Abstract
Background
Glioma surgery at its nascency relied predominantly on visual and tactile feedback for the removal of grossly abnormal tissue. This technique has inherent limitations in delineating infiltrative tumor from normal brain, thus limiting the ability to achieve a gross total resection consistently. Since extent of resection (EOR) is consistently correlated with measures of survival, fluorescence-guided surgery shows promise in improving our ability to treat high-grade gliomas (HGG). 5-Aminolevulinic acid (5-ALA) is a prodrug preferentially metabolized by glioma cells that allows direct, real-time visualization of pathologic tissue through fluorescence under blue light.
Objective
To report the relationship between 5-ALA and EOR in newly diagnosed HGG. To report our institutional experience including nuances of workflow.
Methods
The authors performed a systematic review of the available literature between 1998 and 2018 to isolate studies addressing the impact of fluorescence-guided surgery with 5-ALA on the EOR in newly diagnosed HGG. Search strategy was in adherence to the preferred reporting items for systematic reviews and meta-analyses methodology.
Results
Out of 741 unique articles, eight fulfilled our strict inclusion criteria. Fluorescence-guided resection led to greater EOR in all studies, with six demonstrating statistical significance (p < 0.05). Two studies additionally demonstrated statistically significant increase in progression-free survival in the 5-ALA groups.
Conclusions
5-ALA has an unambiguously positive impact on improving EOR for newly diagnosed HGG. Since the nature of modern glioma surgery includes a complex arsenal of surgical adjuncts, 5-ALA is seldom examined in isolation and can be complemented by intraoperative MRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Primary malignant brain tumors by their inherent nature are incurable, locally infiltrative, and diffusely present through the central nervous system by the time of their initial diagnosis. While the long-term prognosis for patients with high-grade gliomas (HGG) is unanimously dismal, numerous studies have demonstrated a survival benefit to maximizing local control with cytoreduction > 90–98% followed by chemotherapy and radiation [1,2,3]. Progression of disease, despite modern advances in chemotherapy and radiation, most commonly develops within 2 cm of the initial tumor resection margins [4]. The steadfast inevitability of local recurrence has driven numerous advances in glioma surgery, which aim to improve local control of disease and impart a cytoreductive benefit that translates to survival benefit.
By nature of its infiltrative disease, abnormal tumor cells can be found well beyond the margins of what is radiographically abnormal as well as what is grossly abnormal to the unassisted eye. These limitations are neither overcome by simple light microscopy nor appreciable by intraoperative tactile feedback. This brings to light the need to improve identification of abnormal tissue at tumor margins and at the periphery of a gross resection. Several adjunct modalities that have arisen include fluorescein, ex-vivo Raman spectroscopy, intraoperative MRI (iMRI), and 5-aminolevulinic acid (5-ALA). Additional adjuncts that aim to minimize surgical morbidity and maximize EOR include intraoperative mapping and neurophysiological monitoring, whole brain tractography, stereotactic navigation, and functional MRI [5,6,7,8,9,10].
Often times these adjuncts are used not in isolation but simultaneously since each have their inherent benefits and limitations. The objective of this review is to identify the potential role of 5-ALA in the surgical management of newly diagnosed HGG. While the precise mechanism of 5-ALA to protoporphyrin IX accumulation remains elusive, it is hypothesized to relate to both endothelial proliferation as well as metabolic activity. Thus, its uptake and conversion in higher-grade tumor cells is more avid than surrounding tissue [11]. While we would ideally aim to review a collection of high quality studies that focused on 5-ALA as the sole surgical adjunct, the nature of advanced glioma surgery is one in which multimodal techniques and technologies are used. Thus, there is currently a finite body of evidence examining the effect of 5-ALA in isolation with respect to residual contrast-enhancing tumor on postoperative MRI.
Methods
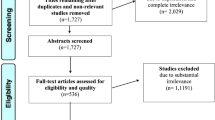
An extensive exploration of the available literature began with a search of PubMed, Web of Science, Cochrane library, and MEDLINE querying the following MeSH (medical subject heading) terms in title and abstracts between March 1998 and June 2018: “5-aminolevulinic acid”, “glioma”, “high-grade glioma”, “glioblastoma”, “resection”, and “fluorescence-guided surgery”. We defined March 1998 as the lower limit of our temporal window as this marks one of the earliest studies investigating FGS with 5-ALA for glioma [12]. Nonetheless, the majority of studies included in the final analysis came after 2007 when the European Medicines Agency officially authorized 5-ALA for use in glioma [13]. Inclusion criteria limited our examination to studies encompassing newly diagnosed high-grade gliomas in which maximal cytoreductive surgery was attempted with a primary outcome of EOR and/or GTR. The preferred reporting items for systematic reviews and meta-analyses (PRISMA) paradigm was used to report our search and screening strategy for finding relevant research articles (Fig. 1).
The studies extracted were further assessed for authorship, publication year, study design, recruitment period, follow-up, sample size, primary outcomes, and secondary outcomes. The quality of evidence was reported according to the Methodology of Guideline Development endorsed by the American Association of Neurologic Surgeons and the Congress of Neurological Surgery [14].
Results
The aforementioned search strategy returned 741 unique articles, of which 141 abstracts were screened for eligibility. While review of the literature produced a plethora of publications, of the 47 full text articles that were reviewed only eight fulfilled our inclusion criteria of tackling (1) newly diagnosed HGG, (2) utilizing 5-ALA, and (3) addressing extent of resection as a primary endpoint. The majority of studies excluded were either reviews, case reports, or studies that did not systematically report extent of resection (or rate of gross total resection as a comparable surrogate) (Table 1).
Search queries of four major electronic databases ultimately yielded a total of eight articles that were included in the final analysis. Two studies were prospective in nature and provided Class I evidence [15, 16], an additional two studies were retrospective case-control studies demonstrating Class II evidence [17, 18], and the remaining four studies were single-center case-series providing Class III evidence (Table 1). The overwhelming theme, consistent among all levels of evidence quality, was that FGS contributed positively towards extent of resection in newly diagnosed high-grade gliomas.
Extent of resection
Eyupoglu’s group out of Germany report their prospective, single center experience of 37 HGG patients that underwent resection using 5-ALA coupled with iMRI; they describe this combination as a “dual intraoperative visualization approach” and explicitly outline their intraoperative protocol. For the subset of lesions that were deemed completely resectable preoperatively, the addition of iMRI to 5-ALA increased the EOR from 71.7 to 100%. When examining the subgroup of lesions near eloquent structures, the addition of iMRI still increases EOR from 57.6 to 71.2% [15]. To contrast these findings, Roder et al. [19] compared each intraoperative tool in isolation only to find much lower rates of GTR; in this retrospective analysis of initial GBM resections, the iMRI arm achieved total resection in 74% of cases while the 5-ALA only group achieved GTR 46% of the time. This once again underscores the complementary nature of iMRI to 5-ALA guided resections.
Survival
Among the studies identified, several included overall survival and progression free survival as secondary outcome measures of treatment success. The Phase III randomized control trial comparing 5-ALA to traditional white-light microscopy demonstrated a statistically significant twofold improvement for 6-month progression-free survival (PFS) (41 and 21%, respectively) [16]. This finding was similarly remonstrated by Kim et al. [20] in their single-surgeon series of 80 consecutive, newly diagnosed HGG patients. In this group, progression free survival was 18 months for the 5-ALA group and 6 months for the white-light control group, each achieving 97 and 84.7% EOR, respectively.
Two other studies within this review also reported on survival outcomes, of note overall survival (OS) and PFS were not significantly different between the combination 5-ALA/iMRI group and only 5-ALA group [18]. This was similar to the Coburger et al. [17] matched case-control study of combined 5-ALA/iMRI compared to iMRI alone where 6-month PFS and OS were not significantly different despite higher rates of GTR in the multimodality group. Strong conclusions can’t be drawn from variables such as survival in these studies because cases and controls were not matched with respect to post-operative interventions, performance status, and adjuvant treatments. Additionally, the iMRI only group from the Schatlo study [18] had a statistically significant selection bias where patients with better preoperative Karnofsky Performance Score (KPS) were more likely to utilize iMRI.
Discussion
Over the past decade, high quality evidence has demonstrated that extent of resection in glioma surgery translates to improvements in overall survival and progression free survival [1, 3]. While the outcome measure of overall survival is relatively straightforward, the implication of progression free survival is that not only are these patients living longer, but also that their functional status and quality of life would remain stable during this period.
For the purpose of this review, we explicitly focus on extent of resection as the primary endpoint in evaluating the efficacy of FGS as a surgical adjunct. In the two case-control studies included in the analysis, the combination of 5-ALA and iMRI yielded a statistically significant increase in EOR in comparison to iMRI alone [17] or 5-ALA alone [18]. This theme is illustrative of the synergy between these two tools. For instance, while the accumulation of fluorophore in glioma cells allows real-time visualization of tumor isles at the boundaries of resection, this same abnormal tissue may not fluoresce if it is behind a mantle of healthy tissue. With the addition of iMRI, we may subsequently appreciate that contrast-enhancing residual tumor still lies immediately beyond direct visualization. This precise scenario of residual contrast enhancement despite resection of all evidently fluorescent tissue is described by Eyupoglu et al. and Prada et al. [8, 15].
FGS has been introduced as a modality in the early 2000s, with 5-ALA gaining approval for use in glioma by 2007 in the European Union and 2017 in the United States of America [13, 21]. Surgery for high-grade gliomas routinely employs a number of surgical adjuncts, including stereotactic navigation and intraoperative MRI. To this effect, we aimed to demonstrate how 5-ALA use contributes towards EOR regardless of what other adjuncts are used. Combining studies of differing methodologies limits our ability to report any quantitative outcome regarding EOR and GTR rates, but it does highlight a persistent, cohesive qualitative analysis that points towards a positive benefit of 5-ALA for improving resection of contrast-enhancing tumor in HGG.
The role of 5-ALA, despite its repeatedly demonstrated benefit for EOR, is not quite at a stage to be heralded as a standard of care modality. Instead, FGS represents an important tool in the surgeon’s armamentarium for select cases based on tumor location, potential surgical radiciality, the patient’s values, and the patient’s aversion to risk.
An inherent limitation of this review is the relative paucity of studies explicitly examining 5-ALA in isolation with respect to HGG. For this reason, several studies were included due to their promising study design despite including some recurrent HGG or intentionally incompletely resected tumors. For these two studies, Tsugu et al. and Della Puppa et al. [22, 23], we focused on the contribution from the HGG subgroup and the newly diagnosed HGG subgroup, respectively.
The majority of the studies selected for final analysis had inclusion criteria that limited patient participation to those that had tumors deemed entirely resectable. This brings to light an inherent selection bias since tumors within or near eloquent areas were preferentially underrepresented. While other surgical adjuncts such as intraoperative cortical mapping and whole-brain tractography may help at the border of eloquent areas, the extent of resection in these instances is influenced by complex factors including the patient’s willingness to accept a neurological deficit and their desire to maximize potential survival advantage. Ultimately, the selection of surgical approach, adjuncts, and goals of surgery in high-grade glioma should be tailored to the values of the individual patient. An example of the ideal candidate for a combined 5-ALA + iMRI resection would be a suspected HGG in the nondominant frontal, temporal, or even occipital lobe.
Institutional experience
As an early adopter of 5-ALA in the United States, we wish to report our institution’s experience and nuances of implementation to support those that may wish to pioneer this tool in their own locality. The application of this modality requires multidisciplinary engagement from intake to discharge of the patient. The presurgical unit is familiar with the low-light requirements for 5-ALA treated patients. Additionally, a neurosurgical intensive-care unit bed is reserved the day prior to scheduled surgery to ensure a low-light environment is available immediately post-operatively. When in transit within the hospital, the patient is cloaked from head to toe in linen coverings to minimize exposed skin surfaces and therefore the risk of phototoxicity and dermatitis [24].
The surgical workflow includes administration of oral 5-ALA (Gleolan, NX Development Corp, Lexington KY, USA) 4 h prior to anesthesia induction [21]. This timing is in anticipation of peak fluorescence culminating 6–8 h after ingestion. While oral bioavailability may be a theoretical limitation, phase 1 dose escalation studies demonstrate safety and efficacy of 5-ALA at doses twofold greater than the generally accepted dose of 20 mg/kg reported in prior studies and recommended by the EMA [21, 25, 26].
To date we have utilized 5-ALA in nine glioma patients, four of which were newly diagnosed HGG (Table 2). Abnormal tissue avidly fluoresced in each instance with pathology unanimously demonstrating HGG by histology in patients newly diagnosed. Out of 44 fluorescent specimens sent for histological analysis, 43 were positive for HGG. The remaining sample that fluoresced but did not contain glioma showed subpial gliosis with reactive changes in a patient with recurrent GBM. Protoporphyrin IX has been known to accumulate in prior areas of inflammation and gliosis around recurrent tumors [9]. In this series of nine consecutive patients, the positive predictive value (PPV) of a fluorescent tissue sample is 97% for all cases, but when recurrent gliomas are excluded, the PPV increases to 100%. This comes as little surprise given the positive predictive value of fluorescent tissue has ranged between 95 and 100% in high quality studies [12, 17, 27,28,29].
Our experience also corroborates the utility of 5-ALA in combination with iMRI affording greater utility than either one alone. In one instance, a 48 year-old female presented with vision changes and was found to have right parietooccipital HGG. Traditional white-light illumination was used to resect grossly abnormal tissue, while 400–410 nm blue-light illumination was utilized intermittently to identify avidly fluorescing tissue until no more fluorescent tissue was visible along all possible working angles within the cavity. The patient was subsequently transferred to the integrated iMRI suite where a significant volume of contrast-enhancing tumor was still visible anterior and medial to the operative bed (Fig. 2a). The patient was brought back for further resection of the residual contrast-enhancing (CE) region to achieve a gross total resection as illustrated by the early postoperative scan (Fig. 2b). Interestingly, this newly identified region of contrast enhancement did not fluoresce under blue-light—histology for this fragment of non-fluorescent tissue showed mildly hypercellular brain parenchyma and fragments of hemostatic gelatin powder but no tumor cells. This finding highlights the inherent differences between using contrast enhanced MRI versus 5-ALA to determine residual tumor. Contrast enhancement is determined by gadolinium extravasation and can be found in conditions other than tumor, such as injured brain caused by surgery. On the other hand, fluorescence is a metabolic marker. As such, at least in this case, fluorescence was a more accurate measure of tumor.
a Intraoperative MRI T1 + contrast depicting residual contrast-enhancing (CE) tissue anterior and medial to the resection cavity. iMRI was performed after all visibly fluorescent tissue had been resected in piecemeal fashion. This CE tissue did not fluoresce under blue-light, pathology showed mildly hypercellular parenchyma but no HGG. b Early postoperative MRI T1 +contrast showing that the previously CE-tissue identified on iMRI was subsequently completely resected
We have also encountered the opposite scenario where iMRI depicts complete resection of CE tissue yet blue-light microscopy demonstrates persistent fluorescence with positive histological margins (Fig. 3). This 60 year-old female with prior history of low-grade glioma who presented with left hemiparesis and likely malignant transformation to HGG in the right frontal precentral gyrus (Fig. 3a). The subsequent resection was performed as an awake craniotomy with 5-ALA, intraoperative neurophysiological motor mapping, as well as iMRI. Towards the limits of our resection, subcortical stimulation yielded motor response at 7 mA and we appeared to be at the boundaries of the tumor based on stereotactic navigation. The remnant tissue, however, was still fluorescing avidly but the decision was made to halt and check the resection status with iMRI given the patient was still spontaneously moving her left upper extremity on command. As shown in Fig. 3b, all contrast-enhancing tumor had been resected yet fluorescent tissue was clearly still visible (Fig. 3c). This was likely reflective of residual non-enhancing lower-grade glioma tissue. (Fig. 3d). Subsequent delayed postoperative MRI after temozolomide and radiotherapy demonstrated numerous subcentimeter foci of disease progression anterior, medial, and superior to the resection cavity infiltrating along the corona radiata.
a Preoperative MRI T1 + contrast depicting right frontal contrast enhancing lesion. b Intraoperative MRI T1 + contrast depicting gross total resection of all enhancing tissue. c View through operative microscope with blue-light demonstrates residual fluorescence, though not as avid as the majority of the tumor. d Intraoperative MRI T2 fluid-attenuated inversion recovery (FLAIR) sequence, likely representing residual non-enhancing lower grade tumor cells
Conclusion
5-ALA has demonstrated an undisputed benefit in providing real-time tumor visualization, high diagnostic accuracy, and minimal deviation from standard operative workflow for HGG resection. Not only has this pro-drug demonstrated its merit in comparison to standard white-light resection, but the addition of iMRI has also shown synergistic benefit when it comes to EOR and consequently survival advantage.
These two tools together overcome numerous limitations of standard glioma surgery, with 5-ALA providing real-time delineation of tumor margins and iMRI revealing hidden nests where tumor may still be obscured.
Abbreviations
- 5-ALA:
-
5-Aminolevulinic acid
- CE:
-
Contrast enhancing
- EOR:
-
Extent of resection
- EMA:
-
European Medicines Agency
- FDA:
-
Food and Drug Administration
- FGS:
-
Fluorescence-guided surgery
- FLAIR:
-
Fluid attenuated inversion recovery
- GTR:
-
Gross total resection
- HGG:
-
High-grade glioma
- iMRI:
-
Intraoperative magnetic resonance imaging
- KPS:
-
Karnofsky Performance Scale
- MRI:
-
Magnetic resonance sequence
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
References
Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE (2014) Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol 32(8):774–782. https://doi.org/10.1200/jco.2013.51.8886
Orringer D, Lau D, Khatri S, Zamora-Berridi GJ, Zhang K, Wu C, Chaudhary N, Sagher O (2012) Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg 117(5):851–859. https://doi.org/10.3171/2012.8.Jns12234
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115(1):3–8. https://doi.org/10.3171/2011.2.Jns10998
Eljamel S (2015) 5-ALA fluorescence image guided resection of glioblastoma multiforme: a meta-analysis of the literature. Int J Mol Sci 16(5):10443–10456. https://doi.org/10.3390/ijms160510443
Gessler F, Forster MT, Duetzmann S, Mittelbronn M, Hattingen E, Franz K, Seifert V, Senft C (2015) Combination of intraoperative magnetic resonance imaging and intraoperative fluorescence to enhance the resection of contrast enhancing gliomas. Neurosurgery 77(1):16–22. https://doi.org/10.1227/neu.0000000000000729 (Discussion 22)
Jenkinson MD, Barone DG, Bryant A, Vale L, Bulbeck H, Lawrie TA, Hart MG, Watts C (2018) Intraoperative imaging technology to maximise extent of resection for glioma. The Cochrane database of systematic reviews. 1:Cd012788. https://doi.org/10.1002/14651858.CD012788.pub2
Kittle D, Mamelak A, Parrish-Novak J, Hansen S, Patil R, Wadhone-Gangalum P, Ljubimova J, Black KL, Butte P (2014) Fluorescence-guided tumor visualization using the tumor paint BLZ-100. Cureus. https://doi.org/10.7759/cureus.210
Prada F, Bene MD, Fornaro R, Vetrano IG, Martegani A, Aiani L, Sconfienza LM, Mauri G, Solbiati L, Pollo B, DiMeco F (2016) Identification of residual tumor with intraoperative contrast-enhanced ultrasound during glioblastoma resection. Neurosurg Focus 40(3):E7. https://doi.org/10.3171/2015.11.Focus15573
Stummer W, Suero Molina E (2017) Fluorescence imaging/agents in tumor resection. Neurosurg Clin N Am 28(4):569–583. https://doi.org/10.1016/j.nec.2017.05.009
Yamada S, Muragaki Y, Maruyama T, Komori T, Okada Y (2015) Role of neurochemical navigation with 5-aminolevulinic acid during intraoperative MRI-guided resection of intracranial malignant gliomas. Clin Neurol Neurosurg 130:134–139. https://doi.org/10.1016/j.clineuro.2015.01.005
Marbacher S, Klinger E, Schwyzer L, Fischer I, Nevzati E, Diepers M, Roelcke U, Fathi AR, Coluccia D, Fandino J (2014) Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus 36(2):E10. https://doi.org/10.3171/2013.12.Focus13464
Stummer W, Stocker S, Wagner S, Stepp H, Fritsch C, Goetz C, Goetz AE, Kiefmann R, Reulen HJ (1998) Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery 42(3):518–525 (Discussion 525–516)
CHMP (2007) Gliolan; INN 5-aminolevulinic hydrochloric acid scientific discussion. London, UK. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000744/WC500021788.pdf
Hadley MN, Walters BC, Grabb PA, Oyesiku NM, Przybylski GJ, Resnick DK, Ryken TC (2002) Methodology of guideline development. Neurosurgery 50(3 Suppl):S2–S6. https://doi.org/10.1097/00006123-200203001-00004
Eyupoglu IY, Hore N, Savaskan NE, Grummich P, Roessler K, Buchfelder M, Ganslandt O (2012) Improving the extent of malignant glioma resection by dual intraoperative visualization approach. PloS ONE 7(9):e44885. https://doi.org/10.1371/journal.pone.0044885
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7(5):392–401. https://doi.org/10.1016/s1470-2045(06)70665-9
Coburger J, Hagel V, Wirtz CR, Konig R (2015) Surgery for glioblastoma: impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PloS ONE 10(6):e0131872. https://doi.org/10.1371/journal.pone.0131872
Schatlo B, Fandino J, Smoll NR, Wetzel O, Remonda L, Marbacher S, Perrig W, Landolt H, Fathi AR (2015) Outcomes after combined use of intraoperative MRI and 5-aminolevulinic acid in high-grade glioma surgery. Neuro-oncology 17(12):1560–1567. https://doi.org/10.1093/neuonc/nov049
Roder C, Bisdas S, Ebner FH, Honegger J, Naegele T, Ernemann U, Tatagiba M (2014) Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: high-field iMRI versus conventional and 5-ALA-assisted surgery. Eur J Surg Oncol 40(3):297–304. https://doi.org/10.1016/j.ejso.2013.11.022
Kim SK, Choi SH, Kim YH, Park C-K (2014) Impact of fluorescence-guided surgery on the improvement of clinical outcomes in glioblastoma patients. Neurooncol Pract 1(3):81–85. https://doi.org/10.1093/nop/npu011
Kaufman MB (2017) Pharmaceutical approval update. Pharm Ther 42(11):673–683
Della Puppa A, Ciccarino P, Lombardi G, Rolma G, Cecchin D, Rossetto M (2014) 5-Aminolevulinic acid fluorescence in high grade glioma surgery: surgical outcome, intraoperative findings, and fluorescence patterns. BioMed Res Int 2014:232561. https://doi.org/10.1155/2014/232561
Tsugu A, Ishizaka H, Mizokami Y, Osada T, Baba T, Yoshiyama M, Nishiyama J, Matsumae M (2011) Impact of the combination of 5-aminolevulinic acid-induced fluorescence with intraoperative magnetic resonance imaging-guided surgery for glioma. World Neurosur 76(1–2):120–127. https://doi.org/10.1016/j.wneu.2011.02.005
Teixidor P, Arraez MA, Villalba G, Garcia R, Tardaguila M, Gonzalez JJ, Rimbau J, Vidal X, Montane E (2016) Safety and efficacy of 5-aminolevulinic acid for high grade glioma in usual clinical practice: a prospective cohort study. PloS ONE 11(2):e0149244. https://doi.org/10.1371/journal.pone.0149244
Cozzens JW, Lokaitis BC, Moore BE, Amin DV, Espinosa JA, MacGregor M, Michael AP, Jones BA (2017) A phase 1 dose-escalation study of oral 5-aminolevulinic acid in adult patients undergoing resection of a newly diagnosed or recurrent high-grade glioma. Neurosurgery 81(1):46–55. https://doi.org/10.1093/neuros/nyw182
Stummer W, Stepp H, Wiestler OD, Pichlmeier U (2017) Randomized, prospective double-blinded study comparing 3 different doses of 5-aminolevulinic acid for fluorescence-guided resections of malignant gliomas. Neurosurgery 81(2):230–239. https://doi.org/10.1093/neuros/nyx074
Diez Valle R, Tejada Solis S, Idoate Gastearena MA, Garcia de Eulate R, Dominguez Echavarri P, Aristu Mendiroz J (2011) Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neurooncol 102(1):105–113. https://doi.org/10.1007/s11060-010-0296-4
Idoate MA, Diez Valle R, Echeveste J, Tejada S (2011) Pathological characterization of the glioblastoma border as shown during surgery using 5-aminolevulinic acid-induced fluorescence. Neuropathology 31(6):575–582. https://doi.org/10.1111/j.1440-1789.2011.01202.x
Roberts DW, Valdes PA, Harris BT, Fontaine KM, Hartov A, Fan X, Ji S, Lollis SS, Pogue BW, Leblond F, Tosteson TD, Wilson BC, Paulsen KD (2011) Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J Neurosurg 114(3):595–603. https://doi.org/10.3171/2010.2.Jns091322
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Lee has consulting agreements with Medtronic and Monteris. Dr. Kalkanis has consulting agreements with Arbor Pharmaceuticals and Synaptive Medical. Dr. Haider and Dr. Lim declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Haider, S.A., Lim, S., Kalkanis, S.N. et al. The impact of 5-aminolevulinic acid on extent of resection in newly diagnosed high grade gliomas: a systematic review and single institutional experience. J Neurooncol 141, 507–515 (2019). https://doi.org/10.1007/s11060-018-03061-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03061-3