyama H, Tago M, Kato N, Toyoda T, Kenjyo M, Hirota S, Shioura H, Inomata T, Kunieda E, Hayakawa K et al (2007) Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys 68:1388–1395. https://doi.org/10.1016/j.ijrobp.2007.03.048
Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, Greenspoon J, Parney IF, Laack NNI, Ashman JB et al (2017) Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 18:1049–1060. https://doi.org/10.1016/S1470-2045(17)30441-2
Adler JR, Chang SD, Murphy MJ, Doty J, Geis P, Hancock SL (1997) The Cyberknife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg 69:124–128
Leksell L (1951) The stereotaxic method and radiosurgery of the brain. Acta Chir Scand 102:316–319
Gregucci F, Fiorentino A, Corradini S, Figlia V, Mazzola R, Ricchetti F, Ruggieri R, Alongi F (2018) Linac-based radiosurgery or fractionated stereotactic radiotherapy with flattening filter-free volumetric modulated arc therapy in elderly patients. Strahlentherapie und Onkol. https://doi.org/10.1007/s00066-018-1405-0
Thomas EM, Popple RA, Wu X, Clark GM, Markert JM, Guthrie BL, Yuan Y, Dobelbower MC, Spencer SA, Fiveash JB (2014) Comparison of plan quality and delivery time between volumetric arc therapy (rapidarc) and gamma knife radiosurgery for multiple cranial metastases. Neurosurgery 75:409–417. https://doi.org/10.1227/NEU.0000000000000448
Hofmaier J, Bodensohn R, Garny S, Hadi I, Fleischmann DF, Eder M, Dinc Y, Reiner M, Corradini S, Parodi K et al (2019) Single isocenter stereotactic radiosurgery for patients with multiple brain metastases: dosimetric comparison of VMAT and a dedicated DCAT planning tool. Radiat Oncol 14:103. https://doi.org/10.1186/s13014-019-1315-z
Rades D, Janssen S, Bajrovic A, Khoa MT, Veninga T, Schild SE (2017) A matched-pair analysis comparing whole-brain radiotherapy with and without a stereotactic boost for intracerebral control and overall survival in patients with one to three cerebral metastases. Radiat Oncol 12:10–15. https://doi.org/10.1186/s13014-017-0804-1
Kessel KA, Bohn C, Engelmann U, Oetzel D, Bougatf N, Bendl R, Debus J, Combs SE (2014) Five-year experience with setup and implementation of an integrated database system for clinical documentation and research. Comput Methods Prog Biomed 114:206–217. https://doi.org/10.1016/j.cmpb.2014.02.002
Kocher M, Wittig A, Piroth MD, Treuer H, Seegenschmiedt H, Ruge M, Grosu AL, Guckenberger M (2014) Stereotactic radiosurgery for treatment of brain metastases: a report of the DEGRO working group on stereotactic radiotherapy. Strahlentherapie und Onkol 190:521–532. https://doi.org/10.1007/s00066-014-0648-7
Soffietti R, Abacioglu U, Baumert B, Combs SE, Kinhult S, Kros JM, Marosi C, Metellus P, Radbruch A, Freixa SSV et al (2017) Diagnosis and treatment of brain metastases from solid tumors: guidelines from the European Association of neuro-oncology (EANO). Neuro Oncol 19:162–174. https://doi.org/10.1093/neuonc/now241
Rief H, Bischof M, Bruckner T, Welzel T, Askoxylakis V, Rieken S, Lindel K, Combs S, Debus J (2013) The stability of osseous metastases of the spine in lung cancer—a retrospective analysis of 338 cases. Radiat Oncol 8:200. https://doi.org/10.1186/1748-717X-8-200
Scharp M, Hauswald H, Bischof M, Debus J, Combs SE (2014) Re-irradiation in the treatment of patients with cerebral metastases of solid tumors: retrospective analysis. Radiat Oncol 9:4. https://doi.org/10.1186/1748-717X-9-4
Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343–346
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Prabhu S, Loghin M, Gilbert MR, Jackson EF (2011) Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol 79:1487–1495. https://doi.org/10.1016/j.ijrobp.2009.12.061
Shuto T, Akabane A, Yamamoto M, Serizawa T, Higuchi Y, Sato Y, Kawagishi J, Yamanaka K, Jokura H, Yomo S et al (2018) Multiinstitutional prospective observational study of stereotactic radiosurgery for patients with multiple brain metastases from non–small cell lung cancer (JLGK0901 study—NSCLC). J Neurosurg 129:86–94. https://doi.org/10.3171/2018.7.GKS181378
Yamamoto M, Serizawa T, Higuchi Y, Sato Y, Kawagishi J, Yamanaka K, Shuto T, Akabane A, Jokura H, Yomo S et al (2017) A multi-institutional prospective observational study of stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901 study update): irradiation-related complications and long-term maintenance of mini-mental state examination scores. Int J Radiat Oncol Biol Phys 99:31–40. https://doi.org/10.1016/j.ijrobp.2017.04.037
Karlsson B, Hanssens P, Wolff R, Söderman M, Lindquist C, Beute G (2009) Thirty years’ experience with Gamma Knife surgery for metastases to the brain: clinical article. J Neurosurg 111:449–457. https://doi.org/10.3171/2008.10.JNS08214
Harris KB, Corbett MR, Mascarenhas H, Lee KS, Arastu H, Leinweber C, Ju AW (2017) A single-institution analysis of 126 patients treated with Stereotactic radiosurgery for brain metastases. Front Oncol 7:1–6. https://doi.org/10.3389/fonc.2017.00090
Nagtegaal S, Claes A, Suijkerbuijk K, Schramel F, Snijders T, Verhoeff J (2019) Comparing survival predicted by the diagnosis-specific graded prognostic assessment (DS-GPA) to actual survival in patients with 1–10 brain metastases treated with stereotactic radiosurgery. Radiother Oncol 138:173–179. https://doi.org/10.1016/j.radonc.2019.06.033
Hamel-Perreault E, Mathieu D, Masson-Cote L (2019) Factors influencing the outcome of stereotactic radiosurgery in patients with five or more brain metastases. Curr Oncol 26:e64–e69. https://doi.org/10.3747/co.26.4244
Fritz C, Borsky K, Stark LS, Tanadini-Lang S, Kroeze SGC, Krayenbühl J, Guckenberger M, Andratschke N (2018) Repeated courses of radiosurgery for new brain metastases to defer whole brain radiotherapy: feasibility and outcome with validation of the new prognostic metric brain metastasis velocity. Front Oncol 8:1–9. https://doi.org/10.3389/fonc.2018.00551
Ali MA, Hirshman BR, Wilson B, Carroll KT, Proudfoot JA, Goetsch SJ, Alksne JF, Ott K, Aiyama H, Nagano O et al (2017) Survival patterns of 5750 stereotactic radiosurgery-treated patients with brain metastasis as a function of the number of lesions. World Neurosurg 107(944–951):e1. https://doi.org/10.1016/j.wneu.2017.07.062
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary J et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672. https://doi.org/10.1016/S0140-6736(04)16250-8
Kocher M, Soffietti R, Abacioglu U, Villà S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C et al (2011) Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952–26001 study. J Clin Oncol 29:134–141. https://doi.org/10.1200/JCO.2010.30.1655
Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, Bohnet S, Zhou C, Lee KH, Nogami N et al (2018) CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol 36:3290–3297. https://doi.org/10.1200/JCO.2018.78.3118
Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, Wilmott JS, Edwards J, Gonzalez M, Scolyer RA et al (2018) Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 19:672–681. https://doi.org/10.1016/S1470-2045(18)30139-6
Rulli E, Legramandi L, Salvati L, Mandala M (2019) The impact of targeted therapies and immunotherapy in melanoma brain metastases: a systematic review and meta-analysis. Cancer. https://doi.org/10.1002/cncr.32375
Shepard MJ, Xu Z, Donahue J, Eluvathingal Muttikkal TJ, Cordeiro D, Hansen L, Mohammed N, Gentzler RD, Larner J, Fadul CE et al (2019) Stereotactic radiosurgery with and without checkpoint inhibition for patients with metastatic non-small cell lung cancer to the brain: a matched cohort study. J Neurosurg. https://doi.org/10.3171/2019.4.jns19822
Singh C, Qian JM, Yu JB, Chiang VL (2019) Local tumor response and survival outcomes after combined stereotactic radiosurgery and immunotherapy in non-small cell lung cancer with brain metastases. J Neurosurg. https://doi.org/10.3171/2018.10.jns181371
Rades D, Kueter JD, Veninga T, Gliemroth J, Schild SE (2009) Whole brain radiotherapy plus stereotactic radiosurgery (WBRT + SRS) versus surgery plus whole brain radiotherapy (OP + WBRT) for 1–3 brain metastases: results of a matched pair analysis. Eur J Cancer 45:400–404. https://doi.org/10.1016/j.ejca.2008.10.033
Rades D, Kueter JD, Pluemer A, Veninga T, Schild SE (2009) A matched-pair analysis comparing whole-brain radiotherapy plus stereotactic radiosurgery versus surgery plus whole-brain radiotherapy and a boost to the metastatic site for one or two brain metastases. Int J Radiat Oncol Biol Phys 73:1077–1081. https://doi.org/10.1016/j.ijrobp.2008.05.035
Rades D, Janssen S, Dziggel L, Blanck O, Bajrovic A, Veninga T, Schild SE (2017) A matched-pair study comparing whole-brain irradiation alone to radiosurgery or fractionated stereotactic radiotherapy alone in patients irradiated for up to three brain metastases. BMC Cancer 17:1–7. https://doi.org/10.1186/s12885-016-2989-3
Viani G, Godoi Da Silva L, Viana B, Rossi B, Suguikawa E, Zuliani G (2016) Whole brain radiotherapy and stereotactic radiosurgery for patients with recursive partitioning analysis i and lesions < 5 cm3: a matched pair analysis. J Cancer Res Ther 12:770–774. https://doi.org/10.4103/0973-1482.179092
Malouff T, Bennion NR, Verma V, Martinez GA, Balkman N, Bhirud A, Smith T, Lin C (2016) Which prognostic index is most appropriate in the setting of delayed stereotactic radiosurgery for brain metastases? Front Oncol 6:1–8. https://doi.org/10.3389/fonc.2016.00248
Badakhshi H, Engeling F, Budach V, Ghadjar P, Zschaeck S, Kaul D (2018) Are prognostic indices for brain metastases of melanoma still valid in the stereotactic era? Radiat Oncol 13:1–6. https://doi.org/10.1186/s13014-017-0951-4
Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, Tsiouris AJ, Cohen J, Vortmeyer A, Jilaveanu L et al (2016) Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 17:976–983. https://doi.org/10.1016/S1470-2045(16)30053-5
Kluger HM, Chiang V, Mahajan A, Zito CR, Sznol M, Tran T, Weiss SA, Cohen JV, Yu J, Hegde U et al (2019) Long-Term Survival of Patients With Melanoma With Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial. J Clin Oncol 37:52–60. https://doi.org/10.1200/JCO.18.00204
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745–751. https://doi.org/10.1016/S0360-3016(96)00619-0
Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W (2008) A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys 70:510–514. https://doi.org/10.1016/j.ijrobp.2007.06.074
Yamamoto M, Kawabe T, Higuchi Y, Sato Y, Nariai T, Watanabe S, Barfod BE, Kasuya H (2014) Validity of prognostic grading indices for brain metastasis patients undergoing repeat radiosurgery. World Neurosurg 82:1242–1249. https://doi.org/10.1016/j.wneu.2014.08.008
Farris M, McTyre ER, Cramer CK, Hughes R, Randolph DM, Ayala-Peacock DN, Bourland JD, Ruiz J, Watabe K, Laxton AW et al (2017) Brain metastasis velocity: a novel prognostic metric predictive of overall survival and freedom from whole-brain radiation therapy after distant brain failure following upfront radiosurgery alone. Int J Radiat Oncol Biol Phys 98:131–141. https://doi.org/10.1016/j.ijrobp.2017.01.201
Yamamoto M, Aiyama H, Koiso T, Watanabe S, Kawabe T, Sato Y, Higuchi Y, Kasuya H, Barfod BE (2019) Validity of a recently proposed Prognostic Grading Index, brain metastasis velocity, for patients with brain metastasis undergoing multiple radiosurgical procedures. Int J Radiat Oncol Biol Phys 103:631–637. https://doi.org/10.1016/j.ijrobp.2018.10.036
Fujimoto D, von Eyben R, Gibbs IC, Chang SD, Li G, Harsh GR, Hancock S, Fischbein N, Soltys SG (2018) Imaging changes over 18 months following stereotactic radiosurgery for brain metastases: both late radiation necrosis and tumor progression can occur. J Neurooncol 136:207–212. https://doi.org/10.1007/s11060-017-2647-x
Kohutek ZA, Yamada Y, Chan TA, Brennan CW, Tabar V, Gutin PH, Jonathan Yang T, Rosenblum MK, Ballangrud Å, Young RJ et al (2015) Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J Neurooncol 125:149–156. https://doi.org/10.1007/s11060-015-1881-3
Jagannathan J, Bourne TD, Schlesinger D, Yen CP, Shaffrey ME, Laws ER, Sheehan JP (2010) Clinical and pathological characteristics of brain metastasis resected after failed radiosurgery. Neurosurgery 66:208–217. https://doi.org/10.1227/01.NEU.0000359318.90478.69
Campos B, Neumann J-O, Hubert A, Adeberg S, El Shafie R, von Deimling A, Bendszus M, Debus J, Bernhardt D, Unterberg A (2020) Analysis of a surgical series of 21 cerebral radiation necroses. World Neurosurg. https://doi.org/10.1016/j.wneu.2020.02.005
Lohmann P, Kocher M, Ceccon G, Bauer EK, Stoffels G, Viswanathan S, Ruge MI, Neumaier B, Shah NJ, Fink GR et al (2018) Combined FET PET/MRI radiomics differentiates radiation injury from recurrent brain metastasis. NeuroImage Clin 20:537–542. https://doi.org/10.1016/j.nicl.2018.08.024
Peng L, Parekh V, Huang P, Lin DD, Sheikh K, Baker B, Kirschbaum T, Silvestri F, Son J, Robinson A et al (2018) Distinguishing true progression from radionecrosis after stereotactic radiation therapy for brain metastases with machine learning and radiomics. Int J Radiat Oncol Biol Phys 102:1236–1243. https://doi.org/10.1016/j.ijrobp.2018.05.041
Kim JM, Miller JA, Kotecha R, Xiao R, Juloori A, Ward MC, Ahluwalia MS, Mohammadi AM, Peereboom DM, Murphy ES et al (2017) The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J Neurooncol 133:357–368. https://doi.org/10.1007/s11060-017-2442-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare they have no conflicts of interest relevant to the scope of this manuscript.
Ethical approval
All procedures and analyses performed in the present work were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Ethics approval for the study and a waiver of written informed consent was granted by the Heidelberg University ethics committee on April 12th, 2018 (#S-172/2018). Patient confidentiality was maintained by anonymizing patient data to remove any identifying information.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
El Shafie, R.A., Celik, A., Weber, D. et al. A matched-pair analysis comparing stereotactic radiosurgery with whole-brain radiotherapy for patients with multiple brain metastases. J Neurooncol 147, 607–618 (2020). https://doi.org/10.1007/s11060-020-03447-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03447-2