Abstract
Purpose
Children with unresectable brainstem-infiltrated ganglioglioma have poor progression-free survival when treated with conventional chemotherapy and radiation regimens. The BRAFV600E mutation occurs in a large number of gangliogliomas, making them amenable for targeted therapy using mutation-specific kinase inhibitors. However, limited data exists on the effectiveness and best treatment duration of these inhibitors in this tumor setting.
Method
Retrospective description of three cases of childhood brainstem ganglioglioma with BRAFV600E mutation treated in the long-term with Dabrafenib, a specific BRAFV600E kinase inhibitor.
Results
Dabrafenib resulted in rapid tumoral regression and significant and durable clinical and radiological improvement. However, all patients had rapid clinical and radiological relapse within days to weeks following treatment discontinuation but showed similar rapid and sustained therapeutic response when Dabrafenib was re-introduced. This targeted therapy has been well tolerated despite its long-term use of 4.8 to 5.5 years in the three patients.
Conclusion
Dabrafenib is effective and seemingly safe and well tolerated in our three patients. We observed sustained chemosensitivity even when re-introducing this kinase inhibitor after its discontinuation after 2 years of therapy. These cases indicate the need to re-evaluate the timing and means of Dabrafenib discontinuation in pediatric patients with BRAFV600E mutated gangliogliomas and better assess the future implications of its long-term use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ganglioglioma (GG) are rare dysplastic neuronal lineage glial cells tumors representing 1–4% of all pediatric central nervous system (CNS) tumors. Most patients usually present with low-grade histology. Review of 348 children’s with GG from surveillance, epidemiology, and results (SEER) cohort between 2004 and 2010 indicates the cerebral hemisphere as the more frequent location for this tumor, with only 3,7% of the cases occurring in the brainstem [1]. Median age at diagnosis is 4 years in tumors in the posterior fossa tumors (PF) and 10.2 years in supratentorial tumors (ST) in a recent case series [2].
GGs are usually associated with good prognosis due to their amenability to complete surgical resection [3, 4]. However, in some cases especially in tumors of the PF this is not possible due to tumor location and the infiltrative nature of the disease specifically in this location. Moreover, in these cases in need of adjuvant therapy, recent studies indicate that GG have a poor response to chemotherapy and radiation therapy [5,6,7]. The patient’s event-free survival is thus highly dependent on the extent of surgical resection, which varies with the anatomical location. Indeed, five-year overall survival is 96% for non-brainstem GG compared to 80% for patients with brainstem GG in the SEER review, notwithstanding the significant morbidity associated with local progression and adjuvant therapies in the later setting [1]. Using recent markers uncovered in low-grade gliomas, PF GG had previously been classified into two distinct molecular subgroups: classical GG where BRAFV600E mutation is identified in 43% of cases and atypical GG where BRAF duplication KIAA1549-BRAF fusion is seen in 82% of cases. Interestingly, a case series focusing on 13 GG of the brainstem, the most debilitating tumor location, identifies BRAFV600E mutation in 54% cases [8].
BRAF is an essential mediator in the MAP kinase signaling. The BRAFV600E mutation is the most common mutation and leads to constitutive kinase activation. It is highly prevalent in melanoma, but has also been described in other cancers including papillary thyroid cancer and colon carcinoma [9]. Specific inhibitors targeting this mutation namely Vemurafenib and Dabrafenib, have FDA approval for their use in melanoma [10]. They are now being used with success in clinical trials targeting these other cancers and, more recently, are being evaluated in clinical trials in pediatric cancers carrying this mutation including brain tumors. These inhibitors represent thus a potential novel therapy in BRAF-mutant GG refractory to surgical resection and standard chemotherapy. There have been recent few case reports of success in gliomas including GG, however other reports indicate possible resistance to these inhibitors following their use [11]. Also, while this therapy may be efficacious, there is no data on its long-term use and side-effects associated with long-term therapy especially in children. Moreover, data on optimal treatment duration and its discontinuation are currently lacking in the literature for pediatric GG. Here we report three cases of pediatric patients with GG successfully treated with Dabrafenib. These children were treated respectfully since 2014 and 2015 and are still successfully undergoing this therapy. They are part of the rare pediatric cases to have undergone this long-term therapy and on which follow-up clinical information is available.
Methods
Medical charts from all pediatric patients with GG who received Dabrafenib were retrospectively reviewed. Demographic data, pathology, tumor location, toxicity and progression-free survival (PFS) were retrieved. BRAFV600E was confirmed by PCR. Whole exome sequencing and RNA sequencing was conducted on case 1 and 2 based on material availability in these patients. Immunohistochemical analysis of TP53, P16 and H3K27M were retrospectively performed on all three cases as previously described [12]. Tumor response evaluation was based on modified RANO criteria (Response Assessment in Neuro-Oncology). The grading of toxicities is based on Common Terminology criteria for Adverse Events Version 4.0 (CTCAE). Local ethic committee approved this study.
Case series
Case 1
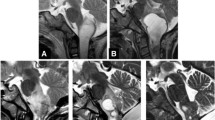
A 26-month-old female with a past medical history of asthma and overweight consulted the pediatric emergency room due to progressive sleep apnea and fatigability. Apart from macrocrania, physical exam was normal. Sleep monitoring confirmed mixed apnea, predominantly central. A brain MRI showed a cervicomedullary tumor extending to the brainstem, medulla and C1–C4 spinal cord. The patient underwent a debulking of the cervicomedullary tumor and histopathology confirmed a ganglioglioma WHO grade 1. The patient was started on weekly Vinblastine. The patient’s central sleep apnea improved initially but fatigue and minor intermittent dysphagia persisted. Ten months later, the bulbar component of the tumor increased significantly in size on the MRI. A second partial resection of the exophytic component of the brainstem tumor was done. Pneumopathy with deterioration of swallowing was noticed during the next few weeks, the patient required Bipap and gastrostomy for supportive care. Chemotherapy with Vincristine-Carboplatin was started. After 4 months of treatment, follow-up MRI showed significant tumoral progression with C6 infiltration. The patient underwent a third partial resection followed by local radiation of 54 Gy. Histopathology confirmed recurrent ganglioglioma with increased Ki-67 ranging from 2 to 10% with a BRAFV600E mutation identified in all three resections. One month later, left hemiparesis, daytime apnea, increased weakness, decreased fine motor skills and significant swallowing difficulty was present. Insertion of catheters into the cervical syrinx cavity was done to decompress the mass effect on the brainstem and a tracheostomy was needed for continuous ventilation. An MRI done 6 months following radiation therapy confirmed the progression of the enhancing component with surrounding oedema (Fig. 1a).
a Sagittal T2 Spine MRI following significant progression of the ganglioglioma before the treatment with Dabrafenib. b Sagittal T2 Spine MRI showing significant reduction of the tumor enhancement in the cervicomedullary region following 21 months of Dabrafenib. c Sagittal T2 Spine MRI showing a rapid growth of the bulbar component of the lesion 6 days following discontinuation of Dabrafenib. d Sagittal T2 Spine MRI showing a significant reduction of the tumor 1 month following re-introduction of Dabrafenib
The patient started Dabrafenib 50 mg twice daily (3.75 mg/kg/day) on a compassionate basis, at 2-years post-diagnosis. Over the next few weeks, she demonstrated significant global clinical improvement and was even able to enter kindergarten. Imaging showed significant reduction of the volume of the tumor and enhancement. Due to the lack of data on long-term effect of BRAF inhibitor and tumor response, the treatment was discontinued after 24 months (Fig. 1b). Six days after discontinuation, the patient was readmitted with increasing neurological symptoms, bradycardia and worsening of nocturnal apnea. The MRI done showed enhancing component of the bulbar lesion that tripled in size when compared to previous MRI at 21 months (Fig. 1b, c) and Dabrafenib twice daily at 50/100 mg was restarted. The patient recovered quickly, she left intensive care unit 48 h later and went back into clinical remission over several days. One month later, the MRI was comparable to the previous imaging before the discontinuation of Dabrafenib (Fig. 1d). Currently, this 10-year-old girl has a very good quality of life and has now been receiving Dabrafenib since 5.5 years without side effects. Retrospective analyses of the three resections showed the BRAFV600E mutation to be the only pathogenic event in all three tumor samples.
Case 2
A 15-year-old boy presented with weakness of the left upper extremity, sleep apnea, unstable gait and a modified voice due to a partial paralysis of the left vocal cord. The MRI revealed a cervicomedullary lesion. He underwent partial resection of the tumor. Pathology showed ganglioglioma WHO grade I with BRAFV600E mutation. He was observed for the next 4 months with seemingly stable disease before he rapidly progressed. He was then treated by focal radiation therapy of 54 Gy, which had mainly temporary benefits. Within seven months, he complained of progressive left hemiparesis, dysphagia, and ataxia, major changes to his voice and increased sleep apnea. Imaging revealed significant progression of the cervicomedullary ganglioglioma (Fig. 2a). Based on the presence of the BRAF mutation, he was started on Dabrafenib 150 mg po BID (5 mg/kg/day). Within 2 weeks of starting therapy, there was a drastic improvement in his gait, sleep apnea and voice. The treatment was very well tolerated, allowing the patient to resume his regular level of activity. A follow-up MRI showed significant partial response (Fig. 2b). The patient experienced grade I skin toxicities (light erythema of palms, arms and face, acne, an episode of tinea pedis). High blood pressure was also noted (grade II) and was treated by enalapril, but value above the 95th percentile preceded the start of Dabrafenib and was likely due to patient’s overweight problems (BMI 34 kg/cm2) and sleep apnea. Dabrafenib was stopped after a scheduled 24-month period. Six weeks after discontinuing Dabrafenib, his initial symptoms recurred, and an MRI confirmed disease progression (Fig. 2c). Dabrafenib was quickly restarted and within one month the MRI showed strong radiological and clinical response. Under this therapy, he continues to show stable disease following the significant response observed at treatment re-initiation and we are now over 2½ years after restarting Dabrafenib (Fig. 2d).
a Sagittal T2 Spine MRI before Dabrafenib treatment. b Sagittal T2 Spine MRI after 10 months on Dabrafenib showing radiographic regression. c Sagittal T2 Spine MRI showing an increased size of the lesion 6 weeks following discontinuation of Dabrafenib. d Sagittal T2 Spine MRI showing a stable partially regressed tumor 1 year after re-introducing Dabrafenib therapy
Case 3
A six-year-old boy presented with neck pain and progressive right arm weakness. He had an MRI for a suspected brachial plexus injury, which showed a lesion in the lower brainstem extending from the craniomedullary junction to C7 (Fig. 3a). A biopsy was conducted confirming the radiological diagnosis of ganglioglioma WHO grade I and identifying a BRAFV600E mutation. Based on extensive disease and the debilitating surgical procedure with known limited efficacy of conventional therapies in brainstem ganglioglioma, and our experience in case 1 and 2, upfront targeted therapy was discussed as the child had motor loss of his right hand and arm and a level of right leg weakness. The reported rapid effectiveness of specific BRAF kinase inhibitors in small case series but also in our experience, led us to offer this therapeutic option after discussion of the case in a multi-disciplinary meeting which included neurosurgeons, radiation oncologists, radiologists, pathologists, neuro-oncologists and members of the Ethics Review Board. This targeted therapy was duly presented to the family with emphasis on potential risks and its yet unknown benefits including the uncertainty of treatment duration and long-term side-effects. The parents opted for the use of targeted therapy and the child was started on Dabrafenib post-operatively at a dose of 75 mg po bid (5 mg/kg/day). After initiating treatment, he showed a rapid improvement of strength in his right hand. Follow-up imaging revealed partial regression (~ 50%) of the tumor and its enhancement within 23 days of initiation and continuous response at 12 months (Fig. 3b). The patient experienced minor cutaneous toxicity, including a maculopapular rash of the lower extremities, skin hyperpigmentation, paronychia (all grade I), one episode of erythema nodosum (grade II) and focal panniculitis of the lower extremities, which spontaneously resolved. After remaining stable clinically and on imaging for two years while on Dabrafenib, the patient was subsequently tapered off. A follow-up MRI 6 weeks post-discontinuation of treatment revealed significant progression in tumor size and enhancement (Fig. 3c). Clinically, the patient also had mild progression of his right arm weakness and headache. He was immediately re-started on Dabrafenib, after which follow-up imaging at 11 weeks showed significant tumoral regression. The patient is still on treatment since 2017 and shows no signs of progression (Fig. 3d). The only side-effects noted are a curly hair and a tendency to overweight which is being corrected with dietary measures. The proposed therapeutic plan is to taper to half dose daily and follow-up with the aim of potentially identifying the minimal therapeutic dose required to control tumor progression.
a Sagittal T2 Spine MRI showing a lesion in the lower brainstem, starting at the craniocervical junction until the C7 junction before Dabrafenib treatment. b Sagittal T2 Spine MRI sagittal showing significant tumor size and enhancement regression after 12 months of Dabrafenib therapy. c Sagittal T2 Spine MRI 6 weeks post-discontinuation of Dabrafenib showing rapid tumoral growth and progressing enhancement. d Sagittal T2 Spine MRI 1 year follow-up of stable partially regressed tumor size and enhancement after restarting Dabrafenib
Discussion
Brainstem GG are rare tumors and therapeutic decisions are challenging. As per a literature review from 180 cases of GG, there are five times more recurrences with brainstem GG compared to supratentorial GG tumors [13]. Dahiya et al. showed a worse progression free survival in BRAFV600E positive GG with a respective PFS of 80% and 58% at 150 months. Rush and al. showed successful treatment with Vemurafenib but a larger clinical trial is needed to confirm longer PFS in Vemurafenib versus single agent Vinblastine (PFS = 42% at 5 years) [2, 8, 14,15,16]. For now, molecular targeted therapy is rarely used or necessary in the treatment of ganglioglioma. However, in the case of GG not amenable to surgical resection or refractory to conventional chemotherapy, new targeted therapy for BRAFV600E mutated tumor is a promising opportunity.
Gliomas with BRAFV600E mutation are particularly sensitive to BRAF inhibitors in adults. Shih et al. reported a 21-year-old man with a progressive and refractory BRAFV600E mutated ganglioglioma who was treated with Dabrafenib. The patient had a partial response after 2 months of treatment [17]. Chamberlain et al. reported on four adult patients with recurrent BRAFV600E mutated pleiomorphic xanthoastrocytomas (PXA) treated with Vemurafenib. Stable disease was documented in two patients, partial response in one patient and progressive disease in one patient [18]. Even for high-grade gliomas (HGG) there is some evidence that the use of BRAF inhibitors could be beneficial. Robinson et al. reported complete clinical regression of a BRAFV600E mutant glioblastoma 6 months after treatment with Vemurafenib [19]. Two other patients with BRAFV600E HGG treated with Vemurafenib experienced a partial response [20]. Meletath et al. reported the case of a 25 year-old man with HGG BRAFV600E mutated arising from a ganglioglioma, treated with tumor radiation field and Dabrafenib, who is still in complete remission at 2 years of follow-up [21].
In children, several glioma entities show significant percentage of tumors harboring BRAFV600E. These include GG, extra-cerebellar pilocytic astrocytomas, PXAs, and HGG and may benefit from this targeted therapy. Indeed, BRAFV600E mutated pediatric LGG are seemingly therapeutically distinct from other LGG. The 10-years PFS is 27% in this setting vs 60% with conventional therapy, showcasing the need for a different therapeutic approach and novel therapies [22]. Similarly to adults, responses to BRAF inhibitors have also been reported in children with BRAF-mutant gliomas. Brown et al. reported successful treatment of a recurrent anaplastic PXA with BRAFV600E mutation with Dabrafenib [23]. A 2-month-old infant with BRAFV600E mutated hypothalamic chiasmatic glioma responded to Dabrafenib [24]. In a phase I study of Dabrafenib in pediatric patients with BRAF mutated LGG, HGG and other solid tumors, Kieran et al. demonstrated that 75% of HGG patients responded completely or partially and 14 out of 15 LGG patients showed a partial response or stable disease [25].
In our series, all three patients showed rapid and sustained clinical and radiological response, within the first 2 weeks for clinical symptoms and 4 weeks for the first MRI. A significant clinical and radiological improvement were achieved and sustained at 4.8 to 5.5 years follow-up for our three patients, even in one patient who was previously aggressively treated. Radiological responses where in the range of 70 to 85% in the three cases, with major decrease of enhancement and tumor observed within the first months and stable disease on follow-up imaging. Treatment was and still is well tolerated in all three patients with the main noted side effects being skin lesions at treatment initiation that regressed in all cases and curly hair with a tendency to overweight that can be managed with dietary and exercise measures. Dabrafenib was started as a first line therapy for one patient (case 3) after surgical biopsy. This decision was supported by the fact that surgical options were limited and the known relative lack of action of standard adjuvant therapies either chemotherapy or radiation therapy, also mirrored by our prior experience in case 1 and 2. This prompted us following the agreement of our review ethic’s board to present this therapeutic alternative to the family. Moreover, based on preliminary results from the ongoing phase I/II study of this inhibitor in pediatric solid tumors, it was believed that a BRAF inhibitor could improve rapidly his clinical status and hopefully avoid significant loss of motricity in his dominant right hand, which we rapidly observed following treatment initiation. Discontinuation of Dabrafenib led to a dramatic rapid relapse in all three cases: over a few days for the first patient and few weeks later for the other two. All were successfully treated with the reintroduction of the same BRAF inhibitor. This is suggestive of maintenance of chemosensitivity of the tumor even after relapse and has significant biological implications for BRAFV600E positive gangliogliomas. This contrasts with recent reports mainly in PXA or in GG with loss of p16 where therapeutic resistance to this targeted therapy was observed after a few month [11]. This maybe due to our small sample size or more possibly to the fact that in all three cases no other pathogenic alteration was identified on the tumor exome and transcriptome, while expression of the p16 checkpoint inhibitor was maintained in all our cases. Indeed, PXA and lower grade gliomas with worse prognosis often harbor loss of p16 which was not the case in our patient set [26, 27].
Currently, limited data is available with regards to the appropriate timing of discontinuation of Dabrafenib therapy, especially in the pediatric population. Aguilera et al. reported a patient with refractory brainstem ganglioglioma treated with Vemurafenib, which had successfully been retreated with the same drug after recurrence post-discontinuation [28]. Bautista et al. reported a patient with HGG having a good response to Vemurafenib but only a partial response with retreatment following discontinuation [20]. Skrypek et al. reported a BRAFV600E positive optic pathway glioma treated with the combination of Vemurafenib and Vinblastine which rapidly lost its response at discontinuation of treatment but similar to our case series still showed therapeutic response following the reintroduction of the same chemotherapy [29]. Multiple teams have also shown successful short-term responses to BRAFV600E inhibitor for LGG [16, 17, 30]. This is similar to what is observed in patients with targeted therapy with Everolimus for astrocytomas in tuberous sclerosis who relapse at the discontinuation of the mTOR inhibitor but still respond when the drug is re-introduced [31].
Conclusions
These cases add to the ongoing evidence that clinical and radiological responses can be rapidly achieved using targeted inhibitors in patients with brainstem GG harboring the BRAFV600E mutation. Furthermore, we show that abrupt discontinuation of Dabrafenib can induce a rapid relapse as all three paediatric patients rapidly relapsed despite the 24 months of clinical stability under therapy. We also show that chemosensitivity and response can be maintained after reinitiating the same treatment following relapse without the need for another adjuvant therapy or dose increase. An international collaboration and a prospective study are needed to obtain a larger database of pediatric patients treated with BRAFV600E positive tumors, and to define the benefit of targeted therapy at relapse and even at initial diagnosis. We should explore the best duration of treatment, the possibility to slowly wean off the drug after several months of maximal efficacy and to collate data to learn more about the potential side effects its long-term use may have.
Change history
25 November 2019
In the original article, the author names were published incorrectly. The names are correct in this publication.
References
Dudley RW, Torok MR, Gallegos DR, Mulcahy-Levy JM, Hoffman LM, Liu AK et al (2015) Pediatric low-grade ganglioglioma: epidemiology, treatments, and outcome analysis on 348 children from the surveillance, epidemiology, and end results database. Neurosurgery 76(3):313–319
Lindsay AJ, Rush SZ, Fenton LZ (2014) Pediatric posterior fossa ganglioglioma: unique MRI features and correlation with BRAF V600E mutation status. J Neurooncol 118(2):395–404
Lang FF, Epstein FJ, Ransohoff J, Allen JC, Wisoff J, Abbott IR et al (1993) Central nervous system gangliogliomas. Part 2: clinical outcome. J Neurosurg 79(6):867–873
Miller DC, Lang FF, Epstein FJ (1993) Central nervous system gangliogliomas. Part 1: Pathology. J Neurosurg 79(6):859–866
Im SH, Chung CK, Cho BK, Wang KC, Yu IK, Song IC et al (2002) Intracranial ganglioglioma: preoperative characteristics and oncologic outcome after surgery. J Neurooncol 59(2):173–183
Rumana CS, Valadka AB (1998) Radiation therapy and malignant degeneration of benign supratentorial gangliogliomas. Neurosurgery 42(5):1038–1043
Cherlow JM, Shaw DWW, Margraf LR, Bowers DC, Huang J, Fouladi M et al (2019) Conformal radiation therapy for pediatric patients with low-grade glioma: results from the children's oncology group phase 2 study ACNS0221. Int J Radiat Oncol Biol Phys 103(4):861–868
Donson AM, Kleinschmidt-DeMasters BK, Aisner DL, Bemis LT, Birks DK, Levy JM et al (2014) Pediatric brainstem gangliogliomas show BRAF(V600E) mutation in a high percentage of cases. Brain Pathol 24(2):173–183
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S et al (2002) Mutations of the BRAF gene in human cancer. Nature 417(6892):949–954
Valpione S, Carlino MS, Mangana J, Mooradian MJ, McArthur G, Schadendorf D et al (2018) Rechallenge with BRAF-directed treatment in metastatic melanoma: a multi-institutional retrospective study. Eur J Cancer 91:116–124
Levy JM, Thompson JC, Griesinger AM, Amani V, Donson AM, Birks DK et al (2014) Autophagy inhibition improves chemosensitivity in BRAF(V600E) brain tumors. Cancer Discov 4(7):773–780
Bechet D, Gielen GG, Korshunov A, Pfister SM, Rousso C, Faury D et al (2014) Specific detection of methionine 27 mutation in histone 3 variants (H3K27M) in fixed tissue from high-grade astrocytomas. Acta Neuropathol 128(5):733–741
Puget S, Beccaria K, Blauwblomme T, Roujeau T, James S, Grill J et al (2015) Biopsy in a series of 130 pediatric diffuse intrinsic Pontine gliomas. Childs Nerv Syst 31(10):1773–1780
Dahiya S, Haydon DH, Alvarado D, Gurnett CA, Gutmann DH, Leonard JR (2013) BRAF(V600E) mutation is a negative prognosticator in pediatric ganglioglioma. Acta Neuropathol 125(6):901–910
Pan CC, Chen X, Xu C, Wu WH, Zhang P, Wang Y et al (2016) Brainstem gangliogliomas: prognostic factors, surgical indications and functional outcomes. J Neurooncol 128(3):445–453
Rush S, Foreman N, Liu A (2013) Brainstem ganglioglioma successfully treated with vemurafenib. J Clin Oncol 31(10):e159–e160
Shih KC, Shastry M, Williams JT, Jelsma PF, Abram SR, Ayyanar K et al (2014) Successful treatment with dabrafenib (GSK2118436) in a patient with ganglioglioma. J Clin Oncol 32(29):e98–e100
Chamberlain MC (2013) Salvage therapy with BRAF inhibitors for recurrent pleomorphic xanthoastrocytoma: a retrospective case series. J Neurooncol 114(2):237–240
Robinson GW, Orr BA, Gajjar A (2014) Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer 14:258
Bautista F, Paci A, Minard-Colin V, Dufour C, Grill J, Lacroix L et al (2014) Vemurafenib in pediatric patients with BRAFV600E mutated high-grade gliomas. Pediatr Blood Cancer 61(6):1101–1103
Meletath SK, Pavlick D, Brennan T, Hamilton R, Chmielecki J, Elvin JA et al (2016) Personalized treatment for a patient with a BRAF V600E mutation using Dabrafenib and a tumor treatment fields device in a high-grade glioma arising from ganglioglioma. J Natl Compr Canc Netw 14(11):1345–1350
Lassaletta A, Zapotocky M, Mistry M et al (2017) Therapeutic and prognostic implications of BRAF V600E in pediatric low-grade gliomas. J Clin Oncol 35:2934–2941
Brown NF, Carter T, Mulholland P (2017) Dabrafenib in BRAFV600-mutated anaplastic pleomorphic xanthoastrocytoma. CNS Oncol 6(1):5–9
Lassaletta A, Guerreiro Stucklin A, Ramaswamy V, Zapotocky M, McKeown T, Hawkins C et al (2016) Profound clinical and radiological response to BRAF inhibition in a 2-month-old diencephalic child with hypothalamic/chiasmatic glioma. Pediatr Blood Cancer 63(11):2038–2041
Kieran MW, Hargrave DR, Cohen KJ, Aerts I, Dunkel IJ, Hummel TR et al (2015) Phase 1 study of dabrafenib in pediatric patients (pts) with relapsed or refractory BRAF V600E high- and low-grade gliomas (HGG, LGG), Langerhans cell histiocytosis (LCH), and other solid tumors (OST). J Clin Oncol. 33(15_suppl):10004
Koelsche C, Sahm F, Wohrer A, Jeibmann A, Schittenhelm J, Kohlhof P et al (2014) BRAF-mutated pleomorphic xanthoastrocytoma is associated with temporal location, reticulin fiber deposition and CD34 expression. Brain Pathol 24(3):221–229
Reis GF, Pekmezci M, Hansen HM, Rice T, Marshall RE, Molinaro AM et al (2015) CDKN2A loss is associated with shortened overall survival in lower-grade (World Health Organization Grades II–III) astrocytomas. J Neuropathol Exp Neurol 74(5):442–452
Aguilera D, Janss A, Mazewski C, Castellino RC, Schniederjan M, Hayes L et al (2016) Successful retreatment of a child with a refractory brainstem ganglioglioma with vemurafenib. Pediatr Blood Cancer 63(3):541–543
Skrypek M, Foreman N, Guillaume D, Moertel C (2014) Pilomyxoid astrocytoma treated successfully with vemurafenib. Pediatr Blood Cancer 61(11):2099–2100
del Bufalo F, Carai A, Figa-Talamanca L, Pettorini B, Mallucci C, Giangaspero F et al (2014) Response of recurrent BRAFV600E mutated ganglioglioma to vemurafenib as single agent. J Transl Med 12:356
Krueger DA, Care MM, Holland K et al (2010) Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med 363:1801–1811
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Philippe, L., Maria, K., Tariq, A. et al. Efficacy of Dabrafenib for three children with brainstem BRAFV600E positive ganglioglioma. J Neurooncol 145, 135–141 (2019). https://doi.org/10.1007/s11060-019-03280-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03280-2