Abstract
Axitinib is a small molecule tyrosine kinase inhibitor with high affinity and specificity for the family of vascular endothelial growth factor receptors. It has previously demonstrated anti-tumor activity in a small cohort of patients with recurrent glioblastoma (rGB). We conducted a non-comparative randomized phase II clinical trial investigating axitinib monotherapy versus axitinib plus lomustine (LOM) in patients with rGB. Primary endpoint was 6 month progression-free survival (6mPFS). Patients who progressed on axitinib-monotherapy were allowed to cross-over. Between August 2011 and July 2015, 79 patients were randomized and initiated axitinib monotherapy (n = 50; AXI) or axitinib plus lomustine (n = 29; AXILOM). Median age was 55y [range 18–80], 50M/28F. Baseline characteristics were well balanced between study arms. Nineteen patients in the AXI-arm crossed-over at the time of progression. Treatment was generally well tolerated. AXILOM patients were at higher risk for grade 3/4 neutropenia (0 vs. 21%) and thrombocytopenia (4 vs. 29%). Best Overall Response Rate (BORR) in the AXI-arm was 28 vs. 38% in the AXILOM-arm. 6mPFS was 26% (95% CI 14–38) versus 17% (95% CI 2–32) for patients treated in the AXI versus AXILOM-arms, respectively. Median overall survival was 29 weeks (95% CI 20–38) in the AXI-arm and 27.4 weeks (95% CI 18.4–36.5) in the AXILOM-arm. MGMT-promoter hypermethylation and steroid treatment at baseline correlated significantly with PFS and OS. We conclude from these results that axitinib improves response rate and progression-free survival in patients with rGB compared to historical controls. There is no indication that upfront combination of axitinib with LOM improves results (European Clinical Trials Database (EudraCT) Study Number: 2011-000900-16).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma is the most frequent and deadliest intrinsic brain tumor [1]. Standard first-line treatment has been established since 2005 and consists of maximal safe resection followed by fractionated radiotherapy (30 × 2 Gy) with concurrent daily temozolomide and 6 cycles of adjuvant temozolomide. Despite advances in treatment, outcome remains poor with a median progression-free survival (PFS) of 6.9 months and a median overall survival (OS) of 14.6 months [2]. At recurrence, no treatment has demonstrated to improve survival of glioblastoma patients in a randomized clinical trial. Cytotoxic salvage therapies resulted in a best overall response rate of 5–10%, 6-month PFS rates of 9–21%, and a median OS of 25–30 weeks [3, 4].
Glioblastoma is known as a highly vascular tumor that is characterized by profound neo-angiogenesis. Vascular endothelial growth factor (VEGF) and VEGF-receptors are key molecular mediators of this tumor associated neo-angiogenesis. By binding to its tyrosine kinase cell surface receptors (VEGFR-1, VEGFR-2 and VEGFR-3), VEGF has a mitogenic effect on endothelial cells and increases endothelial permeability leading to the tumor associated edema [5]. Since neo-angiogenesis plays such an important pathophysiological role in glioblastoma biology, targeting of the VEGF pathway has been considered a therapeutic priority.
Bevacizumab, an anti-VEGF monoclonal antibody, demonstrated anti-tumor activity as a single agent in patients with recurrent glioblastoma (rGB) with objective response rates in the range of 28–35%, median PFS of 11–17 weeks and median OS of 26–37 weeks [6]. In 2009, bevacizumab was approved as monotherapy for rGB by the US Food and Drug Administration despite absence of a proven benefit on overall survival [7, 8]. A phase II trial undertaken in the Netherlands indicated that the combination of bevacizumab and lomustine could possibly improve survival of rGB patients compared to bevacizumab or lomustine alone [9]. The phase III EORTC 26101 study was designed as the confirmatory trial of this finding. In this trial, the combination of bevacizumab plus lomustine was associated with a significant improvement of progression-free survival but not of overall survival compared to lomustine alone [10].
Axitinib is an orally available high affinity tyrosine kinase inhibitor of the VEGF-receptors, blocking VEGF-R-1, -2 and -3 at picomolar concentrations [11]. It is approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of patients with recurrent metastatic renal cell carcinoma [12]. Axitinib has an anti-angiogenic and survival prolongation effect in preclinical orthotopic glioblastoma models and inhibits tumor growth in a glioblastoma xenograft model with primary resistance to bevacizumab [13, 14]. We recently reported that axitinib demonstrated anti-tumor activity with manageable toxicity in a small cohort of rGB patients treated in a non-comparative randomized phase II clinical trial (AxiG-trial) [15]. A partial tumor response was observed in three out of six patients from this trial who were treated with lomustine at the time of progression on axitinib-monotherapy (unpublished results). Following an amendment, the AxiG-trial was continued to further investigate the anti-tumor activity of axitinib monotherapy versus the combination of axitinib with lomustine. Patients in the axitinib monotherapy arm were able to cross over to the combination arm at the time of progression.
Materials and methods
Study design
The axitinib for recurrent glioblastoma study (AxiG) was originally designed as a multicenter non-comparative two-arm phase II trial randomizing patients between axitinib and the treating physician’s best alternative choice of therapy. Forty-four patients were included in the first part of this study (22 in each arm), and the results of this trial were published in March 2016 [15]. The study was then amended to allow for inclusion of 2 × 26 additional patients, randomized between axitinib monotherapy and the combination of axitinib and lomustine (CCNU). The sample size was calculated based on a Fleming one-stage design with a type I error of 0.10 and a type II error of 0.20 [16]. The primary endpoint was the number of patients that are free from progression after 6-months (6mPFS). The combination of axitinib and lomustine would be considered to be of interest for further evaluation if 6mPFS was equal to or higher than 50% and considered as insufficiently active if the observed 6mPFS was less than 30%. Randomization was stratified according to performance status (World Health Organization Performance Status (WHO-PS) ≤ 1, ie “fully active” or “restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature”, and WHO-PS > 1) and whether or not the patient was considered to be refractory to alkylating chemotherapy. Patients were considered to be refractory to alkylating chemotherapy if they had been diagnosed with progression while being treated with an alkylating chemotherapeutic agent or within 3 months after stopping such treatment.
Patients allocated to the axitinib monotherapy arm were allowed to cross over to the combination arm at the time of progression on axitinib monotherapy. This final analysis includes all patients treated within the axitinib monotherapy arm (22 patients treated in the first part of the trial that was reported on previously [15] plus 28 patients who initiated axitinib monotherapy in the second part of the trial) as well as the patients treated with axitinib in combination with lomustine in the second part of the trial. Trial design is illustrated in Fig. 1, adhering to the Consolidated Standards of Reporting Trials (CONSORT) [17].
The ethical committees of all participating centers and the Belgian competent authorities approved the protocol, patient information brochure and informed consent document. All patients provided written informed consent before study participation.
Patients
Eligible patients needed to be diagnosed with histologically confirmed glioblastoma (WHO grade IV glioma), aged ≥ 18 years or older and have tumor recurrence or progression following prior treatment with surgery, radiation therapy and temozolomide. A measurable tumor lesion on gadolinium-enhanced T1-MRI of the brain was required. An interval of at least 3 months was needed after ending prior radiotherapy as well as an interval of at least 4 weeks after the last administration of a cytotoxic treatment or any other kind of anti-glioblastoma treatment. There was no limit to the number of prior recurrences that patients had experienced before being considered for inclusion in this trial. Main exclusion criteria included previous treatment with axitinib or any other VEGF(R)-targeted drug (including, but not limited to bevacizumab, cediranib, sunitinib), prior treatment with alkylating chemotherapy other than temozolomide (including lomustine), uncontrolled hypertension, history of ischemic cerebrovascular accident or intracranial hemorrhage within 12 months prior to study drug administration, history of bleeding diathesis, coagulopathy or recent arterial or venous thromboembolism. Full patient in- and exclusion criteria as well as endpoints and objectives of the trial are in the supplementary material.
Study treatment, safety- and tumor response assessments
In accordance with dose levels set in phase I trials in various other cancer types, patients initiated treatment with axitinib at a dose of 5 mg twice daily, taken orally with food [18]. Dose adjustments were based on individual tolerability and performed according to protocol defined guidelines for increase or decrease of dosing. In the absence of any grade > 3 toxicity or hypertension, axitinib dosing was sequentially increased to 7 and 10 mg BID. Axitinib dosing was interrupted and dose reduced to 3 or 2 mg BID in case of grade ≥ 3 toxicity. Axitinib treatment was stopped in case of disease progression, unacceptable toxicity, or the patient’s request to discontinue treatment.
Patients in the combination arm received axitinib as described above, combined with lomustine, taken orally. In order to minimize hematological adverse events in this combinatorial regimen, dosing of lomustine was set at 90 mg/m2 every 6 weeks.
Clinical visits were planned every 2 weeks. Assessments included medical history, physical and neurological examination and blood analyses (including hematology, chemistry, FT4 and TSH analysis). MR imaging (including Gadolinium enhanced T1- and non-enhanced T2/FLAIR weighted images) was performed at baseline and every 6 weeks thereafter.
Reporting of data and statistical analysis
Baseline patient characteristics, molecular analysis of patient’s tumor samples, treatment disposition, response rates according to the Response Assessment in Neuro-Oncology (RANO) criteria and adverse events are reported using descriptive statistics [19]. Best overall response rate (BORR) is defined as the sum of confirmed (ie on two subsequent MRI evaluations at least 4 weeks apart) complete responses (CR) and partial responses (PR). Chi square test is used to determine presence or absence of correlation between data. Severity of treatment related adverse events are reported according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE Version 4.03) [20].
Progression-free survival (PFS) and overall survival (OS) are estimated using the Kaplan–Meier method. PFS was defined as the time from randomization to the first event of objective tumor progression according to the RANO criteria or death and censored on the last date the patient was known to be alive and progression-free [19]. For patients who had crossed over to the combination arm after progression under axitinib therapy, a PFS2 was defined as the time of randomization until the time of second progression (after addition of lomustine) according to the RANO criteria. In case these patients had shown only progressive disease after addition of lomustine to their treatment, the date of progression remained the date of first PD (and PFS2 = PFS). OS was defined as the time from randomization to the time of the first documentation of death due to any cause and censored on the last date the patient was known to be alive. The log rank test is used to detect differences in PFS or OS between treatment groups but also between patients with differing baseline characteristics (univariate analysis). SPSS software was used for statistical analysis. 95% confidence intervals were generated as is standard with the software used.
Molecular analysis
MGMT promoter methylation analysis was performed by MethyLight as described by Eads et al. [21] For next-generation-sequencing, genomic DNA was extracted from formalin fixed paraffin embedded (FFPE) tissues using the QIAamp FFPE tissue kit (Qiagen, Antwerp, Belgium) according to the manufacturer’s instructions. For library construction, 10 ng of DNA was amplified using the Cancer hotspot panel v2 (AmpliSeqTM, Life Technologies, Gent, Belgium) for sequencing of 2850 hotspot mutations in 50 genes. Sequencing was performed on a PGM™ sequencer [15, 22, 23].
Results
Baseline patient characteristics
Between August 2011 and March 2016, 101 patients were recruited at six different medical centers and initiated study treatment following randomization to the axitinib monotherapy-arm (AXI; 50 patients) or the axitinib plus lomustine combination arm (AXILOM; 29 patients). The outcome of 22 patients randomized to the study arm with the best alternative therapy was reported previously (Fig. 1) [15].
Baseline patient characteristics and molecular data obtained on archival tumor material are summarized in Tables 1 and 2. The demographic, clinical and molecular characteristics were well balanced between both study arms. Prior therapy for rGB has consisted of at least one neurosurgical resection in 47%, radiation therapy in 14%, and at least one line of medical treatment in 39% of patients. Seventy-eight percent of patients were considered alkylator refractory because they had been diagnosed with progression while being treated with an alkylating chemotherapy or within 3 months after stopping such treatment.
Archival tumor samples were obtained from 64 out of 79 patients (81%). Samples were uncontributive in 2 of these 64 patients (3%) for MGMT methylation status analysis and IDH-1 mutation analysis, and in 5 of the 64 patients (8%) for mutational and amplification analysis. (Table 2) Taking only patients into account with informative test-results, 31% were characterized by MGMT promoter methylation and 13% by IDH-1-mutation. EGFR amplification was found in 36% and EGFR mutation in 15%. Additional mutations were found only in a small number of cases, except for p53 mutations which were detected in 36% of patients.
Treatment disposition
All patients allocated to the axitinib treatment arm initiated treatment according to the protocol. Among the patients allocated to the axitinib plus lomustine arm, two patients did not initiate lomustine treatment (protocol deviation). At the time of this analysis, 4 patients are continuing axitinib therapy.
The axitinib dose was maintained at 5 mg bid in 44% of patients and could be increased to a maximum of 7 mg bid in 15.2% and 10 mg bid in 17.7% of patients, respectively. The axitinib dose had to be decreased due to adverse events to 3 mg in 24.1% and to 2 mg bid in 6.4% of patients. The axitinib dose modifications were not significantly different between the two study arms. In the AXI-arm, median duration of axitinib therapy was 20 weeks, with a range from 1 to 111 weeks. In the AXILOM-arm, axitinib was administered for a median period of 14 weeks with a range from 2 to 80 weeks. Lomustine was administered for a median number of 2 cycles (range 1–5) in the AXILOM arm. In 6 out of 29 (20%) patients, lomustine treatment was interrupted for adverse events.
Nineteen out of 50 (38%) patients assigned to the AXI-arm crossed over to AXILOM treatment at the time of progression. There was no significant difference in baseline characteristics (baseline WHO-PS, use of steroids at baseline, biopsy versus resection at baseline, number of previous lines of treatment, primary versus secondary GB, MGMT methylation status and IDH mutation status) between patients who crossed over and those who did not.
Safety
Both axitinib monotherapy and the combination of axitinib and lomustine were generally well tolerated with respectively 36% and 39% of the population experiencing a grade ≥ 3 treatment related adverse event (Table 3). The most frequent axitinib related adverse events were grade 1 or 2 fatigue, diarrhea, oral mucositis/hypersensitivity, hoarseness and anorexia. No patient stopped axitinib treatment because of unacceptable toxicity. Patients treated with axitinib plus lomustine had a higher incidence of thrombo- and neutropenia. Six patients discontinued lomustine treatment in the absence of disease progression because of recurrent treatment related adverse events following dose reduction.
Tumor response rates and steroid treatment
The best overall tumor response rate (BORR%) was 28% for axitinib monotherapy and 38% for the combination of axitinib and lomustine; disease control rate (DCR) was 52 and 45%, respectively (Table 4; Fig. 2). BORR% after crossover and considering all patients who had Lomustine added to axitinib after progression included 3 partial responses (16%); stable disease (SD) was documented in 5 additional patients (26%).
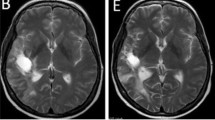
Case illustration. a–f upper row and f lower row: gadolinium-enhanced T1-images. a–e lower row: T2 images. A 48-year-old woman had recurrence of glioblastoma after surgical resection and standard radiochemotherapy followed by a second surgical intervention for recurrence. She was randomized to the trial in May 2015 (a) and initiated treatment with axitinib. A complete response was observed on MRI of the brain (b). The patient developed wound healing problems that necessitated a reconstructive surgical intervention (removal of bone flap and flap surgery). Axitinib was interrupted during 4 weeks (5 days before the intervention and 3 weeks after) in order to be able to safely perform the intervention and for the surgical wound to heal adequately. The MRI scan performed after 4 weeks of treatment interruption of axitinib showed recurrence of contrast-enhanced glioblastoma (c). Rechallenge with axitinib led to radiological complete response (d). Follow-up MRI until October 2016, 17 months after initiation of treatment, shows a durable complete resonse (e). The patient started deteriorating clinically in December 2016 with development of aphasia and right-sided hemiplegia, and MRI in January 2017 showed local progressive disease but also development of new contrast-enhancing lesions in the periventricular area and mesencephalic area (f)
Forty-nine out of 79 patients (62%) were using corticosteroids at the time of randomization. Of these, 27 patients (55%) were able to decrease their steroid dosing and 5 (10%) were able to completely stop steroid treatment. Steroid dosing was kept stable in ten patients (20.4%) and needed to be increased in seven patients (14%).
Survival
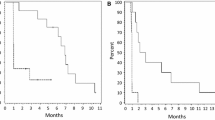
The 6-month progression-free survival rate was 26% (95% CI 13–38) in patients treated with axitinib and 24.1% (95% CI 8–39) in patients who initiated treatment with axitinib plus lomustine (Fig. 3a). Median PFS was 12.4 weeks (95% CI 11–13) and 13 weeks (95% CI 6–20) for axitinib as monotherapy or combined with lomustine, respectively. PFS was not significantly different between both study arms (log-rank p = 0.421).
a Progression-free survival of patients treated with axitinib as single agent (blue line) and patients treated with the combination of axitinib and lomustine (green line); b progression-free survival incorporating the progression-free time gained upon addition of lomustine after progression in the axitinib monotherapy arm (PFS2); c overall survival
PFS did not differ significantly between the first 22 and subsequent 26 patients treated with axitinib. Median PFS following cross-over (n = 19 patients) was 12 weeks (95% CI 5–19) with a 6mPFS of 21.1% (95% CI 0.03–0.39). When PFS was recalculated to include the PFS following cross-over, a difference was observed between both study arms (log-rank p = 0.026, hazard ratio 0.58 with 95% CI 0.35–0.94) (Fig. 3b).
At the time of analysis, 69 patients have died (all except one due to progression of their glioblastoma). Median follow-up for surviving patients on the axitinib arm (n = 5) is 109 weeks (range 68–206) and 85 weeks (range 28–128) for surviving patients on the axitinib plus lomustine arm (n = 5). Median overall survival was 29 weeks (95% CI 20–38) in patients treated with axitinib and 27.4 weeks (95% CI 18.4–36.5) in patients who initiated treatment with axitinib plus lomustine. Six-month Overall Survival was 54% (95% CI 40–68) and 53% (95% CI 34–73), respectively. (Fig. 3c) There was no significant difference between study arms in terms of overall survival (log-rank p = 0.932).
Progression-free and overall survival were significantly longer in patients with MGMT-promoter hypermethylatation (log-rank p = 0.014 and 0.018, respectively) and in patients who were not on steroid treatment at baseline (log-rank p = 0.009 and 0.006, respectively). Progression-free but not overall survival was shorter in patients who were considered alkylator refractory (log-rank p = 0.028). This was observed for both study arms. Other clinical or molecular baseline characteristics, listed in Tables 1 and 2, were not correlated with survival.
Discussion
This trial provides clinical evidence that axitinib as a monotherapy has anti-tumor activity in patients with recurrent GB and that treatment can be administered with an acceptable safety profile. There is no indication that upfront combination of axitinib with lomustine improves the tumor response rate or survival and the risk for hematological toxicity is increased.
The BORR of 28% and 6mPFS of 26% (95% CI 13.9–38.1) observed for the heavily pretreated population compares favorably to the outcome of historical control cohorts treated with cytotoxic agents and is in range with the results obtained with bevacizumab in comparable populations [3, 4, 7, 8, 24, 25]. As illustrated in Table 5, results mostly compare favorably to trials with other anti-angiogenic agents such as sunitinib, pazopanib, aflibercept or cediranib [10, 26,27,28,29]. Overall survival does not seem to improve compared to historical controls treated with salvage therapy.
The addition of lomustine to axitinib therapy did not improve outcome. Our findings are in line with the results of the EORTC 26101 and REGAL study where neither a survival advantage was found for the combination of lomustine with bevacizumab or with cediranib in patients with recurrent glioblastoma [10, 28].
In patients where lomustine was added to axitinib-treatment at the time of progression, 8 out of 19 patients remained free from progression for at least 12 weeks and cumulative PFS on axitinib and following cross-over compares favorably to upfront combination therapy. Notwithstanding, this did not translate into an improved overall survival. The addition of a control arm where only lomustine is administered would most likely not change our conclusions. In the phase III EORTC 26101 trial, the combination of bevacizumab with lomustine showed an improved progression-free survival but not overall survival compared to lomustine alone [10].
Adverse events due to axitinib treatment were generally mild, manageable and in line with the known safety profile in patients with advanced renal cancer. Patients who were treated with lomustine were more at risk for grade 3 or 4 hematological adverse events (mainly thrombopenia but also neutropenia). Therefore, upfront combination of axitinib plus lomustine does not deserve further clinical evaluation.
The clinical results obtained with axitinib seem comparable to those obtained with bevacizumab and tolerability is comparable [15]. However, the small molecule axitinib has some distinct advantages over bevacizumab. It is orally dosed and its short half-life (2–6 h compared to 3 weeks for bevacizumab) allows for rapid reversal of its effects and quick normalization of wound healing properties in case of urgent surgical interventions or wound issues. Treatment-related adverse events, although generally being mild, are rapidly reversible upon treatment interruption (24–72 h dosing interruption is sufficient in the majority of cases in order to recover from side-effects).
Given the demonstrated activity of axitinib in patients with rGB it can be considered an attractive candidate for new combinatorial regimens. The beneficial effect of VEGF- and VEGFR-targeted therapy on the immune suppressive environment in cancer has been demonstrated previously. (ref Terme, Tartour, VEGFA, …) Our research group previously demonstrated that axitinib increases infiltration of immune cells and reduces the suppressive capacity of monocytic myeloid-derived stem cells (MSDCs) in an intracranial mouse melanoma model [30]. It was also demonstrated that the T-cell function is preserved in patients with recurrent glioblastoma with a good clinical response on axitinib, in contrast to the reduced T-cell function in patients with progressive disease [31]. Based on these observations, we conclude that the impact of axitinib on tumor growth and survival is most likely not restricted to direct anti-angiogenic effects but also involves beneficial effects on tumor immunity. Of note, the combination of axitinib with immune-modulatory agents such as anti-CTLA-4 or PD-1/PD-L1 inhibiting monoclonal antibodies has been shown to be synergistic in preclinical models [32,33,34,35]. In patients with advanced renal cell carcinoma, the combination of axitinib with avelumab (an IgG1 antibody directed against the programmed death receptor ligand-1) was found to be tolerable and associated with encouraging rate of tumor response [36]. A phase II trial with the combination of axitinib and avelumab for patients with recurrent glioblastoma is planned (European Clinical Trials Database (EudraCT) number 2017-000200-23).
References
De Angelis LM (2001) Brain tumors. N Engl J Med 344:114–123. doi:10.1056/NEJM200101113440207
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. doi:10.1016/S1470-2045(09)70025-7
Wong ET, Hess KR, Gleason MJ et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572–2578
Lamborn KR, Yung WKA, Chang SM et al (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 10:162–170. doi:10.1215/15228517-2007-062
Puputti M, Tynninen O, Sihto H et al (2006) Amplification of KIT, PDGFRA, VEGFR2, and EGFR in gliomas. Mol Cancer Res 4:927–934. doi:10.1158/1541-7786.MCR-06-0085
Reardon DA, Turner S, Peters KB et al (2011) A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw 9:414–427. doi:10.6004/jnccn.2011.0038
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740. doi:10.1200/JCO.2008.19.8721
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745. doi:10.1200/JCO.2008.16.3055
Taal W, Oosterkamp HM, Walenkamp AME et al (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15:943–953. doi:10.1016/S1470-2045(14)70314-6
Wick W, Brandes A, Gorlia T, Al E (2015) Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neuro Oncol 17 (suppl 5):v1
Escudier B, Gore M (2011) Axitinib for the management of metastatic renal cell carcinoma. Drugs R D 11:113–126. doi:10.2165/11591240-000000000-00000
Albiges L, Gizzi M, Carton E, Escudier B (2015) Axitinib in metastatic renal cell carcinoma. Expert Rev Anticancer Ther 15:499–507. doi:10.1586/14737140.2015.1033408
Lu L, Saha D, Martuza RL et al (2014) Single agent efficacy of the VEGFR kinase inhibitor axitinib in preclinical models of glioblastoma. J Neurooncol 121:91–100. doi:10.1007/s11060-014-1612-1
Kratzsch T, Gruenwald V, Vajkoczy P, Kuhn S (2013) Use of axitinib, a new-generation tyrosine kinase inhibitor, to decrease glioblastoma growth despite primary resistance to the VEGF-antibody bevacizumab. ASCO Meeting Abstracts 2013; 31 2077
Duerinck J, Du Four S, Vandervorst F et al (2016) Randomized phase II study of axitinib versus physicians best alternative choice of therapy in patients with recurrent glioblastoma. J Neurooncol 128:147–155. doi:10.1007/s11060-016-2092-2
Fleming TR (1982) One-sample multiple testing procedure for phase II clinical trials. Biometrics 38:143–151
Moher D, Schulz KF, Altman DG (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. The Lancet 357:1191–1194. doi:10.1016/S0140-6736(00)04337-3
Rugo HS, Herbst RS, Liu G et al (2005) Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol 23:5474–5483. doi:10.1200/JCO.2005.04.192
Wen PY, Macdonald DR, Reardon D a et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:10.1200/JCO.2009.26.3541
National Cancer Institute (2009) Common terminology criteria for adverse events (CTCAE) v4.0
Eads CA, Danenberg KD, Kawakami K et al (2000) MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res 28:E32. doi:10.1093/nar/28.8.e32
D’Haene N, Le Mercier M, De Nève N et al (2015) Clinical validation of targeted next generation sequencing for colon and lung cancers. PLoS ONE 10:e0138245. doi:10.1371/journal.pone.0138245
Le Mercier M, D’Haene N, De Nève N et al (2015) Next-generation sequencing improves the diagnosis of thyroid FNA specimens with indeterminate cytology. Histopathology 66:215–224. doi:10.1111/his.12461
Raizer JJ, Grimm S, Chamberlain MC et al (2010) A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer 116:5297–5305. doi:10.1002/cncr.25462
Duerinck J, Clement P, Bouttens F et al (2015) Patient outcome in the Belgian medical need program on bevacizumab for recurrent glioblastoma. J Neurol 262:742–751. doi:10.1007/s00415-014-7633-z
Hutterer M, Nowosielski M, Haybaeck J et al (2014) A single-arm phase II Austrian/German multicenter trial on continuous daily sunitinib in primary glioblastoma at first recurrence (SURGE 01–07). Neuro Oncol 16:92–102. doi:10.1093/neuonc/not161
Iwamoto FM, Lamborn KR, Robins HI et al (2010) Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02). Neuro Oncol 12:855–861. doi:10.1093/neuonc/noq025
Batchelor TT, Mulholland P, Neyns B et al (2013) Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 31:3212–3218. doi:10.1200/JCO.2012.47.2464
de Groot JF, Lamborn KR, Chang SM et al (2011) Phase II study of aflibercept in recurrent malignant glioma: A North American Brain Tumor Consortium Study. J Clin Oncol 29:2689–2695. doi:10.1200/JCO.2010.34.1636
Du Four S, Maenhout SK, De Pierre K et al (2015) Axitinib increases the infiltration of immune cells and reduces the suppressive capacity of monocytic MDSCs in an intracranial mouse melanoma model. Oncoimmunology 4:e998107. doi:10.1080/2162402X.2014.998107
Du Four S, Maenhout SK, Benteyn D et al (2016) Disease progression in recurrent glioblastoma patients treated with the VEGFR inhibitor axitinib is associated with increased regulatory T cell numbers and T cell exhaustion. Cancer Immunol Immunother 65:727–740. doi:10.1007/s00262-016-1836-3
Zhang X, Fang X, Gao Z et al (2014) Axitinib, a selective inhibitor of vascular endothelial growth factor receptor, exerts an anticancer effect in melanoma through promoting antitumor immunity. Anticancer Drugs 25:204–211. doi:10.1097/CAD.0000000000000033
Stehle F, Schulz K, Fahldieck C et al (2013) Reduced immunosuppressive properties of axitinib in comparison with other tyrosine kinase inhibitors. J Biol Chem 288:16334–16347. doi:10.1074/jbc.M112.437962
Bose A, Lowe DB, Rao A, Storkus WJ (2012) Combined vaccine + axitinib therapy yields superior antitumor efficacy in a murine melanoma model. Melanoma Res 22:236–243. doi:10.1097/CMR.0b013e3283538293
Du Four S, Maenhout SK, Niclou SP et al (2016) Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. Am J Cancer Res 6:2514–2531
Larkin J, Rini BI, Nathan P et al (2016) Phase 1b dose-finding study of avelumab (anti-PD-L1)+ axitinib in treatment-naïve patients with advanced renal cell carcinoma. European Society for Medical Oncology Congress, Copenhagen, Denmark, 27 Supplement
Acknowledgements
We would like to acknowledge the patients who consented to participate in this study, their families and professional health care providers. Pfizer Belgium for the provision of axitinib study medication and a research grant. We would also like to thank the data manager Katrien Van den Bossche and Kathleen Mooren (UZ Brussel) for their help with the data collection and analysis, and Ludwig Van den Hove, PhD (Pfizer Belgium), for his support and critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duerinck, J., Du Four, S., Bouttens, F. et al. Randomized phase II trial comparing axitinib with the combination of axitinib and lomustine in patients with recurrent glioblastoma. J Neurooncol 136, 115–125 (2018). https://doi.org/10.1007/s11060-017-2629-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2629-z