Abstract
Purpose
Routine brain MRI surveillance frequently diagnoses small, asymptomatic brain metastases from non-small cell lung cancer (NSCLC) that are effectively treated with stereotactic radiosurgery (SRS). A subset of patients, however, may die prior to the onset of symptoms. This study identifies clinical features that distinguish neurologically-asymptomatic NSCLC brain metastases patients that die prior to routine 3 month follow-up after SRS.
Methods
Retrospective chart review from 2007 to 2017 identified 18 patients with neurologically-asymptomatic NSCLC brain metastases who died < 3 months after SRS. Twenty-eight additional patients meeting criteria and surviving > 6 months after SRS were identified. Clinical factors were examined to determine characteristics correlated with survival using cox proportional hazards and nominal logistic regression models. Logistic regression models using salient factors were trained with 10-fold cross-validation and compared to the graded prognostic assessment (GPA) and score index of radiosurgery (SIR) using the AUC from receiver operant characteristic curves.
Results
The median survival following SRS was 1.4 and 9.2 months for the < 3 months and > 6 months groups, respectively. Age, number of brain metastases, and Karnofsky performance status were associated with overall survival while gender and interval between primary cancer and first brain metastasis diagnoses were associated with < 3 months and > 6 months survival, respectively. Models using GPA and SIR performed poorly compared to preliminary metrics generated in this study for prognosis of both < 3 months and > 6 months survival.
Conclusion
Physicians require data to provide high-value, cost-conscious health care. Clinical metrics can screen patients with asymptomatic NSCLC brain metastases likely to die prior to the standard screening interval and observation could be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain metastases from non-small cell lung cancer (NSCLC) occur in 20–40% of patients at some point in the disease course [1,2,3,4]. Patients with neurologically-symptomatic brain metastases require definitive treatment with options including SRS, tyrosine kinase inhibitors (TKIs), whole-brain radiation therapy (WBRT). However, routine follow up with increasingly sensitive MRI imaging has increased the likelihood of the early detection of small, neurologically-asymptomatic brain metastases [5,6,7]. Currently, there are no standard treatment or observation guidelines for these patients.
The decision to treat these lesions involves a multidisciplinary team guided by patient characteristics, disease profile, and clinical metrics. Several clinical features have been proposed in the literature as predictors of overall survival (OS) in cancer patients with brain metastases [2, 8,9,10,11,12]. In addition, a number of established prognostic metrics are in frequent clinical use including the disease-specific graded prognostic assessment (GPA) and the score index of radiosurgery (SIR) [9, 13]. However, most studies do not stratify their cohorts based on neurologic symptomatic status and therefore, these metrics may fail to capture these patients [12].
At our medical center, NSCLC patients with small, neurologically-asymptomatic brain metastases may be treated with SRS, a choice therapy for several small brain metastases (< 3 cm each, < 12 total) [8, 14, 15]. Our experience suggested that despite treatment with SRS, a subset of NSCLC patients with small, neurologically-asymptomatic brain metastases would die prior to routine 3-month follow-up, and that observation could have been considered. This study aimed to identify clinical and tumor characteristics to identify these patients. We then developed preliminary prognostic metrics to compare to the GPA and SIR.
Methods
We conducted a retrospective chart review of patients with brain metastases treated with SRS (CyberKnife®, Accuray, Sunnyvale, CA, USA) at Stanford Hospital between 2007 and 2017 (Fig. 1). Exclusion criteria included non-NSCLC histology, neurologically-symptomatic brain metastases, loss to follow-up prior to 6-months, and incomplete medical records. We identified 18 patients that met criteria and died within 3-months of SRS (< 3 months group), the standard and National Comprehensive Cancer Network recommended follow-up period [16]. We then identified 29 patients that met criteria and were alive at 6-month follow-up (> 6 months group) and included 9 patients that died between 3 and 6 months of SRS. For each patient, we collected information on disease time-course, therapeutic management, patient demographics, and tumor characteristics. As patients may undergo multiple SRS treatments, clinical features were calculated from the time of the most recent SRS procedure. The IRB at Stanford University approved this study.
All analyses were performed in the R (v3.4.4) statistical environment [17]. Clinical features were compared between survival groups using Fisher exact test for categorical variables and analysis of variance for continuous variables. Disease progression and survival time were estimated using the Kaplan–Meier (KM) method. Univariate and multivariate associations of clinical features and survival were evaluated with Cox proportional hazards regression model. Complimentary analyses were carried out with nominal logistic regression for the < 3 months (vs others) and for the > 6-months (vs others) models, independently. GPA and SIR were excluded from all multivariate analyses. Factors that had a univariate p value < 0.1 were considered for multivariate analysis. Results of all modeling were reported with a 95% confidence interval and a p value of < 0.05 was considered significant.
ROC analysis was carried to determine the prognostic value of our clinical metrics compared to the GPA and SIR. Nominal logistic regression models based on a single variable of the GPA and SIR were generated to examine < 3 months and > 6 months prognostic value. The models were tuned with their respective variables using 10-fold cross-validation over the entire cohort.
Results
Patient and disease characteristics
Patient demographics
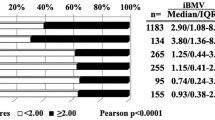
After applying exclusion criteria, 56 (57% female, median age: 64.4 years) patients remained for analysis (Table 1). A history of cigarette smoking was present in 48% of the patients. Patients in the < 3-months group (median age: 72 years) were significantly older than those in the > 6 months (median age: 60) group (p = 0.008). Ethnic composition was largely Asian (48%) and white (45%) with Hispanic/Latino, black/African-American, and others making up the remaining 7%. This demographic composition reflects that expected for our region22.
Primary and extracranial disease profile
At the time of the most recent SRS, 73% of patients had progressive extracranial disease as described on imaging immediately prior to treatment, 25% had liver metastases, and 50% had bone metastases. Of the 56 patients, 47 had available mutation panel testing. In this group, epidermal growth factor receptor (EGFR) mutations were the most prevalent somatic mutation (55%). ALK fusions were rare (7.1%). There were no differences across the groups along these factors.
Brain tumor profile
Overall, patients had a median of 3.5 metastases prior to the most recent SRS. Patients in the < 3-months group presented with a greater number of brain metastases than those in the > 6-months (median: 4.5 vs 2, receptively, p = 0.04). However, when stratified by the two most common extracranial metastases, this difference was only present in patients with bone (median if present: 9 vs 3, p = 0.02; if absent: 2 vs 2, p = 0.39) and liver metastases (median if present: 15 vs 4, p = 0.003; if absent: 2 vs 2, p = 0.36). The difference did not reach significance when stratified by extracranial disease status (if progressive: p = 0.059, if stable: p = 0.11). The largest brain metastasis volumes (median: 0.55 cm3) and total brain metastasis volumes (median: 1.4 cm3) were both distributed similarly across the three groups (p = 0.7 and p = 0.41, respectively). Over half of the largest lesions were localized to either the frontal lobe (32%) or cerebellum (23%) with no differences across groups (p = 0.41). Histology revealed most tumors were adenocarcinomas (94.6%). Further analysis did not stratify by histology.
Prior therapeutic management
The majority (56%) of patients had no history of prior SRS. There was no difference in prior brain radiation between the < 3-months (77%) and the > 6-months groups (56%, p = 0.21). Of the patients that received prior brain radiation (44.6%), the majority (87.9%) received 1–5 rounds of SRS treatment only. Prior to the most recent SRS, patients received rounds of chemotherapy (76.8%), immunotherapy (41%), and/or targeted therapy (54.6%). Of the patients that received EGFR-TKIs as targeted therapy, 87.6% received first generation medications.
Disease time-course and survival
At the time of analysis, 23% of patients were alive. The KM method was used to examine the disease time course divided into three intervals: primary cancer diagnosis to death or last follow-up (median time of 1.13 and 2.75 years for < 3-months and > 6-months groups, respectively; p = 0.02), primary cancer diagnosis to first brain metastasis diagnosis (median time of 0.09 and 1.27 years for < 3-months and > 6-months groups, respectively; p = 0.02), and most recent SRS to time of death or last follow-up (median time of 1.32 and 9.24 months for < 3-months and > 6-months groups, respectively; p < 0.001) (Fig. 2). Time to last SRS from primary cancer diagnosis (median: 15.9 months) and from first brain metastasis diagnosis (median: 4.9 months) did not differ between groups (Table 1).
Kaplan–Meier survival for disease time-course of patients that died within 3 months of SRS and those that survived for greater than 6 months. a OS probability of the two groups from time of primary cancer to death or last follow-up, b from time of primary cancer to brain metastasis diagnosis, and c from time of last SRS to death or last follow-up. In (b), 2 and 5 patients in the < 3-months and > 6-months, respectively, were diagnosed with brain metastases at the time of primary cancer diagnosis. CI confidence interval, GPA graded prognostic assessment, SIR stereotactic index of radiosurgery, OS overall survival, SRS stereotactic radiosurgery
Clinical metric characteristics
The mean KPS recorded prior to the most recent SRS was lower for the < 3-months than the > 6-months group (median: 70 vs 90, respectively, p < 0.001). GPA (median: 2.0 vs 1.0, p = 0.003) and SIR (median: 5.0 vs 4.0, p = 0.004) were also lower for the < 3-months vs > 6-months group (Table 1).
Clinical features associated with survival
In univariate analysis using cox proportional hazard models, age, male gender, the interval between primary cancer and first brain metastasis diagnoses, number of brain metastases, and KPS were associated with survival following the most recent SRS (Table 2). Male gender, number of brain metastases, and KPS remained significant in multivariate analyses.
Nominal logistic regression was carried out to separate factors associated with the < 3-months model (Table S1) and with the > 6-months model (Table S2). In univariate analysis for the < 3-months model, age, male gender, number of brain metastases, and KPS were significantly correlated with < 3-months survival. In the > 6-months model, age, interval between primary cancer and first brain metastasis diagnoses, number of brain metastases, and KPS were correlated with survival > 6-months. In multivariate analysis for the < 3-months model, gender and KPS remained as significant factors. For the > 6-months model, age, and interval between primary cancer and first brain metastasis diagnosis remained as significant factors. GPA and SIR were excluded from multivariate analysis as their values depended on other parameters (KPS, age, gender, number of brain metastases) in the model.
Clinical metrics associated with survival
Common clinical metrics (GPA and SIR) were evaluated using both cox proportional hazards on the whole cohort and nominal logistic regression between the three groups. GPA and SIR were prognostic of overall survival following SRS (Table 2). Additionally, the two metrics were significantly associated with odds of falling into the < 3-months group (vs others) (Table S1) and with odds of falling into the > 6-months group (vs others) (Table S2).
Prognostic value of clinical features and metrics
The < 3-months and > 6-months logistic regression models were trained using 10-fold cross-validation to generate ROCs to compare to logistic regression models trained using GPA and SIR. The < 3-months model was created using age, gender, number of brain metastases, and KPS as factors and the > 6-months model was created using age, interval between primary cancer and brain tumor diagnoses, number of brain metastases, and KPS as factors. These custom models (AUC for < 3-months: 0.84, 95% CI 0.8−0.87; >6-months: 0.77, 95% CI 0.73−0.81) were trained independently and performed better than those using GPA (AUC for < 3-months: 0.72, 95% CI 0.67−0.77; >6-months: 0.7, 95% CI: 0.65−0.74), SIR (AUC for < 3-months: 0.69, 95% CI 0.64−0.73; >6-months: 0.68, 95% CI: 0.64−0.73), or KPS independently (AUC for < 3-months: 0.8, 95% CI 0.8−0.87; >6-months: 0.69, 95% CI 0.64−0.73) (Fig. 3).
ROC curves for prognostic models were developed by training logistic regression models with 10-fold cross-validation on the entire cohort. The < 3 months and > 6 months models were generated based on factors determined to be prognostic in univariate logistic regression (Table S1 and Table S2). These were compared to logistic regression models based on KPS, GPA and SIR to examine prognostic value for a death within 3 months of SRS and for b survival of at least 6 months after SRS. AUC area under the curve, CI confidence interval, GPA graded prognostic assessment, KPS Karnofsky performance status, SIR stereotactic index of radiosurgery, SRS stereotactic radiosurgery
Discussion
Clinical features and metrics
SRS has been an effective treatment option for NSCLC patients with brain metastases, however, a subset of neurologically-asymptomatic patients die within a short period after treatment. In this study, we evaluated associations of clinical features with death < 3-months following SRS in 56 patients with small, neurologically-asymptomatic brain metastases from NSCLC. KPS, age, gender, number of brain metastases, and the time interval between primary cancer and brain tumor diagnoses were significantly associated with survival following SRS. Specifically, KPS and gender were correlated with < 3-months survival and age, interval of brain metastasis from primary cancer diagnoses, and KPS were associated with > 6-month survival.
KPS, age, gender, and number of brain metastases have been previously described as factors associated with survival in NSCLC brain metastases patients undergoing SRS and comprise calculations for the GPA and SIR clinical metrics [9, 13]. Patients in the < 3-months group had a shorter time interval between primary cancer diagnosis and identification of first brain metastasis than did those in the > 6-months group. This may suggest aggressive disease progression processes in the < 3-months group that better overcome the blood–brain barrier or other obstacles to metastasis. Once in the brain, both groups had neurologically-asymptomatic disease with a similar brain metastasis burden and no statistical differences in brain metastasis volumes.
The time interval between primary tumor and brain metastases diagnoses is not captured by GPA or SIR. Furthermore, these metrics fail to account for neurologic-symptom status and tumor genetic profile. For the latter, we collected information on the largest brain metastasis location and somatic mutation profiles of the primary tumors. The distribution of locations for the largest lesions, with an overall bias towards the cerebral cortex (68%) followed by the cerebellum (23%) and subcortical regions (9%), did not differ between groups nor were locations associated with survival. The overall differences may not reflect disease malignancy, and instead, be due to differences in cerebral perfusion [18].
To examine the role of tumor genomic characteristics in survival, mutation panel testing results were collected and analyzed. Of the 47 patients with available mutation panel testing, EGFR mutations were prevalent (55% of patients), an incidence that is higher than the general American population (10–15% of NSCLC brain metastases) [19, 20]. However, 48% of our cohort identified as Asian and high rates of EGFR-mutations have been observed in East Asian NSCLC patients (20–55%) [21,22,23]. In addition, prolonged OS has been observed generally in neurologically-asymptomatic patients and EGFR mutants, regardless of prior treatment [24, 25]. Regardless, the < 3-months and > 6-months groups had statistically similar distributions of EGFR mutations. Similar to the general population, ALK mutations were rare in our cohort. However, the importance of accounting for molecular features of NSCLC is highlighted in recent studies arguing for updates to clinical metrics including the GPA [26]. Spertudo and colleagues (2017) propose the lung-molGPA, a modified GPA metric that includes ALK and EGFR mutations statuses.
Guidance for therapeutic management
Clinical teams have little guidance when considering treatment vs observation for small, asymptomatic brain metastases identified on routine surveillance MRI. Identifying clinical characteristics associated with morbidity prior to neurologic symptom onset may help to avoid interventions that may not otherwise affect overall prognosis or quality of life. Under certain circumstances it may be reasonable for the treatment team to opt for short-interval, follow-up MRI scans rather than immediate treatment. In some patients, the early detection and active therapeutic management of brain metastases, even prior to presentation of neurological symptoms, may improve clinical outcomes and reduce costs [27, 28].
Patients with brain metastases from EGFR-mutant NSCLC benefit from therapeutic regimens other than standard chemotherapy and often see prolonged survival. In fact, small, asymptomatic brain metastases respond well to TKI treatment alone in treatment naïve patients [29]. Liu et al. [29] analyzed overall survival in 96 neurologically-asymptomatic, EGFR-mutant NCSLC patients with brain metastases under three treatment groups: upfront radiation therapy (RT, both WBRT and SRS), delayed RT but upfront TKI treatment, or no RT. They argued for upfront EGFR-TKI followed by RT when appropriate as they found no significant impact on OS with delayed RT.
However, discrepancies exist in the literature over the timing of RT with some suggesting shorter OS with upfront EGFR-TKI and deferred SRS [19, 30]. In these studies, the distribution of patients with and without neurologic symptoms from brain metastases were uneven between treatment branches. Magnuson et al. [30] retrospectively analyzed overall survival in 50 patients with EGFR-mutant NSCLC. In their study cohort, 94% vs 46% of patients were asymptomatic in the upfront-TKI and upfront-RT groups, respectively. In a later retrospective study on 351 patients with EGFR-mutant NSCLC and an identical design, Magnuson et al. [19] had similar distribution with 88% vs 51% of patients being asymptomatic in the upfront-TKI and upfront-RT groups, respectively. These discrepancies highlight the need for further investigation in neurologically-asymptomatic brain metastases patients to better understand treatment response and optimal management.
The landscape of non-surgical and non-SRS NSCLC brain metastasis treatment is rapidly evolving. Erlotinib is a first-line therapy used in most patients treated with EGFR-TKIs in this study as well as studies cited in this discussion. However, EGFR-TKIs represent a non-homogenous group of drugs and recent studies with new agents, such as osimertinib, show superior tumor control and progression free survival [31, 32]. In addition, the updated 2017 guidelines from the NCCN call for routine testing of PD-L1 expression in NSCLC diagnosis, a target of immunotherapy to combat NSCLC. In future studies, these new management strategies need to be taken into account as they will likely affect broad comparisons of efficacy between upfront-SRS and upfront-EGFR-TKI.
Updating clinical metrics
The clinical factors identified in this study could be used to create prognostic metrics that better reflect disease variability among this unique cohort of patients. Zindler et al. [33], using data from 495 NSCLC patients with 1–4, < 4 cm diameter brain metastasis lesions, developed two nomograms using ROC analysis for the prognosis of early death (< 3-months) and long-term survival (> 12-months). Their nomograms summed points from gender, age, presence of extracranial metastases, volume of largest brain metastasis, and the WHO performance scale. These metrics were significantly better at determining death in < 3-months (AUC: 70) than GPA and SIR. They did not report neurologic-symptom status within their cohort, limiting generalizability to our cohort.
However, as shown by the Zindler et al. [33] study and the results from our analysis, there is need to update the current metrics to more dynamic models that (1) better capture inter-patient variability and (2) distinguish factors for early death and long-term survival. As expected, both GPA and SIR were prognostic of overall survival. However, they performed poorly when compared to our more dynamic models. It should be noted that KPS performed quite well in univariate analysis and in model analysis. We argue that by independently selecting factors for early-death and long-term survival, clinical metrics can increase the predictive potential of KPS and other clinical features. Further work is needed to refine clinical features correlated with early death and long-term survival and to better tune these models. External validation of our findings and prospective studies with larger sample sizes are needed to identify optimal management of patients with neurologically-asymptomatic brain metastases.
Study limitations and future directions
Unlike the present study, most of the literature has focused on outcomes following initial treatment of brain metastases, prior to any other intervention for these patient’s intracranial disease. Physicians are often faced with making treatment decisions not only for patients with a new presentation of intracranial disease, but also in patients who continue to see disease progression following prior management. In our study design, OS, clinical features, and clinical metrics were obtained at the time prior to the most recent SRS procedure, providing a clinical context encountered daily by the treatment team. This design limits our ability to directly compare results with prior literature.
Further limitations of this study include those inherent in retrospective designs, including the risk of selection bias. Additionally, patients were excluded if they were lost to follow-up or had incomplete medical records, increasing the risk of selection bias. However, results were consistent following robust analyses using both cox proportional hazard and logistic regression models. Nevertheless, our results were obtained from a small single-institution dataset, resulting in the wide range of values observed in the cohort. Further work on larger cohorts is needed to externally validate the proposed metric and to test our claims.
Finally, while the cause of death could not be reported for the patients in this cohort, to our knowledge, patients did not report neurologic symptoms prior to death. However, the number of intracranial metastases was statistically greater in the < 3-months group compared with the > 6-months group. To further investigate this, the number of intracranial metastases was stratified by measures of systemic disease, namely, presence of bone and liver metastases, the two most common extracranial lesions. The difference in number of intracranial metastases was only significant in the presence of greater systemic disease burden. Therefore, patients who die early following SRS may have an overall increased disease burden. There were no differences in time interval between primary tumor or initial brain metastasis diagnoses and the most recent SRS reducing the risk that < 3-month patients simply had a longer disease time course. Nevertheless, lack of information on exact cause of death remains a major limitation of this manuscript.
Conclusion
Patients with brain metastases from NSCLC are a heterogeneous cohort, with diverse treatment exposures. Consideration of clinical characteristics correlated with decreased survival after SRS of small, asymptomatic NSCLC brain metastases may enable further refinement of high-value, patient-centered care.
References
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Non–Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. Mayo Clin Proc 83:584–594
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2016) Incidence Proportions of Brain Metastases in Patients Diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. https://doi.org/10.1200/JCO.2004.12.149
Hazard LJ, Jensen RL, Shrieve DC (2005) Role of stereotactic radiosurgery in the treatment of brain metastases. Am J Clin Oncol 28:403–410
Gavrilovic IT, Posner JB (2005) Brain metastases: epidemiology and pathophysiology. J Neurooncol 75:5–14. https://doi.org/10.1007/s11060-004-8093-6
Sheehan JP, Yen CP, Nguyen J, Rainey JA, Dassoulas K, Schlesinger DJ (2011) Timing and risk factors for new brain metastasis formation in patients initially treated only with Gamma Knife surgery. Clinical article. J Neurosurg 114:763–768. https://doi.org/10.3171/2010.2.jns091539
Lutterbach J, Cyron D, Henne K, Ostertag CB (2003) Radiosurgery followed by planned observation in patients with one to three brain metastases. Neurosurgery 52:1066–1073; discussion 1073–1064
Korones DN, Butterfield R, Meyers SP, Constine LS (2001) The role of surveillance magnetic resonance imaging (MRI) scanning in detecting recurrent brain tumors in asymptomatic children. J Neurooncol 53:33–38
Tamari K, Suzuki O, Hashimoto N, Kagawa N, Fujiwara M, Sumida I, Seo Y, Isohashi F, Yoshioka Y, Yoshimine T, Ogawa K (2015) Treatment outcomes using CyberKnife for brain metastases from lung cancer. J Radiat Res 56:151–158. https://doi.org/10.1093/jrr/rru092
Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, Bhatt A, Jensen AW, Brown PD, Shih HA, Kirkpatrick J, Gaspar LE, Fiveash JB, Chiang V, Knisely JP, Sperduto CM, Lin N, Mehta M (2012) Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol 30:419–425. https://doi.org/10.1200/jco.2011.38.0527
Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R (1997) Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37:745–751
Elaimy AL, Mackay AR, Lamoreaux WT, Fairbanks RK, Demakas JJ, Cooke BS, Peressini BJ, Holbrook JT, Lee CM (2011) Multimodality treatment of brain metastases: an institutional survival analysis of 275 patients. World J Surg Oncol 9:69. https://doi.org/10.1186/1477-7819-9-69
Gorovets D, Department of Radiation Oncology TMC, Department of Radiation Oncology USA, USA RIH, dgorovets@tuftsmedicalcenter.org, Rava P, Department of Radiation Oncology UMMC, Ebner USA, Department of Radiation Oncology DK, Tybor RIH,USA DJ, Department of Public Health and Community Medicine TUSoM, Cielo USA, D, Department of Neurosurgery RIH, USA, Puthawala Y, Department of Radiation Oncology RIH, Kinsella USA TJ, Department of Radiation Oncology RIH, USA, DiPetrillo TA, Department of Radiation Oncology TMC, USA, Department of Radiation Oncology RIH, USA, Wazer DE, Department of Radiation Oncology TMC, USA, Department of Radiation Oncology RIH, USA, Hepel JT, Department of Radiation Oncology TMC, USA, Department of Radiation Oncology RIH, USA (2015) Predictors for Long-Term Survival Free from Whole Brain Radiation Therapy in Patients Treated with Radiosurgery for Limited Brain Metastases. Frontiers in Oncology 5 https://doi.org/10.3389/fonc.2015.00110
Weltman E, Salvajoli JV, Brandt RA, de Morais Hanriot R, Prisco FE, Cruz JC, de Oliveira Borges SR, Wajsbrot DB (2000) Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys 46:1155–1161
Sheehan JP, Sun MH, Kondziolka D, Flickinger J, Lunsford LD (2002) Radiosurgery for non-small cell lung carcinoma metastatic to the brain: long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg 97:1276–1281. https://doi.org/10.3171/jns.2002.97.6.1276
Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, Yamanaka K, Sato Y, Jokura H, Yomo S, Nagano O, Kenai H, Moriki A, Suzuki S, Kida Y, Iwai Y, Hayashi M, Onishi H, Gondo M, Sato M, Akimitsu T, Kubo K, Kikuchi Y, Shibasaki T, Goto T, Takanashi M, Mori Y, Takakura K, Saeki N, Kunieda E, Aoyama H, Momoshima S, Tsuchiya K (2014) Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 15:387–395. https://doi.org/10.1016/s1470-2045(14)70061-0
Network NCC (2013) The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for Central Nervous System Cancers. Jornnal of the National Comprehensive Cancer Network Version 22013. https://doi.org/10.6004/jnccn.2013.0138
Team RC (2014) R: A language and environment for statistical computing. 3.4.4 edn. R Foundation for Statistical Computing, Vienna
Delattre JY, Krol G, Thaler HT, Posner JB (1988) Distribution of brain metastases. Arch Neurol 45:741–744
Magnuson WJ, Lester-Coll NH, Wu AJ, Yang TJ, Lockney NA, Gerber NK, Beal K, Amini A, Patil T, Kavanagh BD, Camidge DR, Braunstein SE, Boreta LC, Balasubramanian SK, Ahluwalia MS, Rana NG, Attia A, Gettinger SN, Contessa JN, Yu JB, Chiang VL (2017) Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naive Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol 35:1070–1077. https://doi.org/10.1200/jco.2016.69.7144
Li C, Fang R, Sun Y, Han X, Li F, Gao B, Iafrate AJ, Liu XY, Pao W, Chen H, Ji H (2011) Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PLoS One 6:e28204. https://doi.org/10.1371/journal.pone.0028204
Matsumoto S, Takahashi K, Iwakawa R, Matsuno Y, Nakanishi Y, Kohno T, Shimizu E, Yokota J (2006) Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int J Cancer 119:1491–1494. https://doi.org/10.1002/ijc.21940
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M (2004) EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304:1497–1500. https://doi.org/10.1126/science.1099314
Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, Fujisawa T, Feng Z, Roth JA, Herz J, Minna JD, Gazdar AF (2005) Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 97:339–346. https://doi.org/10.1093/jnci/dji055
Iuchi T, Shingyoji M, Itakura M, Yokoi S, Moriya Y, Tamura H, Yoshida Y, Ashinuma H, Kawasaki K, Hasegawa Y, Sakaida T, Iizasa T (2015) Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 20:674–679. https://doi.org/10.1007/s10147-014-0760-9
Eichler AF, Kahle KT, Wang DL, Joshi VA, Willers H, Engelman JA, Lynch TJ, Sequist LV (2010) EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol 12:1193–1199. https://doi.org/10.1093/neuonc/noq076
Sperduto PW, Yang TJ, Beal K, Pan H, Brown PD, Bangdiwala A, Shanley R, Yeh N, Gaspar LE, Braunstein S, Sneed P, Boyle J, Kirkpatrick JP, Mak KS, Shih HA, Engelman A, Roberge D, Arvold ND, Alexander B, Awad MM, Contessa J, Chiang V, Hardie J, Ma D, Lou E, Sperduto W, Mehta MP (2017) Estimating Survival in Patients With Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol 3:827–831. https://doi.org/10.1001/jamaoncol.2016.3834
Lester SC, Taksler GB, Kuremsky JG, Lucas JT, Ayala-Peacock DN, Randolph DM, Bourland JD, Laxton AW, Tatter SB, Chan MD (2017) Clinical and economic outcomes of patients with brain metastases based on symptoms: An argument for routine brain screening of those treated with upfront radiosurgery. Cancer 120:433–441. https://doi.org/10.1002/cncr.28422
Sanchez de Cos J, Sojo Gonzalez MA, Montero MV, Perez Calvo MC, Vicente MJ, Valle MH (2009) Non-small cell lung cancer and silent brain metastasis. Survival and prognostic factors. Lung Cancer 63:140–145. https://doi.org/10.1016/j.lungcan.2008.04.013
Liu S, Qiu B, Chen L, Wang F, Liang Y, Cai P, Zhang L, Chen Z, Liu M, Liu H (2015) Radiotherapy for asymptomatic brain metastasis in epidermal growth factor receptor mutant non-small cell lung cancer without prior tyrosine kinase inhibitors treatment: a retrospective clinical study. Radiat Oncol 10:118. https://doi.org/10.1186/s13014-015-0421-9
Magnuson WJ, Yeung JT, Guillod PD, Gettinger SN, Yu JB, Chiang VL (2016) Impact of Deferring Radiation Therapy in Patients With Epidermal Growth Factor Receptor-Mutant Non-Small Cell Lung Cancer Who Develop Brain Metastases. Int J Radiat Oncol Biol Phys 95:673–679. https://doi.org/10.1016/j.ijrobp.2016.01.037
Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su W-C, Gray JE, Lee S-M, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS (2017) Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. https://doi.org/10.1056/NEJMoa1713137
Goss G, Tsai CM, Shepherd FA, Ahn MJ, Bazhenova L, Crino L, de Marinis F, Felip E, Morabito A, Hodge R, Cantarini M, Johnson M, Mitsudomi T, Janne PA, Yang JC (2018) CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol 29:687–693. https://doi.org/10.1093/annonc/mdx820
Zindler JD, Jochems A, Lagerwaard FJ, Beumer R, Troost EGC, Eekers DBP, Compter I, van der Toorn PP, Essers M, Oei B, Hurkmans CW, Bruynzeel AME, Bosmans G, Swinnen A, Leijenaar RTH, Lambin P (2017) Individualized early death and long-term survival prediction after stereotactic radiosurgery for brain metastases of non-small cell lung cancer: Two externally validated nomograms. Radiother Oncol 123:189–194. https://doi.org/10.1016/j.radonc.2017.02.006
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kakusa, B., Han, S., Aggarwal, S. et al. Clinical factors associated with mortality within three months after radiosurgery of asymptomatic brain metastases from non-small cell lung cancer. J Neurooncol 140, 705–715 (2018). https://doi.org/10.1007/s11060-018-03002-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03002-0