Abstract
Purpose
Diffuse intrinsic pontine glioma (DIPG) is the most severe pediatric solid tumor, with no significant improvement in the past 50 years. Possible reasons for failure to make therapeutic progress include poor understanding of the underlying molecular biology due to lack of tumor material.
Methods
We performed a prospective analysis of children with typical appearance of DIPG who had a stereotactic biopsy in our unit since 2002. Technical approach, complications, histopathological results, and samples processing are exposed. The literature on this subject is discussed.
Results
Reviewing our own 130 cases of DIPG biopsies and previous published data, these procedures appear to have a diagnostic yield and morbidity rates similar to those reported for other brain locations (3.9 % of transient morbidity in our series). In addition, the quality and the quantity of the material obtained allow to (1) confirm the diagnosis, (2) reveal that WHO grading was useless to predict outcome, and (3) perform an extended molecular screen, including biomarkers study and the development of preclinical models. Recent studies reveal that DIPG may comprise more than one biological entity and a unique oncogenesis involving mutations never described in other types of cancers, i.e., histones H3 K27M and activin receptor ACVR1.
Conclusion
Stereotactic biopsies of DIPG can be considered as a safe procedure in well-trained neurosurgical teams and could be incorporated in protocols. It is a unique opportunity to integrate DIPG biopsies in clinical practice and use the biology at diagnosis to drive the introduction of innovative targeted therapies, in combination with radiotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diffuse intrinsic pontine tumors (DIPG), which accounts for 10 to 15 % of all childhood brain tumors [1, 2], represent one of the biggest therapeutic challenges in pediatric neuro-oncology with a median survival of 9 months. Its deep-seated location in the brain stem precludes safe surgical removal. It has been attempted but was abandoned by the majority as it did not improve the patient’s outcome [3].During the last 50 years, no significant improvement has been made. Chemotherapy, so far derived from adult high-grade glioma protocols, has failed to halt or delay significantly tumor growth. Radiotherapy is the only validated treatment but is only transiently efficient.
The development of targeted therapies for these tumors has been hampered by the lack of knowledge of their biology and trials have been carried out based on the misconception that DIPG biology is similar to their adult counterparts [4–6] and to other pediatric supratentorial malignant glioma [4, 7]. The diagnosis is usually based on the association of a short history of less than 2 months, cranial nerve palsies, long tract signs, and ataxia with typical imaging findings. DIPG are usually described as an infiltrating tumor mass of the pons hypointense on T1 and hyperintense on T2 and FLAIR; by definition, at least 50 % of the pons should be involved. Contrast enhancement if any is usually limited and annular.
The biopsy of these tumors has been controversial. Most neurosurgical teams have been reluctant to perform biopsies, because of the potential risks of this procedure and the absence of direct benefit for the patients. Thus, biopsy has generally been limited to lesions with unusual presentation or imaging. Therefore, only very limited data on true newly diagnosed DIPG is available in the literature and confounded by the inclusion of autopsy—usually post-radiotherapy—cases. However, awareness of the urgent need to improve the prognosis of these devastating tumors and the development of newer molecular genetic techniques have led to reconsideration of the role of stereotactic biopsy in DIPG [8–11].
This paper will address the question of the feasibility and the safety of a stereotactic biopsy for DIPG, its diagnostic yield, and its role in redefining this tumor by its molecular signature and profiling targeted therapy.
The safety of DIPG biopsy
Stereotactic biopsies are now completely integrated in the diagnosis and management of several intracranial lesions. Its role in DIPG remains controversial, and currently, the general attitude is not to biopsy these tumors as it has been considered unnecessary.
Stereotactic biopsy of brain stem tumors is an old procedure; it became popular after the first report of this procedure in 1978 [12]. Ten years later, the arguments against brain stem biopsy were strong as it was thought to be useless, dangerous, and to have poor yield [13–15]. The manuscript published by Albright et al. from the Children’s Cancer Group changed the course for pediatric DIPG management as they claimed that “… MRI scans provide images that are virtually diagnostic of brain stem gliomas and yield prognostic information equivalent to that obtainable from biopsies…” [15]. Since then, the neurosurgical world was divided into those in favor of and those against brain stem biopsy. Despite the reluctance of some neurosurgical teams, others continued to perform biopsy of brain stem lesions in children and adults for unusual lesions or for typical ones as part of a trial [8, 16–23].
Interestingly, in 2011, Hankinson et al. reported the results of a survey on the appraisal by pediatric neurosurgeons of the MRI findings of selected examples of DIPG (typical/atypical) [24]. More than 75 % agreement regarding whether a tumor was typical or atypical was found in only 43.8 % of the cases presented. They concluded: “the practice of diagnosing DIPG based on imaging characteristics and clinical history alone does not reach the appropriate threshold to be considered a standard of care.” Sufit et al. reported a series of biopsies from seven diffuse pontine tumors. Two of them were identified as PNETs. They argued, “Since diagnosis by imaging is not reliable and the biology of the tumors is disparate, a biopsy should be performed to enable accurate diagnosis and direct potentially more effective treatments” [25].
A number of papers on stereotactic biopsies in the brain stem have been published in the last 20 years and now represent a significant amount of knowledge. Unfortunately, these reports often involve mixed series of adults and children with a wide range of diagnoses. The details of the series are outlined in Table 1. These mixed series quote the morbidity rates between 0 and 20 % and a mortality rate of 0 to 3 %. However, when data on pediatric patients with DIPG are extracted, the diagnostic yield in these cases ranges from 96 to 100 %, with no mortality and morbidity less than 5 % for the largest series.
Samadani et al. have done a meta-analysis of 13 studies performing stereotactic biopsy of brain stem lesions in 381 children and adults [26]. With a diagnostic yield of 96 %, this study reported one death due to a biopsy of a vascular lesion in an adult and a rate of permanent and transient deficits of 4 and 1 %, respectively. A few years later, a second meta-analysis on pediatric brain stem lesions was published by Pincus et al. [19]. This review of 192 children revealed a diagnostic yield of 94.9 %, 0.7 % mortality, and 4.9 % morbidity rates. Rajshekhar et al. reported a series of 106 stereotactic biopsies in children with brain stem masses. With no mortality or permanent morbidity reported, the authors highlighted that “…this procedure is safe in children and the benefits outweigh the risks in patients who are appropriately selected to undergo this procedure…” [20]. Recently, Wang et al. reported a series of 15 DIPG biopsy with 20 % of transient new or worsening neurological deficits [27]. They used a stealth-guided biopsy with Medtronic device® which may be less accurate than a biopsy with a frame, but they did not report if the patients had a postoperative image to confirm the trajectory and the site of the biopsy and to look for complications.
Importantly, in a large series of 270 stereotactic brain biopsies in adults, it has been shown that increasing numbers of specimens obtained per trajectory in brain stem lesions were not significant risk factors for morbidity [28] and this has been confirmed by our results, presented below.
Necker series
A few years ago, our group started to use stereotactic biopsies of DIPG to obtain both pathological confirmation and immunohistochemical assessment of some specific biomarkers before the inclusion of patients in trials of targeted agents [8, 29]. We excluded from this study the children with atypical presentation (unusual clinical signs or atypical brain stem MRI features). Informed consent was obtained from all children’s parents included in the study. We declined to do the surgical procedure in five cases because they were too young or in very bad condition. In 2007, our team reported the results of a biopsy of 24 children, 2 of whom suffered transient worsening of their neurological deficits [8]. Considering this procedure to be safe and this disease to be not curable, we systematically proposed a biopsy to subsequent patients in order to obtain material that could give clues to the underlying biology of DIPG. It is interesting to note that only two families refused the biopsy.
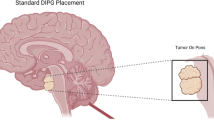
Thus, during a period of 13 years, a total of 130 children with pontine lesions resembling DIPG were operated on by this technique in Necker Hospital. Among them, 68 were females and 62 were males. The age at presentation ranged from 16 months to 16.4 years (median 6.7 years). The majority of them received corticosteroids at least 3 days before the procedure. Using the Leksell stereotactic system, a transcerebellar approach was used in all cases. All the procedures were carried out with the patient in prone position under general anesthesia and the stereotactic coordinates were determined by computed tomography or by MRI. The contrast enhancement was targeted when possible, i.e., when it was not too anterior, close to the pyramidal tract or near the cranial nerves nuclei; in other cases, we targeted the infiltrative part, close to the middle cerebellar peduncle (Fig. 1). Using a single trajectory, the number of samples increased with time (up to 8), allowing for DNA and RNA extraction, as well as cell culture and mouse xenografts. Importantly, increasing the number of samples was not linked with more complications. No mortality or permanent deficit was observed but a transient worsening of neurological deficit occurred in five patients (3.9 %). We recorded a worsening of preexisting ataxia (n = 2), ataxia and VI and VII palsy (n = 2), and VI palsy (n = 1). All the patients had a postoperative image (CT scan or MRI) to confirm the site of the biopsy and to look for complications (Fig. 2). Four patients had a little bleeding at the site of the biopsy that was not associated with a neurological worsening.
The diagnostic yield was 100 % in our series. For the first 92 patients, pathology classified the DIPG according to the WHO grading system. We found 28, 36, and 28 grades II, III, and IV, respectively. For the whole cohort, the median overall survival (OS) and progression-free survival (PFS) were 10.3 and 5.6 months, respectively (Fig. 3a).
Interestingly, the grade was not associated with the OS or the PFS (Fig. 3b). These results have been somewhat controversial, with arguments that the small biopsies might not be representative of the tumor. However, a recent paper on autopsy cases showed similar results [30] and confirm that the WHO grading scheme may not appropriately predict outcome for pediatric DIPG.
Based on our results, we have proposed a national trial (BIOMEDE) used to stratify children to different therapies according to the EGFR, PDGFR, and PTEN status [10, 31]. This trial should be extending to Europe in the next months.
Guidelines for performing biopsies in diffuse intrinsic pontine gliomas
-
1-
Contraindications to the biopsy
The use of a frame for children below the age of 2 is not recommended because of the thickness of the cranial bone. It has to be discussed on a case-by-case basis, eventually helped by a CT scan.
Children with a high risk of complications during and after anesthesia due to a bad clinical condition (with severe swallowing problems, obtundation, breathing difficulties, tetraplegia) should not be biopsied before starting radiotherapy. Biopsy can be performed after radiation therapy or at the time of relapse.
-
2-
How to choose the route?
Two routes have been described for brain stem biopsies: the transcerebellar and the transfrontal approaches. The transfrontal route is longer and allows sampling of a mass located in all the segments of the brain stem. The positioning of the entry site has to be chosen carefully to avoid the ventricles, the vascular structures, and the tentorium. The use of software is recommended for planning this trajectory. The transcerebellar approach is shorter, through the middle cerebellar peduncle and has less eloquent structures in its trajectory [20, 32]. It can be used only for pontine and upper medullary masses and is thus preferred by the authors for DIPG. In a series comparing both routes, no statistically significant differences were reported [33].
-
3-
How to choose the target?
Ideally, the procedure is to take biopsies in both enhancing and non-enhancing regions in one trajectory, which is rarely achievable. The contrast enhancement is targeted when possible, i.e., when it is not too anterior, close to the pyramidal tract or too close to the cranial nerves nuclei; in other cases, we recommend to target the infiltrative part (hyper flair/T2) of the tumor. In case of the transcerebellar approach, we recommend to choose the target in the posterior third of the pons, through the middle cerebellar peduncle.
In case of enhancement surrounding central necrosis, it may be difficult to obtain bulk tissue and there is a potential risk of hemorrhage. On the other hand, depending on the location and the clinical signs of the patient, it can be efficient to decrease the size of this necrotic part, during the stereotactic procedure.
-
4-
The biopsy procedure
The biopsy can be done with a frame or frameless, at the discretion of the neurosurgeon. Under general anesthesia, the procedure is carried out with the patient in prone position for a transcerebellar approach or supine position for a transfrontal one. In case of transcerebellar approach with a frame, it has to be secured as inferiorly as possible onto the skull and its posterolateral post will be removed prior to commencing the surgical procedure. The incision location is recommended at mid-distance between the midline and the mastoid, below the transverse sinus. Importantly, the consistence of the tumor cannot be assessed on imaging, except for the necrotic part. In our experience, it can be very soft or solid, so we recommend gentle aspiration at the start. A side-cutting biopsy needle is used and we can perform, if possible and ideally, four-quadrant biopsies in an enhancing part of the tumor and four-quadrant biopsies in a non-enhancing part in one trajectory. If the enhancing part is not present or cannot be safely biopsied, we recommend to perform four to eight biopsy samples in one trajectory.
-
Two samples are fixed in formaldehyde for histopathological examination.
-
One sample can be used for xenograft and/or culture.
-
The others are immediately snap-frozen in the operating room and stored at −80 °C for molecular biology studies.
-
5-
Quality control of the biopsy
After the procedure, it is recommended to perform a post-op axial T2 sequence to confirm the trajectory and the site of the biopsy and to look for complications.
If MRI cannot be done, at least a CT scan should be performed to look for any complication. An air bubble may be injected at the time of the biopsy to make the site of the biopsy more evident.
Biopsy samples processing
The lack of samples from newly diagnosed DIPG has limited our understanding of their biology and has hindered the development of newer therapies in this devastating tumor. As targeted therapy undoubtedly requires tissue, it could be argued that such advances will only be optimized with the knowledge that biopsy provides in terms of biology and the identification of new targets. In recent papers, authors defend the idea that a biopsy of newly DIPG should be performed to increase our knowledge of tumor biology that could provide clues to improve their prognosis [18, 31, 34]. However, in case of typical DIPG appearance on MRI, biopsy should not be performed only to confirm the diagnosis, as the risk of the procedure even though minimal is not nul. In such typical DIPGs, biopsy should be a part of a well-conducted clinical trial or a research program approved by an ethical committee [18, 23, 31]. The authors agree with the conclusion of Wilkinson and Harris, who stated that “… Once emotional and social interests are taken into account there seems little doubt that brain stem biopsy could be lawful even if there was no benefit to the child’s medical interests…” [35].
Limited preliminary studies and early clinical trials have shown the promise of stereotactic biopsies of DIPG tumors to unravel the biology of the disease and to target drug use according to the discovery of specific genomic alterations.
Although the sample size obtained by stereotactic biopsy of these tumors is limited by the size of the needle, it has been shown that it could provide enough tissue for histopathological diagnosis and immunohistochemical staining. Moreover, the authors have recently shown that this surgical technique could allow multiple biopsies samples (up to 8) to provide enough tissue for further genomic analyses and stem cell culture [10, 31, 36]. In the author’s series, one or two biopsies were used for histological diagnosis and immunohistochemistry. The remaining biopsies were snap-frozen with cytological control smears directly in the operating room, and nucleic acids extracted thereafter. A median of 3.325 μg of DNA (range 0.805 to 21.5 μg) and 2.332 μg of RNA (range 0.048 to 15.84 μg) could be extracted from the biopsies. One to three samples were necessary to obtain enough nucleic acids, depending on the infiltrative rate of the tumor cells. An integrated molecular profiling was carried out and permitted identification of two distinct subgroups of DIPG with specific abnormalities: one showing a mesenchymal gene expression profile and the other a more proliferative gene expression signature. The former group of tumors showed a better survival than the later. The poor prognosis group defined by gene expression profiling showed an oligodendroglial differentiation that could be correlated with an adverse prognosis in another validation cohort. In addition, this group of tumors showed platelet-derived growth factor receptor (PDGFRA) amplification with or without mutation in the external domain and MET gain that could represent relevant therapeutic targets. This study is the first to comprehensively define the biological alterations of DIPG at diagnosis, allowing the discovery of novel therapeutic targets [10]. The material obtained allowed also screening for oncogenic mutations with known targeted drugs available. Our group could therefore identify PI3KCA mutations in 15 % of DIPG, offering another relevant therapeutic target [31]. Finally, cultures of DIPG could be derived as cell line or as neurospheres [36]. They represent irreplaceable tools for preclinical studies of new therapeutic agents as well as for understanding the oncogenesis of DIPG.
Our team also has also shown that a full biological workout, including whole genome sequencing was feasible with the DIPG stereotactic biopsy material [37]. Recent pivotal studies demonstrated that DIPGs appear to be a specific and unique biological entity. Indeed, DIPG almost always have a mutation in the regulatory tail of one of the histone H3 variants [38] that is already present at diagnosis [37] and that has a broad impact on the epigenetic control of these cells [39]. This suggests that this mutation has a driver role in the oncogenesis of DIPG and that it could serve as a diagnostic marker [30]. While this mutation is presently not reversible with any medication, these studies have also discovered other alterations that could help to choose specific individualized targeted therapies: PDGFRA amplification and mutation, MET amplification and mutations, mTOR pathway alterations, and ACVR1 mutations [5, 7, 10, 30, 31, 37, 40–42]. In addition, some of the alterations could be linked to a better prognosis, e.g., ACVR1 mutations [37] or to a worse prognosis, e.g., PDGFRA activation [10] and H3.3 mutation [5]. Biopsies at diagnosis could therefore be used for prognostication and for the choice of targeted therapies. Finally, preclinical models have been established from these tumors and will serve the development of new therapeutics [43–45].
In keeping with our experience, recently, there has been a worldwide resurgence of interest in pediatric brain stem biopsy in hopes that molecular profiling could help to find new therapeutic targets. To this end, several international consensus conferences on DIPG have been organized in North America and in Europe these last 5 years [1, 46]. There is a growing body of evidence that up-front biopsy in DIPG is now considered rational for the majority as it may alter treatment with targeted therapy and may help in correlate biology with response with appropriate biomarkers. It may also help to guide relapse therapies, to look for active treatment and develop biological relevant models [28].
This worldwide realization will probably lead in the near future to the introduction of up-front stereotactic biopsies as a standard healthcare intervention to stimulate translational research and development of individualized treatment for DIPG and to establish stereotactic biopsies as a standard diagnostic tool for all children suffering from DIPG.
Conclusion
DIPG remains a leading cause of death from pediatric brain tumors. The role of diagnostic biopsy in these tumors has been controversial due to the high eloquence of the brain stem and the lack of direct benefit for the patient. Based on the literature and our own data, stereotactic biopsy of DIPG is approximately as safe and diagnostic as supratentorial biopsy and the amount of tissue obtained allows for molecular biology analysis, including whole genome sequencing. This technique should be offered to these patients and opens new perspectives for the characterization of biological markers that permit to enroll children with newly DIPG in next-generation clinical trials with targeted therapy.
References
Jansen MH, van Vuurden DG, Vandertop WP, Kaspers GJ (2012) Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev 38:27–35. doi:10.1016/j.ctrv.2011.06.007
Warren KE, Killian K, Suuriniemi M, et al. (2012) Genomic aberrations in pediatric diffuse intrinsic pontine gliomas. Neuro-Oncology 14:326–332. doi:10.1093/neuonc/nor190
Epstein F, Wisoff JH (1988) Intrinsic brainstem tumors in childhood: surgical indications. J Neuro-Oncol 6:309–317
Bax DA, Mackay A, Little SE, et al. (2010) A distinct spectrum of copy number aberrations in pediatric high-grade gliomas. Clin Cancer Res 16:3368–3377. doi:10.1158/1078-0432.CCR-10-0438
Paugh BS, Broniscer A, Qu C, et al. (2011) Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol 29:3999–4006. doi:10.1200/JCO.2011.35.5677
Qu HQ, Jacob K, Fatet S, et al. (2010) Genome-wide profiling using single-nucleotide polymorphism arrays identifies novel chromosomal imbalances in pediatric glioblastomas. Neuro-Oncology 12:153–163. doi:10.1093/neuonc/nop001
Zarghooni M, Bartels U, Lee E, et al. (2010) Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol 28:1337–1344. doi:10.1200/JCO.2009.25.5463
Roujeau T, Machado G, Garnett MR, et al. (2007) Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg 107:1–4. doi:10.3171/PED-07/07/001
Robison NJ, Kieran MW (2014) Diffuse intrinsic pontine glioma: a reassessment. J Neurooncol 119:7–15. doi:10.1007/s11060-014-1448-8
Puget S, Philippe C, Bax DA, et al. (2012) Mesenchymal transition and PDGFRA amplification/mutation are key distinct oncogenic events in pediatric diffuse intrinsic pontine gliomas. PLoS One 7:e30313. doi: 10.1371/journal.pone.0030313
Kieran MW (2015) Time to rethink the unthinkable: upfront biopsy of children with newly diagnosed diffuse intrinsic pontine glioma (DIPG). Pediatr Blood Cancer 62:3–4. doi:10.1002/pbc.25266
Gleason CA, Wise BL, Feinstein B (1978) Stereotactic localization (with computerized tomographic scanning), biopsy, and radiofrequency treatment of deep brain lesions. Neurosurgery 2:217–222
Epstein F, McCleary EL (1986) Intrinsic brain-stem tumors of childhood: surgical indications. J Neurosurg 64:11–15. doi:10.3171/jns.1986.64.1.0011
Stroink AR, Hoffman HJ, Hendrick EB, Humphreys RP (1986) Diagnosis and management of pediatric brain-stem gliomas. J Neurosurg 65:745–750. doi:10.3171/jns.1986.65.6.0745
Albright AL, Packer RJ, Zimmerman R, et al. (1993) Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children’s Cancer Group. Neurosurgery 33:1026–1029 discussion 1029–30
Chico-Ponce de Leon F, Perezpena-Diazconti M, Castro-Sierra E, et al. (2003) Stereotactically-guided biopsies of brainstem tumors. Childs Nerv Syst 19:305–310. doi:10.1007/s00381-003-0737-x
Dellaretti M, Touzet G, Reyns N, et al. (2011) Correlation among magnetic resonance imaging findings, prognostic factors for survival, and histological diagnosis of intrinsic brainstem lesions in children. J Neurosurg Pediatr 8:539–543. doi:10.3171/2011.9.PEDS1167
Leach PA, Estlin EJ, Coope DJ, et al. (2008) Diffuse brainstem gliomas in children: should we or shouldn’t we biopsy? Br J Neurosurg 22:619–624. doi:10.1080/02688690802366198
Pincus DW, Richter EO, Yachnis AT, et al. (2006) Brainstem stereotactic biopsy sampling in children. J Neurosurg 104:108–114. doi:10.3171/ped.2006.104.2.108
Rajshekhar V, Moorthy RK (2010) Status of stereotactic biopsy in children with brain stem masses: insights from a series of 106 patients. Stereotact Funct Neurosurg 88:360–366. doi:10.1159/000319044
St George EJ, Walsh AR, Sgouros S (2004) Stereotactic biopsy of brain tumours in the paediatric population. Childs Nerv Syst 20:163–167. doi:10.1007/s00381-003-0897-8
Valdes-Gorcia J, Espinoza-Diaz DM, Paredes-Diaz E (1998) Stereotactic biopsy of brain stem and posterior fossa lesions in children. Acta Neurochir 140:899–903
Puget S, Blauwblomme T, Grill J (2012) Is biopsy safe in children with newly diagnosed diffuse intrinsic pontine glioma? Am Soc Clin Oncol Educ Book 629–633. doi: 10.14694/EdBook_AM.2012.32.629
Hankinson TC, Campagna EJ, Foreman NK, Handler MH (2011) Interpretation of magnetic resonance images in diffuse intrinsic pontine glioma: a survey of pediatric neurosurgeons. J Neurosurg Pediatr 8:97–102. doi:10.3171/2011.4.PEDS1180
Sufit A, Donson AM, Birks DK, et al. (2012) Diffuse intrinsic pontine tumors: a study of primitive neuroectodermal tumors versus the more common diffuse intrinsic pontine gliomas. J Neurosurg Pediatr 10:81–88. doi:10.3171/2012.3.PEDS11316
Samadani U, Judy KD (2003) Stereotactic brainstem biopsy is indicated for the diagnosis of a vast array of brainstem pathology. Stereotact Funct Neurosurg 81:5–9. doi:10.1159/000075097
Wang ZJ, Rao L, Bhambhani K, et al. (2015) Diffuse intrinsic pontine glioma biopsy: a single institution experience. Pediatr Blood Cancer 62:163–165. doi:10.1002/pbc.25224
McGirt MJ, Woodworth GF, Coon AL, et al. (2005) Independent predictors of morbidity after image-guided stereotactic brain biopsy: a risk assessment of 270 cases. J Neurosurg 102:897–901. doi:10.3171/jns.2005.102.5.0897
Geoerger B, Hargrave D, Thomas F, et al. (2011) Innovative therapies for children with cancer pediatric phase I study of erlotinib in brainstem glioma and relapsing/refractory brain tumors. Neuro-Oncology 13:109–118. doi:10.1093/neuonc/noq141
Buczkowicz P, Hoeman C, Rakopoulos P, et al. (2014) Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet 46:451–456. doi:10.1038/ng.2936
Grill J, Puget S, Andreiuolo F, et al. (2012) Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr Blood Cancer 58:489–491. doi:10.1002/pbc.24060
Backlund EO (1971) A new instrument for stereotaxic brain tumour biopsy. Technical note. Acta Chir Scand 137:825–827
Dellaretti M, Reyns N, Touzet G, et al. (2012) Stereotactic biopsy for brainstem tumors: comparison of transcerebellar with transfrontal approach. Stereotact Funct Neurosurg 90:79–83. doi:10.1159/000335502
Khatua S, Moore KR, Vats TS, Kestle JR (2011) Diffuse intrinsic pontine glioma—current status and future strategies. Childs Nerv Syst 27:1391–1397. doi:10.1007/s00381-011-1468-z
Wilkinson R, Harris J (2008) Moral and legal reasons for altruism in the case of brainstem biopsy in diffuse glioma. Br J Neurosurg 22:617–618. doi:10.1080/02688690802482896
Thirant C, Bessette B, Varlet P, et al. (2011) Clinical relevance of tumor cells with stem-like properties in pediatric brain tumors. PLoS One 6:e16375. doi: 10.1371/journal.pone.0016375
Taylor KR, Mackay A, Truffaux N, et al. (2014) Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet 46:457–461. doi:10.1038/ng.2925
Wu G, Broniscer A, McEachron TA, et al. (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44:251–253. doi:10.1038/ng.1102
Bender S, Tang Y, Lindroth AM, et al. (2013) Reduced H3K27me3 and DNA hypomethylation are major drivers of gene expression in K27M mutant pediatric high-grade gliomas. Cancer Cell 24:660–672. doi:10.1016/j.ccr.2013.10.006
Fontebasso AM, Papillon-Cavanagh S, Schwartzentruber J, et al. (2014) Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet 46:462–466. doi:10.1038/ng.2950
Paugh BS, Zhu X, Qu C, et al. (2013) Novel oncogenic PDGFRA mutations in pediatric high-grade gliomas. Cancer Res 73:6219–6229. doi:10.1158/0008-5472.CAN-13-1491
Wu G, Diaz AK, Paugh BS, et al. (2014) The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet 46:444–450. doi:10.1038/ng.2938
Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. (2012) K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 124:439–447. doi:10.1007/s00401-012-0998-0
Monje M, Mitra SS, Freret ME, et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc Natl Acad Sci U S A 108:4453–4458. doi: 10.1073/pnas.1101657108
Veringa SJ, Biesmans D, van Vuurden DG, et al. (2013) In vitro drug response and efflux transporters associated with drug resistance in pediatric high grade glioma and diffuse intrinsic pontine glioma. PLoS One 8:e61512. doi: 10.1371/journal.pone.0061512
Bartels U, Hawkins C, Vezina G, et al. (2011) Proceedings of the diffuse intrinsic pontine glioma (DIPG) Toronto Think Tank: advancing basic and translational research and cooperation in DIPG. J Neurooncol 105:119–125. doi:10.1007/s11060-011-0704-4
Acknowledgments
We thank the Tumorotheque Necker-Enfants Malades and the CRB-ADN (Imagine, AP-HP, Université Paris Descartes).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puget, S., Beccaria, K., Blauwblomme, T. et al. Biopsy in a series of 130 pediatric diffuse intrinsic Pontine gliomas. Childs Nerv Syst 31, 1773–1780 (2015). https://doi.org/10.1007/s00381-015-2832-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-015-2832-1