Abstract
Introduction
Diffuse intrinsic pontine glioma (DIPG) is a type of brain malignancy with a very poor prognosis. Although various radiation and chemotherapy protocols have been attempted, only conventional radiotherapy has yielded improvements in survival. In this study, we aimed to compare proton therapy versus conventional photon radiotherapy in terms of the outcomes of pediatric patients with DIPG.
Methods
This retrospective review included 12 pediatric patients with newly diagnosed DIPG who received a total proton therapy dose of 54 Gy (relative biological effectiveness) in 30 fractions at the University of Tsukuba Hospital between 2011 and 2017 (proton group). We additionally reviewed the medical records of 10 patients with DIPG who previously underwent conventional photon radiotherapy at our institute (historical cohort).
Results
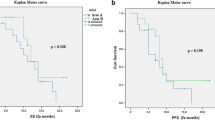
The median progression-free survival (PFS) duration was 5 months (range 1–11 months), and the 6-, 12-, and 18-month PFS rates were 50%, 33%, and 25%, respectively. The median overall survival (OS) duration was 9 months (range 4–48 months), and the 6-, 12-, 18-, and 24-month OS rates were 66.8%, 50%, 41%, and 20%, respectively. There were no significant differences in survival between the proton and historical groups (PFS, p = 0.169 and OS, p = 0.16).
Conclusions
Proton therapy was well tolerated by the majority of patients. No severe adverse events, including radiation necrosis, were recorded. Proton therapy did not yield superior survival outcomes vs. conventional photon radiotherapy in patients with DIPG at our institution. Further research is needed to identify the factors associated with better survival in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain tumors comprise the second most common type of neoplasm arising in children, accounting for approximately 20% of all childhood cancers [1]. In recent decades, advances in our understanding of tumor biology and the available treatment strategies and modalities have led to improved survival outcomes for patients with most types of cancer, including brain tumors. Despite these improvements, brain tumors remain a main cause of cancer-related mortality in children. Moreover, some of these tumors continue to be associated with a poor prognosis.
Diffuse intrinsic pontine glioma (DIPG) is the most aggressive type of brain tumor. These lesions originate in the pons and are diagnosed in patients at median ages ranging from 6 to 8 years [2]. Despite collaborative efforts to improve treatments, the survival of patients with DIPG has remained static over the past decades, and this tumor type is now the main cause of pediatric brain tumor-related deaths. Despite extensive efforts to treat these devastating tumors, including hypofractionated or hyperfractionated radiotherapy and chemotherapies, no previously introduced treatment protocol has led to significantly improved survival outcomes in patients with DIPGs.
Proton therapy is a type of particle radiotherapy that features excellent dose localization and a low level of late toxicity due to a reduction in the irradiation dose to the surrounding normal brain tissues. Accordingly, proton therapy may help to prevent treatment-related late sequelae, including neurocognitive impairments and secondary cancers, in children requiring radiotherapy for brain tumors [3]. In Japan, the public insurance system began to cover the costs associated with proton therapy for childhood cancers in 2016, which led to a subsequent increase in the number of patients undergoing this treatment at our institute. Here, we report our institutional experiences with the administration of first-line proton therapy to consecutive patients with DIPG at the University of Tsukuba Hospital.
Materials and methods
We retrospectively reviewed the medical records, including clinical characteristics, additional treatments, and survival outcomes, of 12 children with DIPG who received proton therapy at the University of Tsukuba Hospital from 2011 to 2017 (proton group). We additionally reviewed the medical records of 10 patients with DIPG who underwent conventional photon radiotherapy at our institute from 1984 to 2004 (historical group). All patients underwent gadolinium-enhanced magnetic resonance imaging (MRI) at the time of diagnosis. Accordingly, a diagnosis of DIPG was based on the following imaging characteristics: an intrinsic central location occupying > 50% of the axial diameter of the pons, an indistinct tumor margin, hypointensity on T1-weighted images, and hyperintensity on T2-weighted images. A biopsy was performed if the radiological diagnosis was uncertain.
Standard fractionated proton therapy was administered using our typical radiation planning volumes. Computed tomography (CT) images of the treatment site were obtained at 3-mm intervals for proton therapy planning. Proton beams with energies of 250 MeV were generated using a booster synchrotron at the Proton Medical Research Center (PMRC). The treatment planning system determined the dose distributions and collimator configuration, bolus, and range-shifter thickness settings. A relative biological effectiveness (RBE) of 1.1 was assumed.
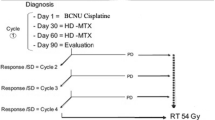
All subjects underwent MRI immediately before starting proton therapy. The gross tumor volume (GTV) was defined as the area of hyperintensity on T2-weighted images. The clinical target volume (CTV) was defined as the GTV plus a 5- to 10-mm margin, and the planning target volume (PTV) was defined as the CTV plus a 2- to 3-mm margin. During proton therapy, a total dose of 54.0 Gy (RBE) in 30 fractions was delivered to the PTV. The exposure dose was adjusted to ≤ 50 Gy (RBE) for the chiasm, and the margin of the lesion was reduced if the dose to an organ at risk exceeded the exposure dose (Fig. 1). Oral temozolomide was administered concurrently at a dosage of 75 mg/m2 for 42 days as a radiosensitizer. After completing proton therapy, all patients were evaluated via physical examinations and MRI. Disease progression was defined as neurological deterioration confirmed by MRI. Recurrence or progression was defined as an increase of at least 25% in the tumor size, as measured by the product of the 2 largest perpendicular dimensions on either the gadolinium-enhanced T1-weighted image, if the tumor was enhanced, or the T2-weighted image. Acute and late radiation-induced toxicities were evaluated according the Common Terminology Criteria for Adverse Events (CTCAE) (version 4.0).
Axial T2-weighted image of a 4-year-old boy who received proton therapy (a). Proton treatment with a total dose of 54 Gy (RBE) to the clinical target volume (red line), with excellent sparing of normal brain tissue (outside of blue line) (b). Axial T2-weighted image of a 6-year-old girl treated with photon radiotherapy (c). Dose distribution demonstrating that most brain tissue was within 40% of the total irradiation dose (green line) (d)
Statistical analyses were performed using SPSS 25.0. Progression-free survival (PFS) was defined as the interval between diagnosis and clinical and/or radiologic tumor progression or death. Overall survival (OS) was defined as the interval between diagnosis and death or the last date of follow-up. The Kaplan–Meier method was used to calculate OS and PFS. A log-rank test was performed to evaluate differences in survival.
Results
Patients treated with proton therapy
The clinical information and outcomes of the 12 children who received proton therapy are presented in Table 1. The median age at diagnosis was 5.8 years (range 4.0–9.9 years). The mean interval from radiological diagnosis to the first radiation treatment dose was 17 days (range 6–27 days), and the mean treatment duration was 47.5 days (range 45–50 days). One patient with an exophytic feature underwent biopsy, which a pathologic analysis identified as a high-grade glioma. Two patients underwent fenestration of intra-tumoral cysts that became enlarged after the completion of proton therapy. Three patients required a ventriculoperitoneal shunt for hydrocephalus after disease progression. Two patients had to stop temozolomide because of severe nausea and vomiting.
Proton therapy was tolerated well by the majority of the patients. The most commonly reported toxicities during treatment were grade ≤ 2 and included alopecia in the irradiated area (n = 12), nausea (n = 4), a decreased lymphocyte count (n = 4), vomiting (n = 2), bullous dermatitis (n = 1), and allergic reaction (n = 1). One patient experienced a grade 3 decrease in the neutrophil count. Four patients discontinued oral temozolomide administration because of toxicities (vomiting, allergy, and neutrocytopenia). No patient required an interruption of proton therapy because of treatment-related toxicity. No deaths related to toxicity occurred during the treatment period.
We used the Karnovsky performance status (KPS) to evaluate the patients’ statuses. Six (50%) patients had a KPS of < 70 at diagnosis. At the end of proton therapy, 2 patients (17%) had a KPS of 70, while 2 became symptom-free and 3 exhibited significant improvements. In 1 patient, the KPS deteriorated during proton therapy, from 60, requires occasional assistance, to 30, severely disabled. After progression, most patients withdrew from chemotherapy and were followed up in a palliative care setting and at home whenever possible. Four patients initiated bevacizumab administration after temozolomide discontinuation upon receiving confirmation of disease progression. One patient whose glioma progressed to disseminated disease underwent proton therapy for a distant lesion. Two patients underwent palliative proton reirradiation at a total dose of 21.6 Gy (RBE) in 12 fractions concomitantly with bevacizumab therapy. One of these patients experienced symptom improvement for 8 months after reirradiation but died 2 months after the second progression.
Historical group
As described in the “Materials and methods”, we also evaluated 10 patients who underwent conventional photon radiotherapy for DIPG at our institute as the historical group. The median age at diagnosis was 8.6 years (range 4.3–15.9 years), and the total irradiation doses ranged from 36 to 51.2 Gy, with a median dose of 54.9 Gy. Concomitant chemotherapy, including ACNU, carboplatin, etoposide, and procarbazine, was administered to 5 patients. One patient discontinued irradiation due to disease progression.
Outcome
In the proton group, the median progression-free survival (PFS) duration was 5 months (range 1–11 months), and the 6-, 12-, and 18-month PFS rates were 50%, 33%, and 25%, respectively. In the same group, the median overall survival (OS) duration was 9 months (range 4–48 months), and the 6-, 12-, 18-, and 24-month OS rates were 66.8%, 50%, 41%, and 20%, respectively. For comparison, the historical group treated with photon radiotherapy had median PFS and OS durations of 5 and 11 months, respectively. A log-rank comparison of the two treatment groups (proton vs. historical) did not reveal significant differences in survival (PFS, p = 0.169 and OS, p = 0.16) (Fig. 2).
Discussion
Despite recent advances in the field of pediatric neuro-oncology, DIPG remains the most challenging disease. Particularly, a diagnosis is often based solely on MRI findings, while radiation is the only treatment proven to prolong PFS effectively and provide transient but limited improvements in symptoms and neurologic function. Undoubtedly, recurrence and progression would eventually lead to death related the burden of disease, as indicated by median PFS and OS durations of 5–9 and 8–12 months in previous reports and 1-, 2-, and 5-year OS rates of approximately 40%, 10%, and almost 0%, respectively.
To date, numerous clinical trials have explored potential treatments for DIPG. However, local irradiation with a total dose of 54 Gy over a 6-week period remains the most standardized treatment protocol, as previous studies of hyperfractionated radiotherapy with total doses of 70.2–72 Gy did not yield further improvements in the patients’ outcomes [4,5,6]. More recently, however, studies reported comparable outcomes with shorter hypofractionated radiotherapy protocols that delivered total doses of 39 Gy (3 Gy/Fr) or 44.8 Gy (2.8Gy/Fr) vs. conventional radiotherapy [7, 8]. The authors of those reports emphasized the advantages of reducing the treatment period and thus reducing the burdens on patients and their families.
Several chemotherapeutic agents have also been tested for the treatment of DIPG. However, the role of chemotherapy in the treatment of pediatric DIPG remains unclear. In a study of the BSG 98 protocol, which combines pre-radiation chemotherapy with delayed irradiation, Frappaz et al. reported a median OS of 17 months [9]. This result was subsequently confirmed by Gokce-Samar et al. [10]. Most other studies, including those of high-dose chemotherapy regimens, failed to demonstrate any additional survival benefits [2]. A recent molecular profiling study identified a mutation of H3K27M as a pathognomonic feature of pediatric glioblastomas, including DIPG [11,12,13]. Although these advances in molecular profiling have led to the development of new targeted approaches to the treatment of DIPGs, their efficacies remain unproven.
Both proton therapy and photon radiotherapy are categorized as forms of low linear energy transfer radiation, and both have a similar RBE. However, the proton beam produces a sharp energy peak called the Bragg peak, which spreads out to cover the tumor volume (spread out of the Bragg peak (SOBP)), while the energy level behind the peak is almost zero. Accordingly, proton therapy enables a reduction in the dose to the normal tissue around the tumor, unlike photon radiotherapy, which would be especially beneficial for a pediatric tumor or a tumor adjacent to highly radiosensitive normal tissue. Proton therapy may therefore provide consistent advantages in terms of reducing the areas receiving low and intermediate (0–40 Gy) doses [14], which in the context of a head and neck tumor could help to preserve intelligence, endocrine function, and hearing [15]. A nationwide retrospective cohort survey in Japan revealed that proton therapy was equally efficient and likely superior in terms of safety when compared with the results of previous studies of other therapies [16]. Accordingly, increasing numbers of patients with brain tumors have been treated using proton therapy.
Most pediatric solid tumors are classified into the highest-priority category for proton therapy. However, high-grade gliomas of the brain and brainstem might be exception, as the poor outcomes and short survival prognoses would likely render patients unable to benefit from the advantages of proton therapy. Furthermore, these tumors are highly likely to infiltrate beyond the initially defined tumor area, and a reduced dose to the surrounding tissues might also reduce the dose to the infiltrating tumor cells. Our results support these concerns, as proton therapy did not improve the PFS and OS outcomes in our study relative to previous reports and did not contribute to a reduction in late complications.
We note that 2 patients in our cohort underwent reirradiation upon disease progression. One patient exhibited a significant improvement in clinical symptoms and maintained an ambulatory status without assistance for 8 months, indicating a benefit from this therapy. Recent reports suggest that reirradiation could potentially improve the clinical course and survival outcomes in both adult and pediatric patients with progressive brain tumors, including DIPG [17, 18]. Moreover, a meta-analysis of reirradiation for DIPG revealed possible extensions of PFS and OS [19]. However, multiple irradiation elicits concerns about the risk of radiation necrosis. Although conventional radiotherapy is frequently the first line of treatment, reirradiation often involves more highly conformal radiation techniques, including intensity-modulated radiation therapy, stereotactic radiosurgery, and proton therapy, to avoid unfavorable acute toxicity [18]. Therefore, proton therapy might be useful for treating recurrent and progressive DIPGs. However, a well-established prospective study is needed for validation.
Radiotherapy is essential for the treatment of DIPG. Our study demonstrated that proton therapy and conventional radiotherapy are equally effective for the treatment of DIPG. However, the advantage of the former therapy, namely the prevention of late complications, is not beneficial in patients with this aggressive disease. Still, proton therapy can be used in a reirradiation setting to prevent radiation necrosis in the surrounding brain tissue. Furthermore, proton therapy will likely become the treatment of choice to avoid late sequelae once advances in treatment yield improved survival outcomes in patients with DIPG. This is the first report of the treatment outcomes of proton therapy for newly diagnosed pediatric DIPG. Although the outcome was not significant, our results will facilitate decisions regarding treatment options. Furthermore, our findings provide novel information about the treatment of pediatric brain tumors.
Conclusions
Proton therapy was well tolerated by the majority of our patients, and no patient required an interruption of proton therapy because of treatment-related toxicity. Theoretically, proton therapy may provide consistent advantages in terms of reducing the areas that receive low and intermediate doses. It will likely become the treatment of choice to avoid late sequelae once advances in treatment have led to improved survival outcomes in patients with DIPG. In this study, proton therapy did not appear to yield survival advantages when compared with previously reported treatment protocols in pediatric patients with DIPG. However, further research is needed to improve the survival outcomes in this patient population.
References
Nakata K, Ito Y, Magadi W, Bonaventure A, Stiller CA, Katanoda K, Matsuda T, Miyashiro I, Pritchard-Jones K, Rachet B (2017) Childhood cancer incidence and survival in Japan and England: a population-based study (1993-2010). Cancer Sci 109:422–434. https://doi.org/10.1111/cas.13457
Hargrave D, Bartels U, Bouffet E (2006) Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7:241–248. https://doi.org/10.1016/S1470-2045(06)70615-5
Mizumoto M, Oshiro Y, Yamamoto T et al (2017) Proton beam therapy for pediatric brain tumor. Neurol Med Chir (Tokyo) 57:343–355. https://doi.org/10.2176/nmc.ra.2017-0003
Allen J, Siffert J, Donahue B et al (1999) A phase I/II study of carboplatin combined with hyperfractionated radiotherapy for brainstem gliomas. Cancer 86:1064–1069
Mandell LR, Kadota R, Freeman C et al (1999) There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys 43:959–964
Jennings MT, Sposto R, Boyett JM et al (2002) Preradiation chemotherapy in primary high-risk brainstem tumors: phase II study CCG-9941 of the Children’s Cancer Group. J Clin Oncol 20:3431–3437. https://doi.org/10.1200/JCO.2002.04.109
Janssens GO, Jansen MH, Lauwers SJ et al (2013) Hypofractionation vs conventional radiation therapy for newly diagnosed diffuse intrinsic pontine glioma: a matched-cohort analysis. Int J Radiat Oncol Biol Phys 85:315–320. https://doi.org/10.1016/j.ijrobp.2012.04.006
Zaghloul MS, Eldebawy E, Ahmed S, Mousa AG, Amin A, Refaat A, Zaky I, Elkhateeb N, Sabry M (2014) Hypofractionated conformal radiotherapy for pediatric diffuse intrinsic pontine glioma (DIPG): a randomized controlled trial. Radiother Oncol 111:35–40. https://doi.org/10.1016/j.radonc.2014.01.013
Frappaz D, Schell M, Thiesse P, Marec-Bérard P, Mottolese C, Perol D, Bergeron C, Philip T, Ricci AC, Galand-Desme S, Szathmari A, Carrie C (2008) Preradiation chemotherapy may improve survival in pediatric diffuse intrinsic brainstem gliomas: final results of BSG 98 prospective trial. Neuro Oncol 10:599–607. https://doi.org/10.1215/15228517-2008-029
Gokce-Samar Z, Beuriat PA, Faure-Conter C, Carrie C, Chabaud S, Claude L, di Rocco F, Mottolese C, Szathmari A, Chabert C, Frappaz D (2016) Pre-radiation chemotherapy improves survival in pediatric diffuse intrinsic pontine gliomas. Childs Nerv Syst 32:1415–1423. https://doi.org/10.1007/s00381-016-3153-8
Schwartzentruber J, Korshunov A, Liu X-Y et al (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature:1–8. https://doi.org/10.1038/nature10833
Khuong-Quang D-A, Buczkowicz P, Rakopoulos P, Liu XY, Fontebasso AM, Bouffet E, Bartels U, Albrecht S, Schwartzentruber J, Letourneau L, Bourgey M, Bourque G, Montpetit A, Bourret G, Lepage P, Fleming A, Lichter P, Kool M, von Deimling A, Sturm D, Korshunov A, Faury D, Jones DT, Majewski J, Pfister SM, Jabado N, Hawkins C (2012) K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 124:439–447. https://doi.org/10.1007/s00401-012-0998-0
Wu G, Broniscer A, McEachron TA, Lu C, Paugh BS, Becksfort J, Qu C, Ding L, Huether R, Parker M, Zhang J, Gajjar A, Dyer MA, Mullighan CG, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Ellison DW, Zhang J, Baker SJ, St. Jude Children's Research Hospital–Washington University Pediatric Cancer Genome Project (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44:251–253. https://doi.org/10.1038/ng.1102
Merchant TE, Hua C-H, Shukla H et al (2008) Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer 51:110–117. https://doi.org/10.1002/pbc.21530
Fukushima H, Fukushima T, Suzuki R, Iwabuchi A, Hidaka K, Shinkai T, Masumoto K, Muroi A, Yamamoto T, Nakao T, Oshiro Y, Mizumoto M, Sakurai H, Sumazaki R (2017) Comorbidity and quality of life in childhood cancer survivors treated with proton beam therapy. Pediatr Int 59:1039–1045. https://doi.org/10.1111/ped.13323
Mizumoto M, Murayama S, Akimoto T et al (2016) Proton beam therapy for pediatric malignancies: a retrospective observational multicenter study in Japan. Cancer Med 5:1519–1525. https://doi.org/10.1002/cam4.743
Navarria P (2019) Re-irradiation for recurrent glioma: outcome evaluation, toxicity and prognostic factors assessment. A multicenter study of the Radiation Oncology Italian Association (AIRO). J Neurooncol 142:59–67. https://doi.org/10.1007/s11060-018-03059-x
Rao AD, Rashid AS, Chen Q et al (2017) Reirradiation for recurrent pediatric central nervous system malignancies: a multi-institutional review. Int J Radiat Oncol Biol Phys 99:634–641. https://doi.org/10.1016/j.ijrobp.2017.07.026
Lu VM, Welby JP, Mahajan A, Laack NN, Daniels DJ (2019) Reirradiation for diffuse intrinsic pontine glioma: a systematic review and meta-analysis. Childs Nerv Syst 35:739–746. https://doi.org/10.1007/s00381-019-04118-y
Acknowledgments
We would like to thank Editage (www.editage.com) for the English language editing.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by AM, MM, TT, HF, and SI. The first draft of the manuscript was written by AM, MM, and EI and all authors commented on previous versions of the manuscript. HS, and AMa critically supervised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Ethics Committee of the University of Tsukuba Hospital.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muroi, A., Mizumoto, M., Ishikawa, E. et al. Proton therapy for newly diagnosed pediatric diffuse intrinsic pontine glioma. Childs Nerv Syst 36, 507–512 (2020). https://doi.org/10.1007/s00381-019-04420-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-019-04420-9