Abstract
The classification, treatment and prognosis of high-grade gliomas has been shown to correlate with the expression of molecular markers (e.g. MGMT promotor methylation and IDH1 mutations). Acquisition of tumor samples may be obtained via stereotactic biopsy or open craniotomy. Between the years 2009 and 2013, 22 patients initially diagnosed with HGGs via stereotactic biopsy, that ultimately underwent open craniotomy for resection of their tumor were prospectively included in an institutional glioma database. MGMT promotor analysis was performed using methylation-specific (MS)-PCR and IDH1R132H mutation analysis was performed using immunohistochemistry. Three patients (13.7%) exhibited IDH1R132H mutations in samples obtained via stereotactic biopsy. Tissue derived from stereotaxic biopsy was demonstrated to have MGMT promotor methylation in ten patients (45.5%), while a non-methylated MGMT promotor was demonstrated in ten patients (45.5%); inconclusive results were obtained for the remaining two patients (9%) within our cohort. The initial histologic grading, IDH1R132H mutation and MGMT promotor methylation results were confirmed using samples obtained during open craniotomy in all but one patient; here inconclusive MGMT promotor analysis was obtained in contrast to that which was obtained via stereotactic biopsy. Tumor samples acquired via stereotactic biopsy provide accurate information with regard to clinically relevant molecular markers that have been shown to impact patient care decisions. The profile of markers analyzed in our cohort was nearly concordant between those samples obtained via stereotactic biopsy or open craniotomy thereby suggesting that clinical decisions may be based on the molecular profile of the tumor samples obtained via stereotactic biopsy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prognosis of patients suffering from high-grade gliomas (HGGs) remains poor with a median survival of approximately 12 months in population based studies [1]. Malignant gliomas account for about 70% of all primary brain tumors in adult patients and recent work has centered on understanding molecular markers in an effort to personalize prognostics/treatment regimens for patients in need [2]. Such work has led to the identification of molecular markers capable of stratifying HGG patients into subgroups thereby influencing treatment decisions [3].
One of the major molecular markers developed in glioma patients in recent years is the promoter methylation status of the gene encoding the enzyme O6-methyl-guanine-DNA methyltransferase (MGMT); the methylation status of the promoter is predictive of a better response to temozolomide (TMZ) as an adjuvant post-operative chemotherapeutic agent [4]. In line with such findings, the NOA-08 study demonstrated the value of treatment decisions based on MGMT promoter methylation status in elderly patients with GBM [5]. The clinical significance of such findings highlights the importance of MGMT promotor methylation assessment and currently, MGMT promotor methylation assessment is the most commonly performed molecular analysis in neuro-oncologic patients suffering from GBM [6].
A second major molecular marker employed in the diagnosis/management of both primary and secondary GBM patients is that of isocitrate dehydrogenase 1 (IDH1) mutations [7, 8]. The most common IDH1 mutation is located on chromosome 2q33 at amino acid residue 132 and is predominately found in grade II/III gliomas as well as in secondary GBM (sGBM) (~85%) [9]. Accordingly, such findings imply, that the presence of an IDH1 mutation may be used as a diagnostic and/or prognostic marker indicative of sGBM [10]. Recently, novel therapies targeting IDH1 mutations have shown promising results for tumor therapy [11, 12]; it is therefore tempting to speculate that IDH1R132 mutational status may result in clinically relevant treatment decisions.
It is prudent to note that HGGs are frequently unresectable due in part to proximity with eloquent and/or critical areas within the brain. In these patients, stereotactic biopsies, for both histological confirmation of the clinico-radiological diagnosis and/or evaluation of a tumors molecular marker profile are necessary. Accordingly, we performed histology, and assessed MGMT promotor methylation and IDH1R132 mutational status in specimens derived from patients who underwent stereotactic biopsies due to inconclusive preoperative imaging. Those patients that ultimately underwent open surgery for their now diagnosed glioma (i.e. via the stereotactic sample) were included within our analysis. Please note, biopsy was only performed because of inconclusive preoperative imaging (e.g. differential diagnosis of cerebral lymphoma having not been ruled out). Specimens derived from open craniotomy were ultimately compared to those that had been derived from stereotaxic biopsy in an effort to understand if results derived from both methods would in fact correlate.
Patients and methods
All patients diagnosed with intracranial gliomas are entered into a prospective data registry at the University of Frankfurt. This study was approved by the University Hospital Institutional Review Board (reference # 04/09 SNO 01/08).
Attending neuropathologists participated in every stereotactic biopsy in an effort to confirm the pathology of the lesion. Stereotactic trajectories were planned by the attending neurosurgeon who would perform the procedure. During planning the surgeon accounted for tumor location, contrast enhancement, peritumoral edema, central necrosis and patient history. The number of biopsies, trajectories and all results from histopathological and molecular analyses were prospectively entered into our institutional glioma database.
Stereotactic planning was based on magnetic resonance imaging (MRI) acquired using a 3T scanner (Siemens Medical Solutions, Erlangen, Germany) which employed 3D isovolumetric T2 weighted and T1 weighted sequences with a spatial resolution of 1 mm3. T1 weighted magnetization-prepared rapid-acquisition gradient echo (MPRAGE) sequencing (TR = 1900 ms; TE = 2.7 ms; inversion time = 900 ms; flip angle = 9°; field of view = 256 × 256 mm2) was performed after intravenous injection of gadobutrol (1 mmol/ml) at a dose of 0.1 ml/kg body weight [13].
For all stereotactic biopsies, a stereotactic frame was utilized (Leksell Coordinate Frame G; Elekta Instruments, Stockholm, Sweden). On the day of surgery, cranial computed tomography (CT) with 1.5 mm slice thickness was performed after fixation of the head to the frame. Automated image co-registration with the preoperative MRI and trajectory planning was performed using iPlan software (BrainLAB, Feldkirchen, Germany). After skin incision and burr hole trepanation, the biopsy needle was inserted into the border of the lesion. Serial biopsies were obtained using micro-forceps with a diameter of 1.4 mm. The procedure was performed or supervised by one of three neurosurgeons with clinical expertise in stereotactic neurosurgical procedures.
Intraoperatively, single specimens (n = 1–3) were selected for smear preparation and stained with methylene blue in an effort to provide a preliminary intraoperative neuropathological diagnosis and to confirm that an adequate sample was retrieved from the pathologic areas for subsequent diagnostic procedures [14]. The remaining samples were fixed in formalin for 24–48 h before further processing. All tumor specimens were evaluated using classic hematoxylin & eosin (HE) staining and were classified according to the World Health Organization (WHO) Classification of Tumors of the Central Nervous System (CNS) [15]. Immunohistochemical (IHC) analyses were employed for the analysis of IDH1 (R132H). Microscopic evaluation of samples was performed using an Olympus BX50 light microscope (Olympus, Hamburg, Germany).
Two to 3 (median: 3) tumor specimens displaying the largest amount of vital tumor tissue (goal: >70% vital tumor tissue) were selected for methylation specific (MS)-PCR. Briefly, four slides of 10 μm thickness cut from each paraffin block were deparaffinized using xylene and two times 96% ethanol. DNA isolation, PCR and gel electrophoresis were performed and interpreted as has been previously described [13].
During open craniotomy, random samples were allocated for both MGMT promotor methylation assessment and IHC for IDH1 mutation analysis. In so doing we provide an unbiased approach for the assessment of molecular markers post-tumor resection.
Statistical analyses
Nominal factors (e.g. type of sample acquisition) related to histology/MS-PCR /IHC results were analyzed using a contingency table followed by χ2 analysis. Cohen’s kappa was analyzed in order to assess correlation between specimens obtained via stereotactic biopsy or via open craniotomy. A significance level of α = 0.05 was selected for all tests and deemed to be significant. Statistical analyses were performed using SPSS software (SAS, Cary, USA).
Results
We analyzed 22 consecutive patients from our institutional glioma database who initially underwent stereotactic biopsies and then went on to have an open resection procedure for their definitively diagnosed intracranial glioma (Fig. 1).
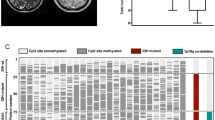
Illustrative case. a Sagittal and axial MR imaging of an exemplary patient. Based on imaging, no definitive diagnosis of glioma was made. b Stereotactic biopsy and sample acquisition using a trajectory tangential to the contrast enhancement was performed and a WHO °IV glioblastoma diagnosed. Open craniotomy and resection of the tumor was performed, followed by postoperative MRI demonstrating complete resection of the tumor. WHO World health organization
The median age of patients included within our study was 58 years (IQR 48–66 years). Thirteen patients were male (59.1%) and nine patients were female (40.9%). The Karnofsky Performance Score (KPS) of the patients included within our analyses is presented in Table 1 and ranged from 100 to 50. Three patients (13.6%) had a histopathologic diagnosis of WHO°III lesions (anaplastic astrocytomas) and 19 patients (86.4%) had a histopathologic diagnosis of WHO°IV (GBM). Only one patient (4.5%) had a prior history of glioma, while 21 patients (95.5%) had been newly diagnosed with glioma. The median time interval between stereotactic biopsy and open craniotomy was 13 days (IQR 9–17 days). It is important to note that patients included within this study cohort underwent both procedures without receiving additional therapy (i.e. between the stereotactic biopsy and open craniotomy). The adjuvant therapeutic regimen after open craniotomy (45.6%) consisted of concomitant radio/chemotherapy and temozolomide for 12 patients; four patients (18.2%) were participants in experimental trials, whilst the remaining eight patients (36.4%) had received other therapies (Table 1).
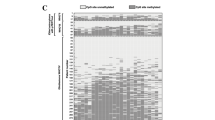
Within our cohort, MS-PCR of stereotactic biopsies yielded conclusive results in 20 (90.9%) of the included cases with both methylated and non-methylated MGMT promotors having been described (i.e. n = 10 patients for each of the aforementioned). Of note, inconclusive MGMT promoter methylation status was described in two (9.1%) of the patients analyzed (Fig. 2c).
Correlation between stereotactic and open surgical sample acquisition. a Correlation of conventional histopathologic grading, b MGMT promotor methylation analysis and c IDH1 mutation were assessed in samples acquired by both stereotactic biopsy and open craniotomy. A highly significant correlation was observed in all three parameters; MGMT O6-methylguanine-DNA methyl-transferase, IDH Isocitrate dehydrogenase, WHO World health organization; data comparison was facilitated by employing Cohen’s κ. p values are indicated for the respective parameters
We went on to examine if conclusive MGMT promoter methylation was associated with the pattern in which the stereotaxic samples were acquired. Hence, we dichotomized trajectories into those which were directed at the lesion center (“centered”) and those which were tangentially directed at the lesion border (“tangential”). No significant differences were observed with regard to a higher level of conclusive results in MS-PCR obtained from patients biopsied via a tangential stereotactic trajectory (92.9% conclusive MS-PCR results) in comparison to those patients biopsied via centered trajectories (87.5% conclusive MS-PCR results; Table 2).
No significant differences were observed in the total number of tumor specimens taken by the neurosurgeon (p = 0.88) between the patients showing conclusive (median 17; IQR 14–19) and those showing inconclusive results (median 17; IQR 14–18) in MS-PCR for MGMT promoter methylation (data not shown). In line with such findings, the absolute number of paraffin-embedded samples (p = 0.9) taken for intraoperative neuropathological diagnosis was not associated with conclusiveness of results in MS-PCR.
Critically within our cohort, no complications with the exception of minor bleeding were observed. In 6 patients (27.3%) the attending surgeon documented an intraoperative observation of blood effusion in the trajectory path. All six patients had minor postoperative signs of hemorrhage evident on postoperative CT scan; none of the other patients had postoperative imaging suggestive of bleeding. In total, two patients (9.1%) developed temporary neurological worsening, yet recovered over the observed clinical course (i.e. no patient suffered a permanent deficit).
In the samples acquired via stereotactic biopsy, conventional histology revealed WHO°III tumors in 3 (12.4%) patients and WHO°IV GBM in 19 patients (87.6%). The same results were obtained, when using samples acquired via open craniotomy. Cohen’s kappa, when comparing both modes of sample acquisition, was 1 (p < 0.001; Fig. 2a).
Assessment of molecular markers consisted of IHC staining of IDH1R132H and MS-PCR for MGMT promotor analysis. Analysis of markers revealed IDH1R132H mutation in three patients in those specimens obtained during both stereotactic biopsy and open craniotomy. The remaining patients were immunonegative using IHC and were considered wildtype with regard to IDH1 mutational status. Between both modes of sample acquisition, Cohen’s kappa was 1 (p < 0.001; Fig. 2b).
Assessment of MGMT promotor methylation using samples obtained via stereotactic biopsy resulted in conclusive results in 20 patients (90.9%). Of these, ten patients displayed a methylated MGMT promotor and ten patients an unmethylated MGMT promotor. In samples acquired via open craniotomy, 19 patients displayed conclusive results with one patient previously diagnosed with a methylated MGMT promotor (using a stereotaxic sample) now having been diagnosed with an inconclusive MGMT promotor status. Cohen’s kappa, when comparing both modes of sample acquisition, was 0.92 (p < 0.001; Fig. 2c).
Discussion
Within the neurosurgical community consensus has emerged to suggest that HGG patients may in fact benefit from the complete resection of their tumor followed by adjuvant radio and chemotherapy [16,17,18,19,20]. Recent literature has also come to suggest that an incomplete tumor resection may not superior to combined radio- and chemotherapy alone [21].
Accordingly, in those patients, where diffuse infiltration of glioma cells into eloquent/critical areas of the CNS may hinder complete resection, tumor samples are nonetheless required for an accurate neuropathological diagnosis. Further, the assessment of such molecular markers will also be required for the development of novel therapeutic regimens and/or evaluation of clinical prognosis.
In the literature, controversial data regarding the tumor homogeneity of both low-grade and high-grade glioma exists; while the intratumoral homogeneity of low grade gliomas with respect to clinically relevant markers has been demonstrated [22], other groups have observed a high level of intratumoral heterogeneity, increasing the risk of undergrading a low-grade glioma (e.g. when missing anaplastic foci in stereotactic sample acquisition) [23, 24]. Further, recent reports have highlighted intratumoral heterogeneity in HGGs with regard to certain molecular markers and this must also be factored into the reliability of stereotactically centered diagnoses [25,26,27,28,29]. With regard to HGG, reports have also suggested that there may be intratumoral heterogeneity with regard to MGMT promotor methylation status [27, 30, 31]. Contrary to such reports, high intratumoral homogeneity for MGMT promotor methylation status has been described for high-grade anaplastic astrocytomas as well as for GBM [23, 24]. One possible explanation for such discordant findings might be the “contamination” with necrotic tissue sample as discussed by Grasbon-Frodl et al. [24].
However, the literature critically lacks analyses confirming the homogeneity of clinically relevant molecular markers in HGGs as well as concordance between samples obtained via stereotactic biopsy and open craniotomy.
Approaches centered around a stereotactic biopsy are much less invasive when compared to open craniotomy procedures [32] which might result in craniotomy-associated complications [33]. Further, the utilization of improved stereotactic techniques for sample acquisition has resulted in a diagnostic neuropathological accuracy of up to 100% [34,35,36,37]; such findings are in line with the work presented herein.
Beyond an accurate histopathological diagnosis, molecular markers are required for GBM patient treatment stratification [38]. While in the past, MGMT promoter methylation status was considered to be the most important marker, novel molecular markers such as IDH1 have emerged [6, 10]. The prognostic value of IDH1 mutations in GBM has been demonstrated with the reduced survival of GBM patients who display a wild-type IDH1 protein [39]. Such diagnostic and therapeutic implications further strengthen the importance of proper tumor sampling by the neurosurgeon, who must provide adequate specimens for both microscopic and molecular pathologic diagnostics [11, 22].
Regarding histologic grading, all patients had the same diagnosis independent of the type of sample acquisition as evidenced by the Cohen’s kappa value of 1. Such a high level of concordance is superior to that which has been published, with correlations between samples acquired via stereotactic surgery and open craniotomy being as low as 63% [40,41,42,43]. Such disparate findings may be due in part to the advancement of novel imaging tools/paradigms (e.g. CT, MRI and/or positron emission tomography [PET]), which are now routinely incorporated into the planning of stereotactic procedures [44,45,46,47].
When evaluating IDH1 mutational status, we were able to demonstrate a high degree of concordance in all patients included within our study when comparing both modes of sample acquisition (i.e. stereotactic biopsy versus open craniotomy) (Cohen’s κ = 1). As per the above-mentioned, IDH1 mutations occur early in the development of gliomas and are observed to be highly homogenous in low-grade gliomas [23, 48]. Such findings clearly imply that stereotactic sample acquisition in low-grade glioma patients would be of value. Beyond low grade gliomas our data also suggest that stereotaxic sample acquisition may be reliably performed if one seeks to determine the IDH1 status of HGGs. This is of particular interest, as recent literature has come to suggest the importance of IDH1 status in the accurate histopathological grading of HGGs in which limited tissue is available [49].
As described, our department relies on MS-PCR analysis for the assessment of MGMT promotor methylation status [13]. The results reported herein fall within the expected range of conclusive results previously published (i.e. 56–100% of all cases) [4, 5]. When we compared samples obtained via stereotactic biopsy or open craniotomy, only one patient displayed a difference in MS-PCR findings. This patient displayed an inconclusive result when the open craniotomy sample was analyzed, while previously having had a conclusive (i.e. methylated) result in the sample acquired via stereotactic biopsy. Our findings result in an exceptionally high correlation as observed by a Cohen’s κ of 0.92. Again, these results may be explained when considering that advanced/novel imaging techniques have been employed and that biopsies were performed using non-centered trajectories in the majority of cases. It is prudent to note that the cohort analyzed within this manuscript is comprised of newly diagnosed glioblastoma patients; however as has been previously demonstrated MGMT promotor methylation may change over time [50]. Hence, extrapolation of the results presented with regard to MGMT promoter methylation should take the aforementioned into consideration.
Conclusions
The value of stereotactic sample acquisition in neurosurgery has become widely acknowledged. In this study, we have demonstrated that the analysis of stereotactically obtained samples provides a high rate of conclusive MGMT promotor methylation and can be used to detect the presence of IDH1 mutations. Further, we have shown for the first time, that there is nearly complete concordance with results obtained from samples derived from open craniotomies with regard to histologic grade, MGMT promotor methylation and IDH1 mutational status in HGGs.
Accordingly, our data indicate that treatment decisions incorporating molecular markers in HGGs may in fact be based on stereotactic biopsies alone. Being that the morbidity of a biopsy is less than that of a craniotomy, a stereotactic biopsy may in fact be employed if molecular profiling is the main reason for surgery (e.g. in non-resectable, eloquent tumors).
Abbreviations
- HGG:
-
High-grade glioma
- MGMT:
-
O6-methyl- guanine-DNA methyltransferase
- IDH:
-
Isocitrate dehydrogenase
- WHO:
-
World Health Organization
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- IHC:
-
Immunohistochemistry
References
Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J, Consortium NC (2010) Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 16:2443–2449. doi:10.1158/1078-0432.CCR-09-3106
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507. doi:10.1056/NEJMra0708126
Tabatabai G, Stupp R, van den Bent MJ, Hegi ME, Tonn JC, Wick W, Weller M (2010) Molecular diagnostics of gliomas: the clinical perspective. Acta Neuropathol 120:585–592. doi:10.1007/s00401-010-0750-6
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. doi:10.1056/NEJMoa043331
Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, Papsdorf K, Steinbach JP, Sabel M, Combs SE, Vesper J, Braun C, Meixensberger J, Ketter R, Mayer-Steinacker R, Reifenberger G, Weller M, Society NOASGoN-oWGoGC (2012) Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 13:707–715. doi:10.1016/S1470-2045(12)70164-X
Holdhoff M, Ye X, Blakeley JO, Blair L, Burger PC, Grossman SA, Diaz LA Jr (2012) Use of personalized molecular biomarkers in the clinical care of adults with glioblastomas. J Neurooncol 110:279–285. doi:10.1007/s11060-012-0968-3
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. doi:10.1056/NEJMoa0808710
Gessler F, Zappi J, Konczalla J, Bernstock JD, Forster MT, Wagner M, Mittelbronn M, Seifert V, Senft C (2017) Secondary glioblastoma: molecular and clinical factors that affect outcome after malignant progression of a lower grade tumor. World Neurosurg. doi:10.1016/j.wneu.2017.02.104
Horbinski C, Kelly L, Nikiforov YE, Durso MB, Nikiforova MN (2010) Detection of IDH1 and IDH2 mutations by fluorescence melting curve analysis as a diagnostic tool for brain biopsies. J Mol Diagn 12:487–492. doi:10.2353/jmoldx.2010.090228
Nobusawa S, Watanabe T, Kleihues P, Ohgaki H (2009) IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res 15:6002–6007. doi:10.1158/1078-0432.CCR-09-0715
Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, Menn O, Osswald M, Oezen I, Ott M, Keil M, Balss J, Rauschenbach K, Grabowska AK, Vogler I, Diekmann J, Trautwein N, Eichmuller SB, Okun J, Stevanovic S, Riemer AB, Sahin U, Friese MA, Beckhove P, von Deimling A, Wick W, Platten M (2014) A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 512:324–327. doi:10.1038/nature13387
Pusch S, Krausert S, Fischer V, Balss J, Ott M, Schrimpf D, Capper D, Sahm F, Eisel J, Beck AC, Jugold M, Eichwald V, Kaulfuss S, Panknin O, Rehwinkel H, Zimmermann K, Hillig RC, Guenther J, Toschi L, Neuhaus R, Haegebart A, Hess-Stumpp H, Bauser M, Wick W, Unterberg A, Herold-Mende C, Platten M, von Deimling A (2017) Pan-mutant IDH1 inhibitor BAY 1436032 for effective treatment of IDH1 mutant astrocytoma in vivo. Acta Neuropathol 133:629–644. doi:10.1007/s00401-017-1677-y
Weise LM, Harter PN, Eibach S, Braczynski AK, Dunst M, Rieger J, Bahr O, Hattingen E, Steinbach JP, Plate KH, Seifert V, Mittelbronn M (2014) Confounding factors in diagnostics of MGMT promoter methylation status in glioblastomas in stereotactic biopsies. Stereotact Funct Neurosurg 92:129–139. doi:10.1159/000360582
Tilgner J, Herr M, Ostertag C, Volk B (2005) Validation of intraoperative diagnoses using smear preparations from stereotactic brain biopsies: intraoperative versus final diagnosis–influence of clinical factors. Neurosurgery 56:257–265 discussion 257–265
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
Eyupoglu IY, Buchfelder M, Savaskan NE (2013) Surgical resection of malignant gliomas-role in optimizing patient outcome. Nat Rev Neurol 9:141–151. doi:10.1038/nrneurol.2012.279
Gessler F, Forster MT, Duetzmann S, Mittelbronn M, Hattingen E, Franz K, Seifert V, Senft C (2015) Combination of Intraoperative Magnetic Resonance Imaging and Intraoperative Fluorescence to Enhance the Resection of Contrast Enhancing Gliomas. Neurosurgery 77: 16–22 discussion 22. doi:10.1227/NEU.0000000000000729
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8. doi:10.3171/2011.2.JNS10998
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987–996 doi:10.1056/NEJMoa043330
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198. doi:10.3171/jns.2001.95.2.0190
Ringel F, Pape H, Sabel M, Krex D, Bock HC, Misch M, Weyerbrock A, Westermaier T, Senft C, Schucht P, Meyer B, Simon M, group SNs (2016) Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol 18:96–104. doi:10.1093/neuonc/nov145
Thon N, Eigenbrod S, Grasbon-Frodl EM, Ruiter M, Mehrkens JH, Kreth S, Tonn JC, Kretzschmar HA, Kreth FW (2009) Novel molecular stereotactic biopsy procedures reveal intratumoral homogeneity of loss of heterozygosity of 1p/19q and TP53 mutations in World Health Organization grade II gliomas. J Neuropathol Exp Neurol 68:1219–1228. doi:10.1097/NEN.0b013e3181bee1f1
Kunz M, Thon N, Eigenbrod S, Hartmann C, Egensperger R, Herms J, Geisler J, la Fougere C, Lutz J, Linn J, Kreth S, von Deimling A, Tonn JC, Kretzschmar HA, Popperl G, Kreth FW (2011) Hot spots in dynamic (18) FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol 13:307–316. doi:10.1093/neuonc/noq196
Grasbon-Frodl EM, Kreth FW, Ruiter M, Schnell O, Bise K, Felsberg J, Reifenberger G, Tonn JC, Kretzschmar HA (2007) Intratumoral homogeneity of MGMT promoter hypermethylation as demonstrated in serial stereotactic specimens from anaplastic astrocytomas and glioblastomas. Int J Cancer 121:2458–2464. doi:10.1002/ijc.23020
Eskilsson E, Rosland GV, Talasila KM, Knappskog S, Keunen O, Sottoriva A, Foerster S, Solecki G, Taxt T, Jirik R, Fritah S, Harter PN, Valk K, Al Hossain J, Joseph JV, Jahedi R, Saed HS, Piccirillo SG, Spiteri I, Euskirchen P, Graziani G, Daubon T, Lund-Johansen M, Enger PO, Winkler F, Ritter CA, Niclou SP, Watts C, Bjerkvig R, Miletic H (2016) EGFRvIII mutations can emerge as late and heterogenous events in glioblastoma development and promote angiogenesis through Src activation. Neuro Oncol. doi:10.1093/neuonc/now113
Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN, Rozenblatt-Rosen O, Suva ML, Regev A, Bernstein BE (2014) Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344:1396–1401. doi:10.1126/science.1254257
Sciuscio D, Diserens AC, van Dommelen K, Martinet D, Jones G, Janzer RC, Pollo C, Hamou MF, Kaina B, Stupp R, Levivier M, Hegi ME (2011) Extent and patterns of MGMT promoter methylation in glioblastoma- and respective glioblastoma-derived spheres. Clin Cancer Res 17:255–266. doi:10.1158/1078-0432.CCR-10-1931
Sottoriva A, Kang H, Ma Z, Graham TA, Salomon MP, Zhao J, Marjoram P, Siegmund K, Press MF, Shibata D, Curtis C (2015) A Big Bang model of human colorectal tumor growth. Nat Genet 47:209–216. doi:10.1038/ng.3214
Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C, Tavare S (2013) Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA 110:4009–4014. doi:10.1073/pnas.1219747110
Parkinson JF, Wheeler HR, Clarkson A, McKenzie CA, Biggs MT, Little NS, Cook RJ, Messina M, Robinson BG, McDonald KL (2008) Variation of O(6)-methylguanine-DNA methyltransferase (MGMT) promoter methylation in serial samples in glioblastoma. J Neurooncol 87:71–78. doi:10.1007/s11060-007-9486-0
Parker NR, Hudson AL, Khong P, Parkinson JF, Dwight T, Ikin RJ, Zhu Y, Cheng ZJ, Vafaee F, Chen J, Wheeler HR, Howell VM (2016) Intratumoral heterogeneity identified at the epigenetic, genetic and transcriptional level in glioblastoma. Sci Rep 6:22477. doi:10.1038/srep22477
Smith JS, Quinones-Hinojosa A, Barbaro NM, McDermott MW (2005) Frame-based stereotactic biopsy remains an important diagnostic tool with distinct advantages over frameless stereotactic biopsy. J Neurooncol 73:173–179. doi:10.1007/s11060-004-4208-3
Gessler F, Bruder M, Duetzmann S, Tritt S, Bernstock JD, Seifert V, Senft C (2017) Risk factors governing the development of cerebral vein and dural sinus thrombosis after craniotomy in patients with intracranial tumors. J Neurosurg. doi:10.3171/2016.11.JNS161871
Aker FV, Hakan T, Karadereler S, Erkan M (2005) Accuracy and diagnostic yield of stereotactic biopsy in the diagnosis of brain masses: comparison of results of biopsy and resected surgical specimens. Neuropathology 25:207–213
Shastri-Hurst N, Tsegaye M, Robson DK, Lowe JS, Macarthur DC (2006) Stereotactic brain biopsy: an audit of sampling reliability in a clinical case series. Br J Neurosurg 20:222–226. doi:10.1080/02688690600875507
Weise LM, Bruder M, Eibach S, Seifert V, Byhahn C, Marquardt G, Setzer M (2013) Efficacy and safety of local versus general anesthesia in stereotactic biopsies: a matched-pairs cohort study. J Neurosurg Anesthesiol 25:148–153. doi:10.1097/ANA.0b013e318274ce41
Jain D, Sharma MC, Sarkar C, Deb P, Gupta D, Mahapatra AK (2006) Correlation of diagnostic yield of stereotactic brain biopsy with number of biopsy bits and site of the lesion. Brain Tumor Pathol 23:71–75. doi:10.1007/s10014-006-0204-y
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. doi:10.1007/s00401-016-1545-1
Molenaar RJ, Verbaan D, Lamba S, Zanon C, Jeuken JW, Boots-Sprenger SH, Wesseling P, Hulsebos TJ, Troost D, van Tilborg AA, Leenstra S, Vandertop WP, Bardelli A, van Noorden CJ, Bleeker FE (2014) The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol 16:1263–1273. doi:10.1093/neuonc/nou005
Glantz MJ, Burger PC, Herndon JE 2nd, Friedman AH, Cairncross JG, Vick NA, Schold SC Jr (1991) Influence of the type of surgery on the histologic diagnosis in patients with anaplastic gliomas. Neurology 41:1741–1744
Chandrasoma PT, Smith MM, Apuzzo ML (1989) Stereotactic biopsy in the diagnosis of brain masses: comparison of results of biopsy and resected surgical specimen. Neurosurgery 24:160–165
Feiden W, Steude U, Bise K, Gundisch O (1991) Accuracy of stereotactic brain tumor biopsy: comparison of the histologic findings in biopsy cylinders and resected tumor tissue. Neurosurg Rev 14:51–56
Jackson RJ, Fuller GN, Abi-Said D, Lang FF, Gokaslan ZL, Shi WM, Wildrick DM, Sawaya R (2001) Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol 3:193–200
Hemm S, Rigau V, Chevalier J, Picot MC, Bauchet L, El Fertit H, Rodriguez MA, Cif L, Vayssiere N, Zanca M, Baldet P, Segnarbieux F, Coubes P (2005) Stereotactic coregistration of 201Tl SPECT and MRI applied to brain tumor biopsies. J Nucl Med 46:1151–1157
Hermann EJ, Hattingen E, Krauss JK, Marquardt G, Pilatus U, Franz K, Setzer M, Gasser T, Tews DS, Zanella FE, Seifert V, Lanfermann H (2008) Stereotactic biopsy in gliomas guided by 3-tesla 1H-chemical-shift imaging of choline. Stereotact Funct Neurosurg 86:300–307. doi:10.1159/000155232
Pirotte B, Goldman S, Massager N, David P, Wikler D, Lipszyc M, Salmon I, Brotchi J, Levivier M (2004) Combined use of 18F-fluorodeoxyglucose and 11C-methionine in 45 positron emission tomography-guided stereotactic brain biopsies. J Neurosurg 101:476–483. doi:10.3171/jns.2004.101.3.0476
Wagner M, Nafe R, Jurcoane A, Pilatus U, Franz K, Rieger J, Steinbach JP, Hattingen E (2011) Heterogeneity in malignant gliomas: a magnetic resonance analysis of spatial distribution of metabolite changes and regional blood volume. J Neurooncol 103:663–672. doi:10.1007/s11060-010-0443-y
Clark O, Yen K, Mellinghoff IK (2016) Molecular pathways: isocitrate dehydrogenase mutations in cancer. Clin Cancer Res 22:1837–1842. doi:10.1158/1078-0432.CCR-13-1333
Kim BY, Jiang W, Beiko J, Prabhu SS, DeMonte F, Gilbert MR, Sawaya R, Aldape KD, Cahill DP, McCutcheon IE (2014) Diagnostic discrepancies in malignant astrocytoma due to limited small pathological tumor sample can be overcome by IDH1 testing. J Neurooncol 118:405–412. doi:10.1007/s11060-014-1451-0
Park CK, Kim JE, Kim JY, Song SW, Kim JW, Choi SH, Kim TM, Lee SH, Kim IH, Park SH (2012) The Changes in MGMT Promoter Methylation Status in Initial and Recurrent Glioblastomas. Transl. Oncol 5:393–397
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Rights and permissions
About this article
Cite this article
Gessler, F., Baumgarten, P., Bernstock, J.D. et al. Assessment of molecular markers demonstrates concordance between samples acquired via stereotactic biopsy and open craniotomy in both anaplastic astrocytomas and glioblastomas. J Neurooncol 133, 399–407 (2017). https://doi.org/10.1007/s11060-017-2448-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2448-2