Abstract
Medulloblastoma (MB) is rare in adults and treatment guidelines are consequently not well-established. Few modern series have reported long-term follow-up and treatment sequelae. We examined long-term outcomes of adult MB patients at one institution. Records of 29 consecutive patients (18 male, 11 female) aged ≥ 18 years who received radiotherapy (RT) for primary MB from 1990 to 2016 were reviewed. Median age at diagnosis was 28 years (range 18–72 years). Seventeen patients were standard risk and 12 were high risk. Nineteen patients had gross total resection, seven had subtotal resection, and three had biopsy only. Median craniospinal irradiation and boost doses were 36 Gy (range 23.4–39.6 Gy) and 55.8 Gy (range 54–59.4 Gy), respectively. Of 24 patients receiving chemotherapy, 20 received concurrent + adjuvant and 4 received adjuvant only. At median follow-up of 9.0 years (range 1.1–20.5 years), five patients recurred: four in the posterior fossa and one in both the posterior fossa and above the tentorium. Five patients died: two of disease progression and three after possible treatment complications (seizure, lobar pneumonia, and multifactorial sepsis). At last follow-up, 23 patients were alive with no evidence of disease. Long-term effects include executive dysfunction (n = 17), weakness/ataxia (n = 16), and depression/anxiety (n = 13). Kaplan–Meier estimates of 10-year overall survival and failure-free survival are 83% (95% confidence interval [CI] 59–93%) and 79% (CI 55–91%), respectively. Despite encouraging disease control in this cohort, long-term sequelae may limit quality of life. Multimodality pediatric regimens using lower RT doses may be considered to reduce treatment-related morbidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medulloblastoma, the second most common malignant pediatric brain tumor, is rare in adults with an estimated incidence of 0.5 per million per year [1, 2]. Medulloblastoma in the adult population has several distinct characteristics compared to its pediatric counterpart: adult tumors are more likely to arise laterally, have poorly defined margins, display desmoplastic histology, and express glial fibrillary acidic protein (GFAP), though the prognostic value of these attributes is unclear [3]. Four molecular subgroups exist in medulloblastoma: Wingless (WNT), Sonic Hedgehog (SHH), Group 3, and Group 4. Compared to children, adults comprise a greater proportion of SHH (60 vs. 30%) and relatively fewer Group 3 (0–5 vs. 25%) subgroup patients [4,5,6,7,8].
Due to the rarity of adult MB, treatment regimens have largely been extrapolated from pediatric protocols [7]. Many series are small retrospective reviews conducted over decades during which technology rapidly developed and patients received heterogeneous management, making outcomes difficult to interpret [9,10,11,12,13,14,15,16,17,18]. Additionally, only two adult prospective trials have been conducted and neither used concurrent nor adjuvant chemotherapy in standard-risk patients, limiting generalizability [5, 19, 20].
Few modern series have reported long-term follow-up and treatment sequelae. Therefore, we report our experience since 1990 with primary adult MB treated with radiotherapy. We describe patient presentation, disease control, and treatment sequelae in the era of magnetic resonance imaging (MRI).
Methods and materials
Patient population
After approval by our Institutional Review Board, we identified consecutive patients meeting the following criteria: (1) age ≥ 18 years at initial diagnosis, (2) histopathologic diagnosis of MB reviewed at our institution, (3) non-recurrent status at presentation, and (4) receipt of RT at our institution since 1990. Twenty-nine patients were identified. Standard-risk disease was defined as no metastasis found on craniospinal MRI or CSF sampling, < 1.5 cm2 post-operative residual tumor, and no disease recurrence on pre-RT imaging. High-risk disease was defined as any metastasis, T3b/T4 disease, > 1.5 cm2 post-operative residual tumor, or recurrence on pre-RT imaging.
Statistical analysis
Statistical analysis was performed with Microsoft Excel 2016 (Microsoft, Redmond, WA) and Stata Version 13.0 (StataCorp, College Station, TX, USA). Failure-free survival (FFS) was calculated from MB diagnosis to first relapse after completing initial therapy. The Kaplan–Meier method was used to assess FFS and overall survival (OS). Survival curves among different patient subgroups (standard vs. high risk, desmoplastic vs. other histologies, surgery-RT interval ≤ 30 vs. >30 days, CSI dose of 23.4 vs. 36+ Gy, boost dose ≥ 55.8 vs. <55.8 Gy, concurrent chemotherapy vs. none, adjuvant chemotherapy vs. none) were compared using the log-rank test with significance defined as p < 0.05.
Results
Presentation
Median age at presentation was 28 (range 18–72) (Table 1). Eighteen patients were men. Median symptom duration before presentation was 4.3 months (range 0.1–36 months); common symptoms were headache (n = 23), nausea/vomiting (n = 18), ataxia (n = 14), and dizziness/vertigo (n = 12). Tumor was midline in 15 patients and lateral in 14 patients. Twenty patients had hydrocephalus.
Surgery, staging, and risk stratification
Nineteen patients underwent gross total resection, seven subtotal resection, and three biopsy (Table 1). Ten patients required cerebrospinal fluid (CSF) diversion: five extra ventricular drain and five ventriculoperitoneal shunt. On histopathological examination, 23 patients had classic MB, 5 had desmoplastic MB, and 1 had anaplastic MB. Staging was performed according to Modified Chang system, with M1 disease representing microscopic tumor cells found in the CSF and M2 disease representing gross nodular seeding demonstrated in the cerebellar/cerebral subarachnoid space or in the third or lateral ventricles [21].
All patients underwent risk factor stratification assessment incorporating age, presence of metastases, extent of resection, and histologic subtype. Seventeen patients were standard risk and twelve high risk. Of high risk patients, 3 had M1 disease (positive LP), 2 had M2 disease (dorsal medulla, infundibulum), 2 had > 1.5 cm2 residual disease, 2 had > 1.5 cm2 residual disease and M2 disease (unilateral cranial nerve VIII, cerebral aqueduct), 1 had T4 disease (extension past foramen magnum), 1 had T3b disease with > 1.5 cm2 residual, and 1 progressed intracranially with infundibular spread before delayed presentation for RT 2 months after surgery.
Of the entire cohort, 5 recent patients had molecular subtyping performed primarily using next generation sequencing with the MSK-IMPACT platform [22], in-situ hybridization, and immunohistochemistry. Of these, 1 WNT subgroup, 2 SHH (1 TP53 wild-type, 1 TP53 mutant) and 2 non-WNT/non-SHH patients were identified (Table 1).
Radiotherapy
Median pre-RT Karnofsky Performance Score (KPS) was 80 (range 40–90). All 29 patients started CSI in 1.8 Gy/day fractions at a median 39 days (range 25–137) after surgery. Twenty-three patients (79%) began RT > 30 days after surgery and five of these (17%) began RT > 60 days after surgery. Two patients (7%) began RT > 90 days after surgery: one due to protracted postoperative morbidity requiring intensive care and one due to delayed presentation to our institution after biopsy and two cycles of etoposide/cisplatin with no response. Twenty-six patients received photon CSI while three received proton CSI. Median CSI dose was 36 Gy (range 23.4–39.6 Gy), with seven standard-risk patients receiving 23.4 Gy. Median boost dose was 55.8 Gy (range 54–59.4 Gy); boost location was posterior fossa in 18 patients, tumor bed in seven patients, and tumor bed + residual/metastasis in four patients. Boost prescription was delivered via opposed lateral fields in four patients, 3D-CRT in six patients, IMRT in 16 patients, and protons in three patients. All patients completed the full course of RT over a median of 43 days (range 36–50 days). Commonly reported acute toxicities during RT ± concurrent chemotherapy included dermatitis and alopecia (n = 29), fatigue (n = 29), nausea/vomiting (n = 23), anemia (n = 18), leukopenia (n = 17), thrombocytopenia (n = 16), and dysphagia (n = 14). During CSI, RBC transfusion was required in four patients and platelet transfusion was required in two patients. One patient developed neutropenic fever requiring admission during CSI treatment.
Chemotherapy
One patient received neoadjuvant (pre-RT) chemotherapy with two cycles of etoposide/cisplatin with no response before presenting to our institution. A total of 20 patients, including 10 of 12 high-risk patients, received chemotherapy concurrent with RT: four received vincristine alone and six received vincristine and carboplatin. Median number of vincristine cycles was six (range 4–8). Nine patients were unable to complete planned concurrent chemotherapy due to peripheral neuropathy.
Twenty-four patients, including all high-risk patients, received combination adjuvant (post-RT) chemotherapy with a median of seven cycles (range 4–8). The most commonly used agents were cisplatin/carboplatin (n = 23), vincristine (n = 15), and lomustine (n = 12). Of these, 12/23 on a platinum-based regimen, 5/15 on vincristine, and 7/12 on lomustine completed the full planned course of treatment without dose reduction. Commonly reported acute toxicities during adjuvant chemotherapy included nausea/vomiting (n = 24), constipation (n = 24), fatigue (n = 24), anemia (n = 22), thrombocytopenia (n = 20), leukopenia (n = 19), neuropathy (n = 17), and ototoxicity (n = 14). During adjuvant chemotherapy, RBC transfusion was required in ten patients and platelet transfusion was required in 11 patients. Six patients developed neutropenic fever requiring admission during adjuvant chemotherapy.
Outcomes
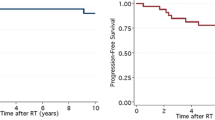
At a median follow-up of 9.0 years (range 1.1–20.5 years), five patients recurred: four in the posterior fossa (all standard-risk, GTR, received CSI to 36 Gy) and one in both the posterior fossa and above the tentorium (high-risk, biopsy only, received CSI to 36 Gy). Of those with recurrence, four received salvage chemotherapy, one with stem cell rescue, two had surgery, and one had tumor bed re-RT. One recently relapsed patient had posterior fossa re-resection and is planned to receive proton re-RT to the resection cavity to a dose of 30 Gy (relative biological effectiveness; RBE) followed by metronomic chemotherapy. Median time to first recurrence was 1.7 years (range 1.0–7.1 years).
Of five patients who died, two died of disease progression and three died of possible treatment-related complications with no evidence of disease (seizure, lobar pneumonia secondary to brainstem dysfunction and leukopenia, multifactorial treatment-related sepsis). The patient with seizures first had an epileptic episode 3.6 years after completing RT and was subsequently controlled on carbamazepine until his death 12 years later, when he experienced fatal head trauma during a seizure. The patient who died of lobar pneumonia developed persistent leukopenia during adjuvant chemotherapy and was found to have biopsy-confirmed posterior fossa necrosis 1.7 years after completing RT. She then developed brainstem lesions on MRI 3 months later and developed dysarthria and hypophonia and died shortly thereafter; an autopsy confirmed pneumonia as the cause of death, presumably due to aspiration secondary to brainstem dysfunction and chronic leukopenia. The patient with multifactorial sepsis developed bilateral arm paresthesias and weakness 13 months after completing CSI to 23.4 Gy with a 54 Gy posterior fossa boost. Craniospinal MRI showed findings questionable for recurrent tumor versus cervical myelopathy with poorly delineated intramedullary T2 hyperintensity suggesting cord edema. The patient was started on IV dexamethasone and high-dose cyclophosphamide, but suffered a protracted course complicated by multifocal osteomyelitis with bacterial and fungal sepsis secondary to prolonged pancytopenia and an infected chemotherapy port. Serial scans over the next 6 months later showed stability of the cervicomedullary lesion. The chemotherapy port was removed and a central line was placed, but the patient’s sepsis recurred and ultimately led to death. Biopsy was unable to be performed and autopsy was declined.
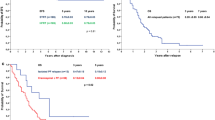
At last follow-up, 23 patients were alive with no evidence of disease and 1 was alive with disease and undergoing treatment. Kaplan–Meier estimates of 10-year OS and (FFS) are 83% (95% confidence interval [CI] 59–93%) and 79% (CI 55–91%), respectively (Fig. 1). Outcomes according to risk group and treatment received are illustrated in Fig. 2. On univariate log-rank analysis (Table 2), desmoplastic histology (p = 0.02) and concurrent chemotherapy (p = 0.004) were associated with superior FFS but not with superior OS. Boost dose of ≥ 55.8 Gy trended towards superior OS (p = 0.06) but not superior FFS. Gender, age at diagnosis, gross total resection, location, histology, adjuvant chemotherapy, risk stratum, and CSI dose were not significantly associated with FFS or OS. Interval to start of RT > 30 days revealed a non-significant association with death; all five deaths, however, occurred in patients with interval > 30 days.
Performance status, treatment sequelae, and quality of life
All patients experienced treatment-related morbidity. The most severe complications were hemiparesis (n = 2) and quadriparesis (n = 2) after surgery, posterior fossa/brainstem RT necrosis (n = 1), and multifactorial treatment-related sepsis (n = 1). Two patients were found on routine MRI to have multiple punctate supratentorial cavernomas: one 2.4 years after 23.4 Gy CSI and the other 4.3 years after 36 Gy CSI. Both patients remain asymptomatic from these lesions. One patient developed suspected vasculopathy manifesting as cortical and basal ganglia stroke with unrevealing cardiac and hematologic workup, 8.1 years after CSI to 39.6 Gy. One patient developed papillary thyroid carcinoma 18.4 years after CSI to 36 Gy; he subsequently had total thyroidectomy and iodine-131 ablation and had no evidence of disease at last follow up. Notably, this patient also developed MB recurrence at 4 years, renal cell carcinoma at 15 years, and prostate adenocarcinoma at 19 years after initial MB diagnosis.
Patients had audiologic testing at baseline, upon exhibiting symptoms, and after the completion of chemotherapy as part of long-term follow-up and data were available for 22 patients. Of these, 8 patients experienced grade 1 ototoxicity, 4 experienced grade 2, and 2 experienced grade 3 [23]. Six patients developed hypothyroidism following therapy. Seventeen patients exhibited executive dysfunction as scored by formal neuropsychiatric testing and/or clinical evaluation incorporating neurological mental status examination ± Montreal Cognitive Assessment (MoCA). Sixteen patients had weakness/ataxia. Thirteen patients were diagnosed with depression/anxiety requiring pharmacologic/behavioral therapy. Median KPS at last follow-up was 80 (range 30–90).
Discussion
Survival
Prospective evidence for adult MB is available from only two trials. Moots et al. report on 11 patients treated with three neoadjuvant cycles of cisplatin, etoposide, and cyclophosphamide followed by 36 Gy CSI with a 54 Gy posterior fossa boost without concurrent or adjuvant chemotherapy. However, outcomes were poor; only two patients had an objective response to pre-RT chemotherapy, two patients progressed on chemotherapy, and 5-year OS and PFS were 27 and 55%, respectively [20]. Brandes et al. report on 10 standard-risk patients receiving RT alone and 26 high-risk patients receiving two cycles of neoadjuvant chemotherapy, RT, and adjuvant chemotherapy. CSI to 36 Gy with a posterior fossa boost to a maximum of 54.8 Gy was given to all patients. Five-year PFS and OS were 72 and 75%, respectively [5, 19]. Our study has several key differences, including several patients treated with CSI to 23.4 Gy, omission of neoadjuvant chemotherapy in all but one patient, and use of concurrent chemotherapy.
Adult MB patients in the present study had comparable OS and FFS as those from prior retrospective studies, which is unsurprising given similar baseline characteristics. Ten-year OS in pediatric MB patients ranges from 65 to 81% but is lower for adult MB patients, ranging from 30 to 76% [2, 5, 9,10,11,12,13,14,15,16,17,18,19, 24,25,26,27,28]. Several studies have found a higher incidence of late relapses in adults as compared to children [5, 26]. Though we only observed one relapse after 5 years, these data underscore the importance of long-term surveillance for adult MB.
Impact of histology and staging
The proportion of patients (5/29) with desmoplastic MB was lower than generally reported and these patients demonstrated superior FFS, consistent with previous studies that have characterized desmoplastic histology as prognostically favorable [11]. Several series have found an association between risk group and outcome, but we did not find risk stratum to be prognostic, possibly due to insufficient sample [9, 12, 13, 15, 24, 25, 29]. One explanation is that patients in our study had complete staging and received risk-adjusted treatment which contributed to favorable outcomes. Brandes et al. found risk group to be prognostic at 5 years but not 10 years, suggesting that its significance may decrease over time when patients are appropriately stratified and treated [5, 19]. We therefore reaffirm the importance of staging and stratification.
Role of radiotherapy and chemotherapy
Studies examining the dose–response relationship have reported superior survival with posterior fossa boost doses in excess of 50–54 Gy, and all our patients received at least 54 Gy [12, 30, 31]. Furthermore, patients with ≥ 55.8 Gy boost demonstrated superior OS on univariate analysis. Pediatric MB studies have shown that RT should begin ≤ 30 days after surgery [32, 33]. In the present study 79% of patients did not receive RT within 30 days, most often due to delayed referral, and these patients showed a trend towards poorer survival. While longer follow-up is needed, these results reinforce the importance of prompt initiation of RT after surgery as in pediatric MB. Past series have also reported RT duration to affect prognosis, with lengthened courses associated with poorer outcomes [34, 35]. However, the median duration of 44 days in the present study is below the 50-day threshold cited in the PNET-3 study. The role of conformal radiotherapy, such as proton CSI, is an active area of investigation in adult MB treatment with preliminary results suggesting lower rates of acute GI and hematologic toxicities. However, longer-term data including survival are lacking [36, 37].
While postoperative RT has a clear role in the treatment of adult MB, the role of chemotherapy is disputed. The largest study examining 468 adult MB patients from the National Cancer Data Base found superior OS for patients receiving adjuvant radiation and chemotherapy [27]. Pediatric guidelines are commonly applied to adults because of the proven benefit of chemotherapy in pediatric trials. Among standard-risk children, chemotherapy and reduced-dose CSI (23.4 Gy) appear to provide superior outcomes as compared to full-dose CSI (36 Gy) without chemotherapy [32, 38]. In our study, 7 of 17 standard-risk patients received CSI to 23.4 Gy with concurrent and adjuvant chemotherapy. None of these patients relapsed, though one died of multifactorial treatment-related sepsis and another of lobar pneumonia likely consequent to aspiration from RT-related brainstem necrosis. A larger series noted no difference in relapse rate in adults treated with 23.4 Gy CSI + posterior fossa boost and concurrent chemotherapy as compared to 36 Gy alone [13]. The study from Brandes et al. showed no difference across risk group in PFS and OS after 7.6 years of follow-up, suggesting a role for chemotherapy in standard-risk patients [19].
Among high-risk patients, there is consensus that chemotherapy improves outcomes. Children with high-risk MB are generally treated with full-dose CSI + concurrent and adjuvant chemotherapy. In our study, all high-risk patients were treated with CSI to ≥ 36 Gy with adjuvant chemotherapy, with 10 also receiving concurrent chemotherapy. One patient who did not receive concurrent chemotherapy died, though this patient had comorbidities preventing resection and required treatment breaks due to toxicity.
Toxicities
Two of the most commonly reported late complications following treatment for MB are ototoxicity and hypothyroidism, for which we observed incidences of 48% (21% Grade 2+) and 21%, respectively. These rates compare favorably with those reported elsewhere [39,40,41]. Another common late complication in our cohort was depression/anxiety; 45% of patients were diagnosed with new-onset depression/anxiety requiring behavioral and/or pharmacologic intervention. The majority of patients in our study experienced executive dysfunction, with many unable to work or live independently. The most severe complication was in a patient who developed cervical myelopathy vs. recurrent tumor 13 months after RT. Though tissue confirmation was unable to be obtained, the patient had stability of a cervicomedullary lesion over a period of months in the setting of steroids and high-dose chemotherapy. This patient had received CSI to 23.4 Gy with a 54 Gy posterior fossa boost, doses not typically associated with the development of myelopathy. While the patient’s records did not show issues with radiation delivery, it is possible the technical complexities of matching whole brain and cervical spine fields contributed to this development, since extreme care must be taken to limit exposure of this sensitive area. Cavernoma, vasculopathy, and second neoplasms are known late complications of RT, and were found in few patients in our sample. The development of papillary thyroid carcinoma in one patient 18 years after MB diagnosis likely had multifactorial etiology, given that this patient also developed several primary neoplasms at other sites.
Molecular subtyping
Molecular subtyping of medulloblastoma has advanced significantly in recent years. The vast majority of adults fall into WNT, SHH, and group 4 subgroups, with an estimated adult MB distribution of 15, 60, and 25%, respectively, and 5-year survival rates of 80, 75, and 45–75%, respectively [7, 42]. With a good prognosis, WNT patients are an attractive target for therapy de-escalation to reduce long-term sequelae. Among SHH patients, TP53 mutation status is a poor prognostic marker. Group 4 patients have a propensity for distant metastatic recurrence [43]. Only five recent patients had molecular testing: 1 WNT, 2 SHH (1 TP53 wild-type, 1 TP53 mutated) and 2 non-WNT/non-SHH. SHH-pathway inhibitors are currently being evaluated in phase I–III trials, and may provide a therapeutic option to chemoradiation protocols currently used for these patients [7, 44]. Despite advances in subtyping, it remains difficult to assess prognosis in adult MB since studies have reported heterogenous treatment, many without chemotherapy. As subtyping becomes routine, further studies may elucidate prognosis based on a combination of clinicopathologic and molecular characteristics. While subtyping information is not yet available for a majority of patients in the present study, a future analysis is planned.
Conclusions
Limitations of our study include the small sample size, retrospective design, heterogeneous patient population and histopathologies represented, probable confounders within the analysis, and lack of molecular data for a majority of patients. However, the long-term data presented demonstrate favorable survival. Nonetheless, multi-institutional prospective trials may provide a clearer depiction of survival and prognostic factors. Taken together with recent series, our results suggest that chemotherapy may contribute to longer-term disease control and should be considered in patients who can tolerate multimodal treatment. As long-term survival improves, late effects of treatment are becoming increasingly important; treatment morbidity and quality of life should be evaluated as endpoints in future trials.
References
Lai R (2008) Survival of patients with adult medulloblastoma: a population-based study. Cancer 112(7):1568–1574. doi:10.1002/cncr.23329
Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2016) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro-oncol. doi:10.1093/neuonc/now207
Giordana MT, Cavalla P, Dutto A, Borsotti L, Chio A, Schiffer D (1997) Is medulloblastoma the same tumor in children and adults? J Neurooncol 35(2):169–176
Douglas JG, Barker JL, Ellenbogen RG, Geyer JR (2004) Concurrent chemotherapy and reduced-dose cranial spinal irradiation followed by conformal posterior fossa tumor bed boost for average-risk medulloblastoma: efficacy and patterns of failure. Int J Radiat Oncol Biol Phys 58(4):1161–1164. doi:10.1016/j.ijrobp.2003.09.010
Brandes AA, Franceschi E, Tosoni A, Blatt V, Ermani M (2007) Long-term results of a prospective study on the treatment of medulloblastoma in adults. Cancer 110(9):2035–2041. doi:10.1002/cncr.23003
Brandes AA, Bartolotti M, Marucci G, Ghimenton C, Agati R, Fioravanti A, Mascarin M, Volpin L, Ammannati F, Masotto B, Gardiman MP, De Biase D, Tallini G, Crisi G, Bartolini S, Franceschi E (2015) New perspectives in the treatment of adult medulloblastoma in the era of molecular oncology. Crit Rev Oncol Hematol 94(3):348–359. doi:10.1016/j.critrevonc.2014.12.016
Brandes AA, Franceschi E (2014) Shedding light on adult medulloblastoma: current management and opportunities for advances. Am Soc Clin Oncol Educ Book. doi:10.14694/EdBook_AM.2014.34.e82
Vigneron C, Entz-Werle N, Lutz P, Spiegel A, Jannier S, Helfre S, Alapetite C, Coca A, Kehrli P, Noel G (2015) Evolution of the management of pediatric and adult medulloblastoma. Cancer Radiother 19(5):347–357. doi:10.1016/j.canrad.2015.03.010 (quiz 358–349, 362)
Prados MD, Warnick RE, Wara WM, Larson DA, Lamborn K, Wilson CB (1995) Medulloblastoma in adults. Int J Radiat Oncol Biol Phys 32(4):1145–1152
Frost PJ, Laperriere NJ, Wong CS, Milosevic MF, Simpson WJ, Pintilie M (1995) Medulloblastoma in adults. Int J Radiat Oncol Biol Phys 32(4):951–957
Carrie C, Lasset C, Alapetite C, Haie-Meder C, Hoffstetter S, Demaille MC, Kerr C, Wagner JP, Lagrange JL, Maire JP et al (1994) Multivariate analysis of prognostic factors in adult patients with medulloblastoma: retrospective study of 156 patients. Cancer 74(8):2352–2360
Abacioglu U, Uzel O, Sengoz M, Turkan S, Ober A (2002) Medulloblastoma in adults: treatment results and prognostic factors. Int J Radiat Oncol Biol Phys 54(3):855–860
Padovani L, Sunyach MP, Perol D, Mercier C, Alapetite C, Haie-Meder C, Hoffstetter S, Muracciole X, Kerr C, Wagner JP, Lagrange JL, Maire JP, Cowen D, Frappaz D, Carrie C (2007) Common strategy for adult and pediatric medulloblastoma: a multicenter series of 253 adults. Int J Radiat Oncol Biol Phys 68(2):433–440. doi:10.1016/j.ijrobp.2006.12.030
Selek U, Zorlu F, Hurmuz P, Cengiz M, Turker A, Soylemezoglu F, Gurkaynak M (2007) Craniospinal radiotherapy in adult medulloblastoma. Strahlentherapie Onkologie : Organ der Deutschen Rontgengesellschaft [et al] 183(5):236–240. doi:10.1007/s00066-007-1563-y
Ang C, Hauerstock D, Guiot MC, Kasymjanova G, Roberge D, Kavan P, Muanza T (2008) Characteristics and outcomes of medulloblastoma in adults. Pediatr Blood Cancer 51(5):603–607. doi:10.1002/pbc.21588
Ertas G, Ucer AR, Altundag MB, Durmus S, Calikoglu T, Ozbagi K, Abanuz H, Altundag K, Demirkasimoglu A (2008) Medulloblastoma/primitive neuroectodermal tumor in adults: prognostic factors and treatment results: a single-center experience from Turkey. Medical oncology 25(1):69–72. doi:10.1007/s12032-007-0044-6
Menon G, Krishnakumar K, Nair S (2008) Adult medulloblastoma: clinical profile and treatment results of 18 patients. J Clin Neurosci 15(2):122–126. doi:10.1016/j.jocn.2007.06.007
Smee RI, Williams JR (2008) Medulloblastomas-primitive neuroectodermal tumours in the adult population. J Med Imaging Radiat Oncol 52(1):72–76. doi:10.1111/j.1440-1673.2007.01915.x
Brandes AA, Ermani M, Amista P, Basso U, Vastola F, Gardiman M, Iuzzolino P, Turazzi S, Rotilio A, Volpin L, Mazza C, Sainati L, Ammannati F, Berti F (2003) The treatment of adults with medulloblastoma: a prospective study. Int J Radiat Oncol Biol Phys 57(3):755–761
Moots PL, O’Neill A, Londer H, Mehta M, Blumenthal DT, Barger GR, Grunnet ML, Grossman S, Gilbert MR, Schiff D (2016) Preradiation chemotherapy for adult high-risk medulloblastoma: a trial of the ECOG-ACRIN cancer research group (E4397). Am J Clin Oncol. doi:10.1097/coc.0000000000000326
Chang CH, Housepian EM, Herbert C Jr (1969) An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology 93(6):1351–1359. doi:10.1148/93.6.1351
Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, Chandramohan R, Liu ZY, Won HH, Scott SN, Brannon AR, O’Reilly C, Sadowska J, Casanova J, Yannes A, Hechtman JF, Yao J, Song W, Ross DS, Oultache A, Dogan S, Borsu L, Hameed M, Nafa K, Arcila ME, Ladanyi M, Berger MF (2015) Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17(3):251–264. doi:10.1016/j.jmoldx.2014.12.006
Brock PR, Knight KR, Freyer DR, Campbell KCM, Steyger PS, Blakley BW, Rassekh SR, Chang KW, Fligor BJ, Rajput K, Sullivan M, Neuwelt EA (2012) Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new international society of pediatric oncology boston ototoxicity scale. J Clin Oncol 30(19):2408–2417. doi:10.1200/jco.2011.39.1110
Silvani A, Gaviani P, Lamperti E, Botturi A, DiMeco F, Franzini A, Ferroli P, Fariselli L, Milanesi I, Erbetta A, Pollo B, Salmaggi A (2012) Adult medulloblastoma: multiagent chemotherapy with cisplatinum and etoposide: a single institutional experience. J Neurooncol 106(3):595–600. doi:10.1007/s11060-011-0696-0
Riffaud L, Saikali S, Leray E, Hamlat A, Haegelen C, Vauleon E, Thierry Lesimple (2009) Survival and prognostic factors in a series of adults with medulloblastomas. J Neurosurg 111(3):478–487. doi:10.3171/2009.1.jns081004
Chan AW, Tarbell NJ, Black PM, Louis DN, Frosch MP, Ancukiewicz M, Chapman P, Loeffler JS (2000) Adult medulloblastoma: prognostic factors and patterns of relapse. Neurosurgery 47(3):623–631 (discussion 631–622)
Kann BH, Lester-Coll NH, Park HS, Yeboa DN, Kelly JR, Baehring JM, Becker KP, Yu JB, Bindra RS, Roberts KB (2016) Adjuvant chemotherapy and overall survival in adult medulloblastoma. Neuro-oncol. doi:10.1093/neuonc/now150
Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A (2013) Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children’s Oncology Group trial A9961. Neuro-oncol 15(1):97–103. doi:10.1093/neuonc/nos267
Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, Allen JC, Stevens KR, Stanley P, Li H, Wisoff JH, Geyer JR, McGuire-Cullen P, Stehbens JA, Shurin SB, Packer RJ (1999) Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J clin Oncol 17(3):832–845. doi:10.1200/jco.1999.17.3.832
Hubbard JL, Scheithauer BW, Kispert DB, Carpenter SM, Wick MR, Laws ER Jr (1989) Adult cerebellar medulloblastomas: the pathological, radiographic, and clinical disease spectrum. J Neurosurg 70(4):536–544. doi:10.3171/jns.1989.70.4.0536
Berry MP, Jenkin RD, Keen CW, Nair BD, Simpson WJ (1981) Radiation treatment for medulloblastoma. A 21-year review. J Neurosurg 55(1):43–51. doi:10.3171/jns.1981.55.1.0043
Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, Bayer L, LaFond D, Donahue BR, Marymont MH, Muraszko K, Langston J, Sposto R (2006) Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 24(25):4202–4208. doi:10.1200/jco.2006.06.4980
Kortmann RD, Kuhl J, Timmermann B, Mittler U, Urban C, Budach V, Richter E, Willich N, Flentje M, Berthold F, Slavc I, Wolff J, Meisner C, Wiestler O, Sorensen N, Warmuth-Metz M, Bamberg M (2000) Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT ‘91. Int J Radiat Oncol Biol Phys 46(2):269–279
Taylor RE, Bailey CC, Robinson KJ, Weston CL, Ellison D, Ironside J, Lucraft H, Gilbertson R, Tait DM, Saran F, Walker DA, Pizer BL, Lashford LS (2004) Impact of radiotherapy parameters on outcome in the International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 study of preradiotherapy chemotherapy for M0-M1 medulloblastoma. Int J Radiat Oncol Biol Phys 58(4):1184–1193. doi:10.1016/j.ijrobp.2003.08.010
Taylor RE, Bailey CC, Robinson K, Weston CL, Ellison D, Ironside J, Lucraft H, Gilbertson R, Tait DM, Walker DA, Pizer BL, Imeson J, Lashford LS (2003) Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children’s Cancer Study Group PNET-3 Study. J Clin Oncol 21(8):1581–1591. doi:10.1200/jco.2003.05.116
Brown AP, Barney CL, Grosshans DR, McAleer MF, de Groot JF, Puduvalli VK, Tucker SL, Crawford CN, Khan M, Khatua S, Gilbert MR, Brown PD, Mahajan A (2013) Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys 86(2):277–284. doi:10.1016/j.ijrobp.2013.01.014
Barney CL, Brown AP, Grosshans DR, McAleer MF, de Groot JF, Puduvalli V, Tucker SL, Crawford CN, Gilbert MR, Brown PD, Mahajan A (2014) Technique, outcomes, and acute toxicities in adults treated with proton beam craniospinal irradiation. Neuro-oncol 16(2):303–309. doi:10.1093/neuonc/not155
Merchant TE, Kun LE, Krasin MJ, Wallace D, Chintagumpala MM, Woo SY, Ashley DM, Sexton M, Kellie SJ, Ahern V, Gajjar A (2008) Multi-institution prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose-intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys 70(3):782–787. doi:10.1016/j.ijrobp.2007.07.2342
Huang E, Teh BS, Strother DR, Davis QG, Chiu JK, Lu HH, Carpenter LS, Mai WY, Chintagumpala MM, South M, Grant WH 3rd, Butler EB, Woo SY (2002) Intensity-modulated radiation therapy for pediatric medulloblastoma: early report on the reduction of ototoxicity. Int J Radiat Oncol Biol Phys 52(3):599–605
Ricardi U, Corrias A, Einaudi S, Genitori L, Sandri A, di Montezemolo LC, Besenzon L, Madon E, Urgesi A (2001) Thyroid dysfunction as a late effect in childhood medulloblastoma: a comparison of hyperfractionated versus conventionally fractionated craniospinal radiotherapy. Int J Radiat Oncol Biol Phys 50(5):1287–1294
Paulino AC (2002) Hypothyroidism in children with medulloblastoma: a comparison of 3600 and 2340 cGy craniospinal radiotherapy. Int J Radiat Oncol Biol Phys 53(3):543–547
Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM (2012) Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol 123(4):465–472. doi:10.1007/s00401-011-0922-z
Remke M, Hielscher T, Northcott PA, Witt H, Ryzhova M, Wittmann A, Benner A, von Deimling A, Scheurlen W, Perry A, Croul S, Kulozik AE, Lichter P, Taylor MD, Pfister SM, Korshunov A (2011) Adult medulloblastoma comprises three major molecular variants. J Clin Oncol 29(19):2717–2723. doi:10.1200/jco.2011.34.9373
Lou E, Schomaker M, Wilson JD, Ahrens M, Dolan M, Nelson AC (2016) Complete and sustained response of adult medulloblastoma to first-line sonic hedgehog inhibition with vismodegib. Cancer Biol Ther 17(10):1010–1016. doi:10.1080/15384047.2016.1220453
Acknowledgements
Funding was provided by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA008748). This work is also supported by a gift from Jack and Susan Rudin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.M.S. reports personal fees from Aesculap, Sanofi, and Baxter. I.J.D. reports personal fees from Bayer, Bristol-Myers Squibb, Ipsen, Eisai, and Pfizer, and grants from Genentech and Parexel (GSK, Novartis). L.M.D reports personal fees from Novartis, GlaxoSmithKline, and Celgene. All payments are outside the submitted work. Other authors declare no conflicts.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and the 1964 Helsinki declaration and its later amendments.
Formal consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
De, B., Beal, K., De Braganca, K.C. et al. Long-term outcomes of adult medulloblastoma patients treated with radiotherapy. J Neurooncol 136, 95–104 (2018). https://doi.org/10.1007/s11060-017-2627-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2627-1