Abstract
Purpose
Non-small cell lung cancer (NSCLC) brain metastases are associated with substantial morbidity and mortality. During recent years, accompanying dramatic improvements in systemic disease control, NSCLC brain metastases have emerged as an increasingly relevant clinical problem. However, optimal surveillance practices remain poorly defined. This purpose of this study was to further characterize the natural history, clinical course and risk factors associated with earlier development of subsequent NSCLC brain metastases to better inform clinical practice and help guide survivorship care.
Methods
We retrospectively reviewed all institutional NSCLC brain metastasis cases treated with radiotherapy between 1997 and 2015. Exclusion criteria included presence of brain metastases at initial NSCLC diagnosis and incomplete staging information. Interval time to brain metastases and subsequent survival were characterized using Kaplan–Meier and multivariate Cox regression analyses.
Results
Among 105 patients within this cohort, median interval time to development of brain metastases was 16 months. Median interval times were 29, 19, 16 and 13 months for Stage I–IV patients, respectively (P = 0.016). Additional independent predictors for earlier development of NSCLC brain metastases included non-adenocarcinomatous histopathology (HR 3.036, P < 0.001), no prior surgical resection (HR 1.609, P = 0.036) and no prior systemic therapy (HR 3.560, P = 0.004). Median survival following intracranial progression was 16 months. Delayed development of brain metastases was associated with better prognosis (HR 0.970, P < 0.001) but not survival following intracranial disease onset.
Conclusions
Collectively, our results provide valuable insights into the natural history of NSCLC brain metastases. NSCLC stage, histology, prior surgical resection and prior systemic therapy emerged as independent predictors for interval time to brain metastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases remain a significant source of morbidity and mortality, cumulatively affecting 10–30% of adult cancer patients nationwide. Most commonly associated with lung cancer, breast cancer, melanoma, renal cell carcinoma and gastrointestinal cancers, brain metastases impact almost 200,000 United States individuals each year [1,2,3,4,5]. Without prompt treatment, potential consequences include not only distressing symptoms including headaches, nausea, vomiting, seizures, delirium and neuropsychiatric changes that may compromise quality of life but also permanent functional deficits, paralysis, coma and even death, emphasizing the importance of early detection of intracranial disease involvement. Lung cancer remains the most common nationwide source of brain metastases, accounting for almost half of all cases [1]. Current projections estimate that approximately 30–50% of lung cancer patients will ultimately develop brain metastases [6, 7], emphasizing the need for further research characterizing their natural history and clinical course to help inform clinical practice during the modern era.
During the past 15 years, accompanying landmark advancements surrounding both the diagnosis and treatment of lung cancer (including nationwide screening recommendations, improved diagnostic approaches using endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and preoperative PET/CT scans for improved staging accuracy, potentially curative treatment options for medically inoperable patients using stereotactic body radiotherapy, novel targeted agents and the advent of immune checkpoint inhibitors), long-term survivorship has become increasingly common. But despite improved systemic disease control, consistent with the ‘sanctuary site’ hypothesis, NSCLC patients often develop brain metastases as isolated recurrences without concurrent systemic progression. Amidst these developments, NSCLC brain metastases have emerged as an increasingly important concern. Meanwhile, optimal surveillance practices for NSCLC brain metastases beyond initial staging MRIs remain poorly defined. Current nationwide guidelines provide no systematic recommendation for interval MRIs in the absence of neurologic symptoms or systemic progression, presumably reflecting the historically poor prognosis of NSCLC. However, early detection and prompt treatment of subsequent brain metastases could not only help prevent associated complications but even potentially improve survival among well-selected patients [8,9,10,11].
While population-based research estimates that the incidence rate for subsequent brain metastases among NSCLC patients is approximately 9% [12], further research is needed toward better understanding the natural history and clinical course of subsequent NSCLC brain metastases. Previously described risk factors associated with higher incidence proportions of subsequent NSCLC brain metastases have included younger age, female gender, tumor histology (specifically, adenocarcinomas) and initial stage [1, 5, 12]. While prophylactic cranial irradiation (PCI) remains highly controversial for NSCLC [13,14,15], prior retrospective studies have also examined risk factors associated with interval development of brain metastases toward identifying potential populations who might warrant such treatment [16,17,18,19,20,21].
Given our limited understanding regarding interval development of NSCLC brain metastases during the modern era, the purpose of this study was to further characterize their natural history and clinical course, as well as risk factors associated with earlier versus delayed development of subsequent brain metastases among NSCLC patients, in order to better inform clinical practice and potentially guide future research regarding optimal surveillance practices. Here, we examined the clinical course of interval brain metastases, characterized accompanying survival trends and investigated both predictive factors and prognostic implications of interval time until development of NSCLC brain metastases using a retrospective institutional cohort with prolonged follow-up.
Methods
This study was Institutional Review Board-approved and conducted in accordance with ethical standards of the 1964 Helsinki Declaration and its later amendments. We retrospectively reviewed institutional charts from patients (aged ≥ 18) diagnosed with biopsy-proven NSCLC who ultimately developed subsequent brain metastases treated using either whole-brain radiotherapy (WBRT) or Gamma Knife stereotactic radiosurgery (GKRS) from 1997 to 2015. Exclusion criteria included incomplete chart information and presence of NSCLC brain metastases at initial cancer diagnosis (e.g., NSCLC patients diagnosed with brain metastases at the time of index diagnosis). We examined baseline demographic information and treatment-related variables including age, race, gender, Karnofsky Performance Status (KPS), anatomic location of primary tumor, histology, grade, molecular alterations (specifically EGFR, KRAS and ALK mutations), primary tumor stage, nodal involvement, extracranial metastases, clinical stage at diagnosis (Stage I, II, III and IV), surgical history of the primary tumor, receipt of systemic therapy, receipt of thoracic radiation and local control at onset of brain metastases. After initial diagnosis, subsequent brain MRIs were performed according to institutional practice at the time of systemic disease progression or the development of new clinical signs or symptoms concerning for intracranial disease. Interval time to development of brain metastases was defined as the time from initial pathologic diagnosis until date of radiographic evidence of intracranial disease. Overall survival was calculated based on the time from initial diagnosis until death, censoring as needed based on last follow-up date.
Baseline patient and treatment characteristics were evaluated using descriptive statistics. Comparisons between groups were made using independent samples t-tests for continuous variables and contingency tables with Pearson’s chi-squared test and two-sided Fisher’s exact tests for categorical variables, respectively. Kaplan–Meier analyses were performed for overall survival and freedom from interval development of brain metastases. Differences between groups were evaluated using Mantel-Cox log-rank, Breslow and Tarone-Ware tests. Cox proportional hazards modeling was used for both interval time to brain metastases and overall survival to estimate hazard ratios with 95% confidence intervals. Univariate Cox regression was used to identify covariates for multivariate regression using threshold P-value < 0.2, which was performed using backward conditional modeling. P-values < 0.05 were considered significant without adjustment for multiple comparisons.
Results
Within this cohort, median follow-up was approximately 10.6 years. Cumulatively, we examined 105 patients who developed subsequent NSCLC brain metastases following a median interval time of 16 months (95% CI 14.0–20.0). Approximately 13.3%, 13.3%, 37.1% and 36.2% were originally diagnosed with Stage I, II, III and IV NSCLC, respectively (Table 1). Patients were predominantly Caucasian individuals (61.0%) with lung adenocarcinomas (81.9%). Median age was 62. Among 72 patients who underwent molecular profiling, 22.2%, 16.7% and 6.9% of tumors were EGFR-, KRAS- and ALK-positive, respectively.
Both patient and treatment characteristics concerning interval development of NSCLC brain metastases are detailed in Table 2. Most patients had successfully maintained durable primary tumor control (72.1%) at the time of intracranial progression. Notably, almost 40% of patients who developed interval NSCLC brain metastases had no other distant metastases at that time. Patients who presented with Stage I and II disease experienced significantly higher incidences of subsequent brain metastases in the absence of other distant disease involvement (64.3%, p = 0.001). Following interval onset of NSCLC brain metastases, commonly administered treatments included whole-brain radiotherapy (WBRT) alone (27.5%), Gamma Knife radiosurgery (GKRS) alone (27.5%) and WBRT plus GKRS (16.5%). Regarding treatment modalities for interval brain metastases, while we detected no significant association between initial stage and number of brain metastases upon onset of intracranial disease involvement, treatment using surgical resection followed by WBRT was significantly more common among early-stage patients (35.7% for Stage I) and less common among individuals with advanced NSCLC (5.1% and 2.6% for Stage III and Stage IV, respectively; P = 0.020).
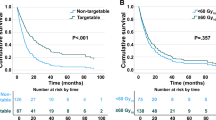
Notably, NSCLC stage was significantly associated with survival duration before interval development of brain metastases (Fig. 1a). Specifically, median interval times were 29 months (IQR 12–61), 19 months (IQR 9–47), 16 months (IQR 10–27) and 13 months (IQR 7–21) among Stage I–IV NSCLC patients, respectively (P = 0.016). Multivariate Cox regression (Table 3) confirmed that more advanced NSCLC stage predicted earlier onset of brain metastases (HR 1.602 for Stage II, HR 2.874 for Stage III and HR 3.501 for Stage IV versus Stage I; P = 0.016). Additional independent predictors for earlier development of NSCLC brain metastases included non-adenocarcinomatous histopathology (HR: 3.036, P < 0.001), no prior surgical resection (HR: 1.609, p P 0.036) and no prior systemic therapy (HR: 3.560, P = 0.004).
Kaplan–Meier analyses for impacts of NSCLC stage on a interval time to NSCLC brain metastases and b survival following onset of brain metastases. a Median interval time to development of brain metastases was 16.0 months (95% CI 12.4–19.6 months) overall with median interval durations of approximately 28.0 months among Stage I patients, 18.0 months among Stage II patients, 15.0 months among Stage III patients and 12.0 months among Stage IV patients, respectively (log-rank P = 0.017). b Median survival following interval development of brain metastases was approximately 16.0 months (95% CI 9.4–22.6 months) overall, with median survivals of 31.0 months among patients who originally presented with Stage I disease, 26.0 months for Stage II disease, 11.0 months for Stage III disease and 8.0 months for Stage IV disease, respectively (log-rank P = 0.042)
Median survival following interval onset of NSCLC brain metastases was approximately 16 months (95% CI 9.4–22.6 months). Notably, median survival following subsequent development of brain metastases was also associated with NSCLC stage (Fig. 1b), with survival durations of 31 months (IQR 23–NR), 26 months (IQR 12–NR), 11 months (IQR 4–43) and 8 months (IQR 4–35) among Stage I–IV patients, respectively (P = 0.042). Multivariate Cox regression revealed that delayed development of NSCLC brain metastases among patients who ultimately developed intracranial disease involvement predicted reduced risk of mortality (HR 0.970, 95% CI 0.957–0.984, P < 0.001) (Supplementary Table 1).
We subsequently examined prognostic factors for survival following interval development of brain metastases (Table 4). On univariate Cox regression, factors that were significantly associated with worsened survival following intracranial disease onset included disease-specific GPA score, older age, lower KPS, more advanced stage at initial diagnosis, concurrent presence of extracranial metastases, local failure at the time of intracranial disease onset, lack of initial surgical resection and non-adenocarcinomatous histology, but not interval time to development of brain metastases (Table 4). Multivariate Cox regression confirmed that lower lung cancer disease-specific GPA scores predicted better survival following onset of brain metastases (HR 0.531, 95% CI 0.390–0.724, P < 0.001), while concurrent extracranial metastases (HR 1.842, 95% CI 1.083–3.132, P = 0.024), local failure at onset of intracranial disease (HR 3.320, 95% CI 1.938–5.688, P < 0.001) and non-adenocarcinomatous histology (HR 3.031, 95% CI 1.643–5.595, P < 0.001) predicted increased risk of subsequent mortality.
Discussion
Particularly during recent years, accompanying dramatic improvements in systemic disease control with the advent of immune checkpoint inhibitors and novel molecularly targeted therapies, NSCLC brain metastases have emerged as an increasingly relevant clinical problem. Despite improving long-term survivorship rates associated with NSCLC, brain metastases remain a tremendous clinical, financial and psychosocial burden. Potential consequences include not only progressive neurocognitive deterioration [22, 23] but also heightened rates of cancer distress [24], diminished health-related quality of life [25, 26] and increased healthcare costs [27]. While prophylactic cranial irradiation has been proposed as a potential solution for NSCLC brain metastases, significant concerns remain given resulting neurocognitive toxicities and potential impacts on quality of life [28]. Conversely, routine intracranial surveillance imaging represents a potential alternative toward minimizing complications from NSCLC brain metastases, facilitating early detection and prompt treatment while avoiding potential neurocognitive decline associated with PCI.
Here, we retrospectively characterized the natural history and clinical course of subsequent NSCLC brain metastases during the modern era using a large institutional cohort with over 10 years of median follow-up, with the goals of helping inform both current practice and future research surrounding potential benefits and costs associated with intracranial surveillance imaging during routine follow-up care. We observed several noteworthy trends regarding the natural history of interval brain metastases. Notably, NSCLC patients originally diagnosed with early-stage disease who developed subsequent brain metastases most often presented with isolated brain metastases (64.3% for Stage I–II patients) in the absence of other distantly involved sites, a particularly encouraging trend given preliminary data supporting the benefits of potentially curative treatment among well-selected patients with oligometastatic disease [10, 29]. Encouragingly, patients originally diagnosed with early-stage NSCLC who developed subsequent brain metastases experienced prolonged survival even after intracranial disease onset, with median survivals of approximately 31 months (IQR 23–N.R.) and 26 months (IQR 12–NR) among Stage I and II patients, respectively. Regarding treatment patterns, we also observed a significantly greater frequency of surgery plus WBRT as a therapeutic modality among patients diagnosed with early-stage NSCLC (35.7% among Stage I patients) compared with locally-advanced and metastastic disease (5.1% and 2.6% among Stage III–IV patients, respectively), which could be explained by higher incidences of limited brain metastases in the setting of oligometastatic disease.
Across the entire cohort, median interval time from initial diagnosis until subsequent onset of brain metastases was 16 months (95% CI 14–20). While historic estimates have previously ranged from 9 to 13 months [16, 18, 20, 30,31,32], few studies have reported on interval time to NSCLC brain metastases during the modern era. Notably, our findings are highly consistent with a large retrospective multiinstitutional study of approximately 2200 brain metastasis patients evaluating prognostic significance of molecular alterations and receipt of tyrosine kinase inhibitors, which similarly reported a median interval time to development of NSCLC brain metastases of approximately 16 months [33]. Multiple retrospective series from China have reported similar estimates during the modern era [34, 35]. Notably, interval time to development of brain metastases was also significantly associated with NSCLC stage at diagnosis, with median durations of 28, 18, 15 and 12 months among Stage I, II, III and IV NSCLC patients, respectively. To our knowledge, this represents the most comprehensive study characterizing the natural history and clinical course of interval NSCLC brain metastases across all stages of disease. Most prior studies have specifically examined predictors of interval brain metastases among locally advanced NSCLC patients toward identifying potential subsets that might benefit from PCI [16, 19,20,21, 32, 34,35,36]. Multiple predictive factors have been identified for interval development of NSCLC brain including younger age [16, 18,19,20, 35, 36], adenocarcinomatous/non-squamous histology [19, 21, 31, 32, 36,37,38,39], advanced stage [30, 35, 38], larger primary tumor size or higher T stage [16, 31, 35, 38], hilar node involvement [16, 21], induction chemotherapy [30, 36, 37] and pretreatment serum tumor markers [36, 39], with potential roles for both immunohistochemical markers and genetic profiling including EGFR, ALK and KRAS mutations [40].
Here, we specifically investigated predictive factors impacting interval time to brain metastases among NSCLC patients who ultimately developed intracranial progression, rather than risk factors for development of NSCLC brain metastases. Within this cohort, more advanced NSCLC stage independently predicted faster onset of interval brain metastases. Interestingly, non-adenocarcinomatous histology also predicted earlier onset of interval brain metastases. While this finding was initially surprising given that adenocarcinomas are associated with increased risk of developing NSCLC brain metastases, one potential explanation could be particularly aggressive biologic features among non-adenocarcinomatous NSCLCs that do produce brain metastases, which could result in faster onset of brain metastases along with increased propensity toward intracranial spread. Both lack of surgical resection and lack of systemic therapy also emerged as independent predictors of earlier onset of NSCLC brain metastases, as although most Stage I–II patients underwent surgery and most Stage III–IV patients received systemic therapy, a minority of patients did not due to noncompliance or concurrent medical comorbidities. Interestingly, regarding the fact that surgical resection was associated with longer freedom from interval NSCLC brain metastases, optimal roles for surgical resection in the management of Stage III NSCLC remain controversial. While randomized controlled data has not demonstrated that surgical resection provides a significant survival benefit for Stage III patients with N2 disease [41, 42], current nationwide guidelines nevertheless emphasize the importance of offering surgical resection for resectable patients given potential opportunities for cure. While our findings do not address potential impacts of surgical resection toward prevention of brain metastases, these results do suggest a potential benefit toward delaying interval time to brain metastases among Stage III NSCLC patients who ultimately develop intracranial disease involvement. While a subset analysis of Stage III NSCLC patients within this cohort demonstrated only a nonsignificant trend toward delayed development of brain metastases with receipt of surgical resection (data not shown), this question warrants further research given potential clinical implications. Encouragingly, receipt of systemic therapy also independently predicted delayed onset of brain metastases, consistent with a growing wealth of evidence over the past decade supporting intracranial activity of tyrosine kinase inhibitors among patients with EGFR- and ALK-mutated NSCLC [43]. Notably, we did not detect a significant association between common lung cancer mutations (including EGFR, ALK and KRAS) and interval time to subsequent brain metastases. However, statistical power may have been limited by our sample size and only partial availability of mutational profiling. Notably, a large multiinstitutional retrospective analysis conducted by Sperduto and colleagues did find a significant association between both EGFR and ALK mutations and prolonged interval times to brain metastases [44].
Compared with historic survival estimates of only 3–6 months among NSCLC brain metastases patients, we observed a median survival of approximately 16 months (95% CI 9.4–22.6) following intracranial disease onset. Encouragingly, Sperduto and colleagues recently conducted a retrospective multiinstitutional review that reported a median survival of approximately 12 months among a modern cohort diagnosed from 2006 to 2014, compared with only 7 months within the original disease-specific GPA cohort [33, 45]. Meanwhile, overall prognostic significance of lung cancer brain metastases remains controversial. Previously, one population-based study identified development of subsequent NSCLC brain metastases as a poor prognostic factor [12]. Multiple retrospective studies have also found the development of brain metastases to be associated with worsened survival [36] and increased risk of death [21] among locally-advanced NSCLC patients. However, a recent multiinstitutional study that examined causes of death among patients with NSCLC brain metastasis found that the majority (82%) ultimately died from nonneurologic causes [44]. Here, despite a small effect size, we found that longer freedom from interval development of brain metastases predicted lower risk of mortality (HR 0.970, 95% CI 0.957–0.984) among NSCLC patients affected by subsequent brain metastases. However, interval time to brain metastases was not associated with survival following intracranial disease onset.
While our results are interesting, several limitations must be considered. Most notably, potential conclusions remain limited given the retrospective nature of our study design, due to resulting selection biases likely impacting both follow-up practices and treatment administration. Moreover, although we hope that our results may prove useful toward informing future research evaluating implementation of intracranial surveillance imaging, we were unable to evaluate true risks of developing NSCLC brain metastases over time due to the fact that this experimental cohort included only NSCLC patients who developed interval brain metastases (rather than a larger cohort encompassing all patients diagnosed with NSCLC, including those who never developed intracranial disease), limiting potential conclusions surrounding implications for screening. Regarding patient selection, we also acknowledge additional selection biases within our cohort given that we identified individuals who developed subsequent NSCLC brain metastases based on their receipt of radiation therapy for intracranial disease (therefore omitting individuals who may have declined treatment, those who may not have received brain radiotherapy due to effective treatment response of brain metastases from systemic therapy and those who may have died before initiating radiotherapy). Moreover, because of limited availability of molecular profiling within our cohort and fairly low mutational incidences, we were unable to control for specific impacts of molecularly targeted agents on interval time to brain metastases among NSCLC patients with EGFR- and ALK-positive tumors. Notably, the advent of immune checkpoint inhibitors have resulted in dramatic practice shifts for NSCLC during recent years, and given our study period, we acknowledge our inability to examine impacts of immune checkpoint inhibitors on interval time to brain metastases as an important limitation. For these reasons, our results should be considered hypothesis generating.
Despite these limitations, our results provide important insights into the natural history and clinical course of subsequent NSCLC brain metastases during the modern era. Given conflicting evidence regarding potential benefits of PCI for NSCLC and substantial concerns regarding long-term neurocognitive toxicity, routine intracranial surveillance imaging could represent an alternative solution, minimizing complications from brain metastases that may impact quality of life through prompt detection and minimally invasive treatment using stereotactic radiosurgery. We hope that these findings will not only help inform clinical practice and patient counseling for individuals with NSCLC, but also future research surrounding potential roles for intracranial surveillance imaging toward preventing symptomatic complications of NSCLC brain metastases that may compromise quality of life.
References
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2014) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol 22(14):2865–2872. https://doi.org/10.1200/JCO.2004.12.149
Wen PY, Loeffler JS (1999) Management of brain metastases. Oncol (willist Park 13(7):941–961
Lassman AB, DeAngelis LM (2003) Brain metastases. Neurol Clin N Am 21:1–23
Ostrom QT, Wright CH, Barnholtz-Sloan JS (2018) Brain metastases: epidemiology. Elsevier, Amsterdam. https://doi.org/10.1016/B978-0-12-811161-1.00002-5
Davis FG, Dolecek TA, Mccarthy BJ, Villano JL (2012) Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol 14(9):1171–1177
Schouten LJ, Rooten J, Huveneers HAM, Twijnstra A (2002) Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94(10):2698–2705. https://doi.org/10.1002/cncr.10541
Sorensen BJB, Hansen HH, Hansen M, Dombernowsky P (1988) Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol 6(9):1474–1480
Gomez DR Jr, Lee GRB, et al (2016) Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 17(12):1672–1682. https://doi.org/10.1016/S1470-2045(16)30532-0
Stephens SJ, Moravan MJ, Salama JK (2018) Managing patients with oligometastatic non-small-cell lung cancer POPULAR. J Oncol Pract 14(1):23–31. https://doi.org/10.1200/JOP.2017.026500
Hu C, Chang EL, Hassenbusch SJ et al (2006) Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer. https://doi.org/10.1002/cncr.21818
Sheehan JP, Sun M-H, Kondziolka D, Flickinger JC, Lunsford LD (2002) Radiosurgery for non-small cell lung carcinoma metastatic to the brain: long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg 97:1276–1281
Goncalves PH, Peterson SL, Vigneau FD et al (2016) Risk of brain metastases in patients with nonmetastatic lung cancer: analysis of the metropolitan detroit Surveillance, Epidemiology, and End Results (SEER) data. Cancer June:1921–1927. https://doi.org/10.1002/cncr.30000
Rusthoven CG (2018) Prophylactic cranial irradiation in non-small-cell lung cancer: the costs outweigh the benefits. J Clin Oncol 36(34):3433–3435. https://doi.org/10.1200/JCO.18.00732
Addeo A, Caparrotti F, Picardi C, Dietrich P-Y (2018) Prophylactic cranial irradiation in stage III non-small-cell lung cancer: overall survival should not necessarily be the final end point. J Clin Oncol 36(34):25–26. https://doi.org/10.1200/JCO.18.00461
Zeng H, Yuan S, Yu J (2018) Prophylactic cranial irradiation in non-small-cell lung cancer: hope or hype? J Clin Oncol 36(34):3431–3433. https://doi.org/10.1200/JCO.18.00617
Hubbs JL, Boyd JA, Hollis D, Chino JP, Saynak M, Kelsey CR (2010) Factors associated with the development of brain metastases. Cancer. https://doi.org/10.1002/cncr.25254
Figlin RA, Piantadosi S, Feld R, Group TLCS (1988) Intracranial recurrence of carcinoma after complete surgical resection of stage I, II and III non-small cell lung cancer. N Engl J Med 318(20):1300–1305
Ceresoli GL, Reni M, Chiesa G et al (2002) Brain metastases in locally advanced nonsmall cell lung carcinoma after multimodality treatment risk factors analysis. Cancer 95(3):605–612. https://doi.org/10.1002/cncr.10687
Gaspar LE, Chansky K, Albain KS et al (2005) Time from treatment to subsequent diagnosis of brain metastases in stage III non-small-cell lung cancer: a retrospective review by the Southwest Oncology Group. J Clin Oncol 23(13):2955–2961. https://doi.org/10.1200/JCO.2005.08.026
Carolan H, Sun AY, Bezjak A et al (2003) Does the incidence and outcome of brain metastases in locally advanced non-small cell lung cancer justify prophylactic cranial irradiation or early detection? Lung Cancer. https://doi.org/10.1016/j.lungcan.2004.12.004
Mamon HJ, Yeap BY, Ja PA (2005) High risk of brain metastases in surgically staged III A non-small-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J Clin Oncol 23(7):1530–1537. https://doi.org/10.1200/JCO.2005.04.123
Chang EL, Wefel JS, Ph D, Ph D, Allen PK, Ph D (2007) A pilot study of neurocognitive function in patients with one to three brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery 60(2):277–284. https://doi.org/10.1227/01.NEU.0000249272.64439.B1
Mehta MP, Rodrigus P, Terhaard CHJ et al (2003) Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol 21(13):2529–2536. https://doi.org/10.1200/JCO.2003.12.122
Cordes M, Scherwath A, Ahmad T et al (2014) Distress, anxiety and depression in patients with brain metastases before and after radiotherapy. BMC Cancer 14(731):1–11
Peters S, Bexelius C, Munk V, Leighl N (2016) The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev 45:139–162. https://doi.org/10.1016/j.ctrv.2016.03.009
Walker MS, Wong W, Ravelo A, Miller PJE, Schwartzberg LS (2018) Effect of brain metastasis on patient-reported outcomes in advanced NSCLC treated in real-world community oncology settings. Clin Lung Cancer 19(2):139–147. https://doi.org/10.1016/j.cllc.2017.10.003
Guerin A, Sasane M, Dea K, Zhang J, Culver K, Nitulescu R (2016) The economic burden of brain metastasis among lung cancer patients in the United States. J Med Econ 19(5):526–536
DeRuysscher D, Dingemans AC, Praag J et al (2018) Prophylactic cranial irradiation versus observation in radically treated stage III Non-small-cell lung cancer: a randomized phase III NVALT-11/DLCRG-02 study. J Clin Oncol. https://doi.org/10.1200/JCO.2017.77.5817
Palma DA, Olson RA, Harrow S et al (2018) Stereotactic ablative radiation therapy for the comprehensive treatment of oligometastatic tumors (SABR-COMET): results of a randomized trial. Int J Radiat Oncol Biol Phys 102(3):S3–S4. https://doi.org/10.1016/j.ijrobp.2018.06.105
Robnett TJ, Machtay M, Stevenson JP, Algazy KM, Hahn SM (2001) Factors affecting the risk of brain metastases after definitive chemoradiation for locally advanced non-small-cell lung carcinoma. J Clin Oncol 19(5):1344–1349
Bajard A, Westeel V, Dubiez A et al (2004) Multivariate analysis of factors predictive of brain metastases in localised non-small cell lung carcinoma. Lung Cancer 45:317–323. https://doi.org/10.1016/j.lungcan.2004.01.025
Chen AM, Jahan TM, Jablons DM, Garcia J, Larson DA (2007) Risk of cerebral metastases and neurological death after pathological complete response to neoadjuvant therapy for locally advanced. Cancer 109(8):1668–1675. https://doi.org/10.1002/cncr.22565
Sperduto PW, Kased N, Roberge D et al (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30(4):419–425. https://doi.org/10.1200/JCO.2011.38.0527
Ding X, Dai H, Hui Z et al (2012) Risk factors of brain metastases in completely resected pathological stage IIIA-N2 non-small cell lung cancer. Radiat Oncol 7(119):1–10
Chang W, Wu Y, Su P, Yang S, Lin C (2018) The impact of EGFR mutations on the incidence and survival of stages I to III NSCLC patients with subsequent brain metastasis. PLoS ONE 13:1–16
Ji Z, Bi N, Wang J et al (2014) Risk factors for brain metastases in locally advanced non-small cell lung cancer with definitive chest radiation. Radiat Oncol Biol 89(2):330–337. https://doi.org/10.1016/j.ijrobp.2014.02.025
Andre F, Grunenwald D, Pujol JL, Girard P, Dujon A, Brichon PY (2001) Patterns of relapse of N2 nonsmall-cell lung carcinoma patients treated with preoperative chemotherapy should prophylactic cranial irradiation be reconsidered? Cancer 91(12):2394–2400
Won Y, Joo J, Yun T et al (2015) Lung Cancer A nomogram to predict brain metastasis as the first relapse in curatively resected non-small cell lung cancer patients. Lung Cancer 88(2):201–207. https://doi.org/10.1016/j.lungcan.2015.02.006
Zhang F, Zheng W, Ying L, Wu J, Wu S (2016) A Nomogram to Predict Brain Metastases of Resected Non-Small Cell Lung Cancer Patients. Ann Surg Oncol 23:3033–3039. https://doi.org/10.1245/s10434-016-5206-3
An N, Jing W, Wang H et al (2018) Risk factors for brain metastases in patients with non-small cell lung cancer. Cancer Med. https://doi.org/10.1002/cam4.1865
Albain KS, Swann RS, Rusch VW et al (2009) Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 374(9687):379–386. https://doi.org/10.1016/S0140-6736(09)60737-6
Meerbeeck JP, Van Kramer GWPM, Schil PEY, Van et al (2007) randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. JNCI. https://doi.org/10.1093/jnci/djk093
Chamberlain MC, Baik CS, Gadi VK, Bhatia S, Chow LQM (2017) Systemic therapy of brain metastases: non-small cell lung cancer, breast cancer and melanoma. Neuro Oncol 19(1):1–24. https://doi.org/10.1093/neuonc/now197
Sperduto PW, Yang TJ, Beal K et al (2016) The effect of gene alterations and tyrosine kinase inhibition on survival and cause of death in patients with adenocarcinoma of the lung and brain metastases. Int J Radiat Oncol Biol Phys 96(2):406–413. https://doi.org/10.1016/j.ijrobp.2016.06.006
Sperduto PW, Yang TJ, Beal K et al (2017) Estimating survival in patients with lung cancer and brain metastases an update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA). JAMA Oncol 55387:827–831. https://doi.org/10.1001/jamaoncol.2016.3834
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Wang reports personal fees and non-financial support from AbbVie, non-financial support from Merck, personal fees from AstraZeneca, personal fees from Doximity, personal fees and non-financial support from Novocure, personal fees and non-financial support from Elekta and personal fees from Wolters Kluwer, outside the submitted work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Smith, D.R., Bian, Y., Wu, CC. et al. Natural history, clinical course and predictors of interval time from initial diagnosis to development of subsequent NSCLC brain metastases. J Neurooncol 143, 145–155 (2019). https://doi.org/10.1007/s11060-019-03149-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03149-4