Abstract
Valproic acid (VPA) is an anti-epileptic drug with properties of a histone deacetylase inhibitor (HDACi). HDACi play a key role in epigenetic regulation of gene expression and have been increasingly used as anticancer agents. Recent studies suggest that VPA is associated with improved survival in high-grade gliomas. However, effects on lower grade gliomas have not been examined. This study investigates whether use of VPA correlates with tumor grade, histological progression, progression-free and overall survival (OS) in grade II, III, and IV glioma patients. Data from 359 glioma patients (WHO II–IV) treated with temozolomide plus an antiepileptic drug (VPA or another antiepileptic drug) between January 1997 and June 2013 at the Massachusetts General Hospital was analyzed retrospectively. After confounder adjustment, VPA was associated with a 28 % decrease in hazard of death (p = 0.031) and a 28 % decrease in the hazard of progression or death (p = 0.015) in glioblastoma. Additionally, VPA dose correlated with reduced hazard of death by 7 % (p = 0.002) and reduced hazard of progression or death by 5 % (p < 0.001) with each 100 g increase in total dose. Conversely, in grade II and III gliomas VPA was associated with a 118 % increased risk of tumor progression or death (p = 0.014), and every additional 100 g of VPA raised the hazard of progression or death by 4 %, although not statistically significant (p = 0.064). Moreover, grade II and III glioma patients taking VPA had 2.17 times the risk of histological progression (p = 0.020), although this effect was no longer significant after confounder adjustment. In conclusion, VPA was associated with improved survival in glioblastoma in a dose-dependent manner. However, in grade II and III gliomas, VPA was linked to histological progression and decrease in progression-free survival. Prospective evaluation of VPA treatment for glioma patients is warranted to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas are the most frequent primary malignant central nervous system tumors [1–3]. Standard treatment for GBMs consists of cytoreductive surgery followed by radiation therapy with concurrent chemotherapy with the alkylating agent temozolomide (TMZ), followed by six cycles of adjuvant TMZ [4–7]. Almost all patients diagnosed with malignant gliomas will experience recurrence, and although there are different options for salvage treatment, so far none of these therapies has demonstrated a clear benefit [8]. Recent studies regarding initial therapy of lower grade gliomas favor similar treatment as GBMs in the form of surgery and chemoradiation [9–12]; however, due to the lack of Class 1 evidence the decision and timing for surgery and irradiation as well as the choice of chemotherapeutic drug still remain controversial [2, 9, 11, 13, 14]. Moreover, patient age [15, 16], epigenetic tumor markers (e.g. MGMT DNA methylation status [17]) and genetic characteristics may influence therapy decisions as well as response to treatment. For example, the standard chemotherapeutic for those high-grade anaplastic gliomas characterized by co-deletion of chromosomes 1p/19q, remains procarbazine, lomustine, and vincristine (PCV) and although sometimes replaced by TMZ, a possible beneficial role of TMZ is yet to be further investigated [18, 19].
Therapy for gliomas can be complicated by seizures, which occur in 30–50 % of high-grade and 60–85 % of low-grade cases, requiring treatment with anti-epileptic drugs (AEDs), such as valproic acid (VPA), phenytoin, lamotrigine, topiramate or levetiracetam [20]. The choice of AED, however, is largely discretionary, although non enzyme-inducing AEDs are generally preferred. In addition to its antiepileptic capabilities, VPA has also been shown to be a potent histone deacetylase (HDAC) inhibitor [21–23]. HDAC inhibitors (HDACi) play a key role in epigenetic regulation of gene expression and have been widely studied for their potential role in cancer therapy. VPA, specifically, has been investigated for its anti-proliferative and differentiating effects in vitro and its potential use in multiple cancers [21, 24, 25]. Moreover, data from recent retrospective studies suggest that combining VPA with TMZ is possibly associated with improved survival in patients with GBM [26–30]. Given these promising findings, this study was initiated to further investigate the influence of VPA treatment and dosage on survival and progression in glioma patients. Hereby we were especially interested to identify whether the previously described beneficial effects of VPA in GBM were also relevant in grade II and III gliomas.
Materials and methods
Patients
Patients with intracranial gliomas (WHO grade II–IV) who were treated at the Massachusetts General Hospital (MGH) with surgery and TMZ between January 1997 and June 2013 were identified through an Institutional Review Board–approved (Protocol #: 2013P000736) electronic database from the MGH Brain Tumor Center. Only patients on AEDs were included in the analysis. 360 patients met these criteria, of which one had to be excluded due to an error in pathologic diagnosis, leading to a final number of 359 patients. Two patient cohorts were assembled for analysis (Fig. 1). The first cohort consisted of 224 GBM patients confirmed by histological grading. 74 of these patients had received VPA while the other 150 patients had taken another AED, serving as a control group. The second cohort consisted of 135 non-GBM glioma patients (WHO grade II and III) of whom 46 patients had received VPA upon diagnosis while 89 patients had taken another AED.
Baseline characteristics of patients were assessed and populated a dataset, including histological subtype, age, sex, tumor maximum diameter, tumor volume, isocitrate dehydrogenase 1 (IDH1) mutation status, epidermal growth factor receptor (EGFR) amplification, O6-DNA methylguanine-methyltransferase (MGMT) promoter methylation status, 1p/19q deletion status [31, 32], extent of surgical resection, Karnofsky Performance Status (KPS), and radiation status.
Patients were studied for binary and dose-dependent influences of VPA on overall survival (OS), progression-free survival (PFS), and time to histological grade progression (TTP). Due to the nature of a retrospective study and the setup of the in-house electronic database, data regarding length of TMZ and VPA treatment as well as compliance could not be directly assessed.
OS, PFS and TTP data were evaluated in months from the time of initial histologically confirmed diagnosis. Hereby, OS is defined as the time from diagnosis to death and PFS is defined as the time from diagnosis to radiographic evidence of recurrence or death. TTP is pertinent only to the analysis of grade II and III gliomas and is here defined as the time to histological evidence of advancement in WHO-grading.
Statistical methods
Patients’ characteristics in the treatment group (VPA) and control group (other AED) were compared using t-tests and Wilcoxon rank sum tests for normally and non-normally distributed continuous variables, respectively. Fisher’s exact tests were used for class variables. Patient survival was last assessed in April 2014. Extended Cox models were fit to model the effects of VPA on OS and PFS with an interaction effect of WHO grade (IV vs. II–III), accommodating time-dependent progression from grade II/III glioma to GBM. VPA was represented both as VPA status (given or not) and as VPA dosage levels (total cumulative VPA dose per patient). There was no minimal length of VPA-treatment. Additional models for OS and PFS were fit, controlling for known prognostic factors if they were found to significantly differ by VPA status in this population. Confounders of interest included tumor location, mutation status, tumor volume at presentation, treatment course, surgical resectability, and KPS. The effect of VPA on TTP in the baseline grade II/III glioma population was analyzed using both univariate and multivariate Cox proportional hazards models, the multivariate model controlling for the same above prognostic factors.
Results
From 1997 to 2013 a total of 359 patients with grade II–IV gliomas were included in our study. 224 of these patients had primary GBM, while 135 had grade II/III gliomas, both diagnosed based on histological grading of surgical tissue. The median total dose of VPA per patient was 90 g for GBM patients (min 0.49 g; max 1825 g) and 710.63 g for grade II/III patients (min 10.5 g; max 4106.25 g). The difference in total applied median doses between the two groups is hereby likely a result of the longer median survival and consequently longer VPA treatment of grade II/III patients in comparison to GBM patients.
Of the 224 GBM patients, 221 received adjuvant radiation therapy and all 224 received TMZ. In addition to adjuvant chemoradiation, 74 GBM patients received VPA, while 150 patients were treated with another AED. In the grade II/III glioma group 110 of 135 patients received adjuvant radiation therapy and all 135 patients were medicated with TMZ. In this group 46 patients were treated with VPA, while 89 patients received another AED.
Supplementary Table 1 presents aggregate patient characteristics of the treatment (+VPA) and control (−VPA) groups for both GBM and grade II/III glioma combined. There were no significant differences by VPA status in regard to gender, age, primary pathology, tumor diameter, mutation status, gross total resection and chemoradiation treatment rates (p ≥ 0.05). However, patients treated with VPA had significantly higher rates of subtotal and multiple resections.
Within the grade II/III glioma group (see Table 1), VPA-treated patients again had significantly worse resection rates (higher rates of subtotal and multiple resections and lower rates of gross total resections) but no other significant differences from −VPA patients. In the GBM-only patient group, +VPA patients had lower rates of biopsy compared to −VPA patients (see Table 2).
Overall survival (OS)
At the end of follow up, only 35 of the initial 224 GBM patients were still alive and 4 had been lost to follow-up. Among the 135 patients with grade II/III gliomas, 69 were still alive at the end of data collection and 6 were lost to follow-up. Median OS was 22 months for +VPA and 14 for −VPA patients with GBM. The median OS in the grade II/III group was 109 months for +VPA and 127 months for −VPA patients.
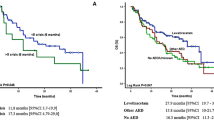
In an extended Cox model, treatment with VPA was associated with a 35 % decrease in the hazard of death in GBM patients (p = 0.004). Conversely, no difference in OS was seen in grade II/III glioma patients (p = 0.335; see Table 3). Even after adjusting for biopsy status, extent of resection and multiple resections with an extended Cox model, use of VPA was still associated with a 28 % decrease in the hazard of death in GBM patients (p = 0.031; see Table 4). Applying the same statistical adjustments to the grade II/III glioma group, we were unable to detect a significant difference in OS (p = 0.280; Table 4). Kaplan–Meier survival curves of +VPA versus −VPA patients of both groups, GBM and grade II/III diagnoses at baseline, are presented in Fig. 2, supporting the notion that VPA use is associated with an OS benefit in GBM but not in grade II/III patients.
Overall survival and progression-free survival of baseline GBM and baseline grade II/III patients receiving VPA or other AED. Kaplan–Meier Survival estimates. a GBM patients who received VPA had a significant benefit in overall survival compared to GBM patients who received other AEDs (no VPA). b VPA treatment did not have any significant influence on overall survival of grade II/III patients. c GBM patients receiving VPA showed benefit in progression-free survival compared to GBM patients who received other AEDs (no VPA). d VPA treatment significantly shortened progression-free survival for grade II/III patients
An association of VPA dose and extent of OS is summarized in Supplementary Table 2. In GBM patients, each 100 g increase of VPA total dose significantly decreased one’s hazard of death by 7 % in the adjusted model (p = 0.002). There was no significant dose-relationship between VPA and OS in grade II/III glioma patients (p = 0.434).
Progression-free survival (PFS)
Among the GBM patients, median PFS was 11 months in the +VPA and 9 months in the –VPA group. For grade II/III patients, median PFS was 44 months and 117 months in the +VPA and –VPA groups, respectively.
In the unadjusted model, VPA was associated with a 33 % decrease in the hazard of progression or death (p = 0.002) among GBM patients. Conversely, among grade II/III glioma patients, taking VPA was associated with a 150 % increase in hazard of progression or death (p = 0.004) (see Table 3). After controlling for the same factors as above, VPA continued to share a beneficial association with PFS among GBM patients (hazard ratio = 0.72; p = 0.015), and was still associated with a 118 % increase in the hazard of progression in grade II/III glioma patients (p = 0.014; see Table 4). Figure 2 shows a Kaplan–Meier estimate of the PFS of baseline GBM patients versus baseline grade II/III glioma patients with and without VPA. Grade II/III glioma patients who received VPA had a faster time to radiographic progression or death as compared to those who did not take VPA. The opposite effect was seen amongst GBM patients.
The dose-response relationship between VPA and PFS is highlighted in Supplementary Table 2. In the adjusted model, every 100 g increase of VPA decreased one’s hazard of death or progression by 5 % among the GBM patients (p < 0.001). Conversely, every additional 100 g of VPA dose in grade II/III glioma patients’ was associated with a 4 % increase in hazard of progression or death in the adjusted model, although not significant after adjusting for confounders (p = 0.064).
Time to histological grade progression (TTP)
Among the 135 grade II/III glioma patients, 39 (29 %) progressed during follow up. Taking VPA was associated with a 117 % increase in one’s hazard of histological progression to a higher grade (p = 0.020), although this effect was no longer significant after controlling for biopsy status, extent of resection and multiple resections (p = 0.226).
Discussion
This study focused on examining the effects of VPA on GBM (grade IV) and grade II/III gliomas treated with surgery, radiation and TMZ. Plus-minus effects and dose-dependent influences of VPA on OS and PFS were evaluated. Furthermore, for grade II/III glioma patients we analyzed the time to histological grade progression (TTP).
In recent years, a number of studies have indicated that high-grade glioma patients treated for seizures showed a modest OS benefit when receiving VPA, instead of another antiepileptic drug, in addition to standard therapy with resection and chemoradiation [27, 29, 30]. Findings from our retrospective analysis are consistent with these reports. However, surprisingly, the VPA associated survival benefit could not be detected in patients presenting with grade II/III gliomas. Notably, VPA was associated with a decrease in PFS and promoted malignant transformation in this patient group, although the latter association was not robust to confounder adjustment.
Effect of VPA on overall survival (OS)
After adjusting for biopsy, subtotal resection, gross total resection and multiple resections, VPA was associated with a 28 % decrease in hazard of death in patients with GBM (p = 0.031). This result supports previous findings of a beneficial use of VPA in GBM patients receiving TMZ. Interestingly, every 100 g increase in VPA dose was associated with a decrease in the hazard of death by 7 % in GBM patients (p = 0.002). A dose dependent effect of VPA on OS has been suggested [29]. Kerkhof et al [29] noted a smaller survival benefit possibly secondary to smaller VPA doses in their GBM patients when compared to the study by Weller et al [30]. Findings from our study support this hypothesis in GBM patients.
As discussed in a number of studies, a possible mechanism by which VPA is associated with improved survival of cancer patients, is dependent on its HDAC inhibitory potential [33, 34]. Suppression of histone deacetylase activity leads to an increased acetylation of histones, which ultimately promotes a more open chromatin configuration. This in turn, has been hypothesized to cause overexpression of tumor suppressor genes that promote growth arrest, differentiation and apoptosis [21, 22, 34]. Moreover, VPA and other HDACi have been shown to enhance the in vitro and in vivo response to irradiation of various cancers [35–38], including gliomas [39, 40]. Interestingly, the initial concern about VPA`s demethylation effect on DNA [41], which was hypothesized to possibly induce MGMT protein and thereby antagonize TMZ treatment, has not substantialized. In fact, a recent in vitro study by Van Nifterik et al [42], as well as our own and the above mentioned clinical studies on GBM patient survival, support a synergistic effect of combined treatment of VPA, TMZ and irradiation in the setting of glioblastoma.
Effect of VPA on progression-free survival (PFS) and time to histological progression (TTP)
VPA was associated with a 28 % decrease in hazard of progression or death in GBM patients (p = 0.015) and a 118 % increase in hazard of progression or death in grade II/III patients (p = 0.014; see Table 4) after adjusting for confounding factors. In contrast to GBM patients for whom VPA had a beneficial dose-response relationship with PFS (p < 0.001; hazard ratio 0.95), incremental VPA doses were associated with a borderline significant 4 % increase in hazard of progression in this patient group (p = 0.064, see Supplementary Table 2).
The beneficial effect of VPA on PFS in GBM patients has previously been suggested by Kerkhof et al, who in their study recorded borderline significant improvement of PFS in GBM patients treated with a minimum of 3 months coexposure of VPA and TMZ (p = 0.06) [29]. However, the significant (p < 0.001) dose dependence of this effect shown in our study has not previously been reported.
When examining TTP, grade II/III patients who took VPA had 2.17 times the hazard of progression to a higher grade, compared to those who did not take VPA (p = 0.020), although the association did not hold after adjusting for confounding factors. Despite these findings, we found that OS for patients with grade II and grade III gliomas was not significantly different when comparing +VPA and −VPA patients of this group (p = 0.280; see Table 4). The similar OS, despite earlier progression, may be explained by the significant PFS and OS benefit of VPA for GBM patients. This observation, where a specific treatment is associated with tumor progression without affecting OS, has been seen in previous studies of radiotherapy for low-grade glioma (LGG), where early radiotherapy compared to delayed treatment prolonged PFS but did not influence OS [14]. In both cases, the discrepancy between a positive or negative effect of treatment on PFS or TTP in contrast to unchanged OS may be explained by differing influences of treatment during different disease stages. In more detail, a benefit of treatment during early disease, might mechanistically lead to earlier clonal selection of a more malignant/resistant phenotype, which in turn decreases PFS once a patient enters late stage disease. In the above mentioned example of early irradiation in LGG, the increase in PFS and unchanged OS might additionally be explained by reaching the maximal irradiation dose at an earlier time point, leaving less effective treatment options (e.g. salvage radiotherapy) for high grade disease. Conversely, as seen in our study, a shortened PFS during lower grade disease stage does not necessarily have to lead to a decrease in OS, as the positive effect of continued treatment during higher-grade disease might balance out the negative effects that this treatment inflicts during early stage disease.
Possible mechanisms of VPA induced progression and malignant transformation
Our findings that VPA treatment of grade II/III patients was associated with shortened PFS and possibly shortened TTP have not been described previously. A possible mechanism explaining these results, may be related to VPA`s HDACi properties. HDACi, such as VPA, have been shown to inhibit expression of the CDK2NA gene [43]. CDK2NA encodes tumor suppressor protein p16 as well as its alternate reading frame, the tumor suppressor protein p14 (p14ARF). Recent studies were able to demonstrate that both, a loss of the CDK2NA locus [44], as well as hypermethylation of p14ARF led to malignant progression of LGGs [45]. The majority of tumors with p14ARF hypermethylation exhibited homozygous co-deletions of p14ARF, p15 (INK4b) and p16 (INK4a). Therefore, VPA-mediated down regulation of CDK2NA could be a possible explanation for the earlier progression/malignant transformation seen in our VPA treated grade II/III glioma group. Additionally, VPA has been reported to induce proteasomal degradation of HDAC II [46]. Expression of HDAC II and IV have been found to be down regulated in high grade gliomas [47] and as a result VPA-associated decrease of HDAC II activity in LGGs may possibly favor malignant transformation into GBM. Furthermore, a recently published preclinical study by Santoro et al [48] on acute promyelocytic leukemia was able to demonstrate that both, HDAC I and, to a lesser degree, HDAC II acted as tumor suppressors during tumor initiation (preleukemic phase), but had oncogenic properties during tumor maintenance (full leukemic stage). In their model, treating preleukemic mice with VPA led to markedly accelerated leukemogenesis, in contrast to the increased survival seen when treatment was started during the full leukemic stage. Interestingly accelerated progression was not seen after treatment of preleukemic Eμ-myc mice, possibly suggesting a tumor-type or oncogene specific effect. In any case, our study is the first to demonstrate the contrary effect of HDACi treatment during early and late stage disease in a clinical setting. Further ongoing investigations will be needed to elucidate possible mechanisms explaining earlier progression and possible malignant transformation of Grade II/III gliomas after VPA treatment. In the meantime we recommend that VPA or other HDACi medication should be thoroughly considered during Grade II/III glioma therapy and avoided if possible.
Study limitations
This study has several limitations to be considered. Due to the nature of a retrospective study and the setup of the in-house electronic database, data regarding length of VPA treatment could not be directly assessed. This is an important shortcoming, since the length of VPA therapy has previously been shown to significantly correlate with OS in high grade gliomas [29]. However, it has to be considered that our electronic database did instead allow for assessment of total VPA dose per patient, which likely correlates well with length of VPA treatment and may in fact be a much better parameter to correlate treatment with disease progression. Optimally, one would also assess length and dose of TMZ treatment as well as serum VPA levels, but unfortunately this data was not available. Especially the lack of information on length of VPA treatment has to be considered when interpreting binary statistics presented in this study.
Another limitation of this study is the imbalance of documented information regarding prognostic molecular markers between the +VPA (documented for 35 % of patients) and –VPA (documented for 57 %) groups in the Grade II/III patient population. Considering the importance of IDH1/2 mutation status on prognosis, this might possibly have led to confounding statistical outcomes.
Conclusions
Our data supports previous findings in which VPA use was associated with improved survival and additionally showed significant improvement of PFS in GBM. Moreover, we demonstrate for the first time that the VPA dose positively correlates with a benefit in survival in this patient group. Surprisingly, in Grade II/III gliomas VPA was associated with a decrease in PFS and more rapid malignant progression, though the latter may be attributed to other factors including extent of resection, number of resections and biopsy status. Despite the limitations of a retrospective analysis, this is a novel finding, which might impact patient management, keeping in mind though that ultimately efficacy on seizures and tolerability remain the most important factors in the decision process regarding the choice of antiepileptic drug. Our findings suggest that further prospective studies are warranted to validate a possible differential effect of VPA in low grade and high-grade glioma patients. Future, prospective studies should optimally also include data on length of VPA treatment as well as dose and length of TMZ therapy.
References
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J (2014) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol 16(Suppl 4):63. doi:10.1093/neuonc/nou223
Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre J-YY (2012) Primary brain tumours in adults. Lancet 379:1984–1996. doi:10.1016/S0140-6736(11)61346-9
Rigau V, Zouaoui S, Mathieu-Daudé H, Darlix A, Maran A, Trétarre B, Bessaoud F, Bauchet F, Attaoua R, Fabbro-Peray P, Fabbro M, Kerr C, Taillandier L, Duffau H, Figarella-Branger D, Costes V, Bauchet L, Société Française de Neuropathologie SFdN, Club de Neuro-Oncologie of the Société Française de N, Association des Neuro-Oncologues d’Expression Française (ANOCEF) (2011) French brain tumor database: 5-year histological results on 25 756 cases. Brain Pathol 21(6):633–644. doi:10.1111/j.1750-3639.2011.00491.x
Hardesty DA, Sanai N (2011) The value of glioma extent of resection in the modern neurosurgical era. Front Neurol 3:140. doi:10.3389/fneur.2012.00140
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95:190–198. doi:10.3171/jns.2001.95.2.0190
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff R-OO, for, of, Groups E, of Group N (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. doi:10.1016/S1470-2045(09)70025-7
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine 352:987–996. doi:10.1056/NEJMoa043330
Price RL, Chiocca EA (2014) Evolution of malignant glioma treatment: from chemotherapy to vaccines to viruses. Neurosurgery 61(Suppl 1):74–83. doi:10.1227/NEU.0000000000000390
Kumthekar P, Raizer J, Singh S (2015) Low-grade glioma. Cancer Treat Res 163:75–87. doi:10.1007/978-3-319-12048-5_5
Pouratian N, Schiff D (2010) Management of low-grade glioma. Curr Neurol Neurosci Rep 10:224–231. doi:10.1007/s11910-010-0105-7
Tandon A, Schiff D (2014) Therapeutic decision making in patients with newly diagnosed low grade glioma. Curr Treat Options Oncol 15:529–538. doi:10.1007/s11864-014-0304-6
Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O’Neill AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA (1999) Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol 17:2762–2771
Klein M (2009) Health-related quality of life aspects in patients with low-grade glioma. Adv Tech Stand Neurosurg 35:213–235
van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, Malmström POO, Collette L, Piérart M, Mirimanoff R, Karim AB, Radiotherapy E, Brain Tumor G, The UKMRC (2004) Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 366:985–990. doi:10.1016/S0140-6736(05)67070-5
Malmström A, Grønberg BHH, Marosi C, Stupp R, Frappaz D, Schultz H, Abacioglu U, Tavelin B, Lhermitte B, Hegi ME, Rosell J, Henriksson R, Nordic (2012) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 13:916–926. doi:10.1016/S1470-2045(12)70265-6
Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, Papsdorf K, Steinbach JP, Sabel M, Combs SE, Vesper J, Braun C, Meixensberger J, Ketter R, Mayer-Steinacker R, Reifenberger G, Weller M, NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society (2012) Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. The Lancet Oncology 13:707–715. doi:10.1016/S1470-2045(12)70164-X
Hegi ME, Diserens A-CC, Gorlia T, Hamou M-FF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff ROO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. doi:10.1056/NEJMoa043331
Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, Souhami L, Laperriere N, Curran W, Mehta M (2013) Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 31:337–343. doi:10.1200/JCO.2012.43.2674
van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre J-YY, Bernsen HJJ, Frenay M, Tijssen CC, Grisold W, Sipos L, Enting RH, French PJ, Dinjens WN, Vecht CJ, Allgeier A, Lacombe D, Gorlia T, Hoang-Xuan K (2013) Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 31:344–350. doi:10.1200/JCO.2012.43.2229
van Breemen MS, Wilms EB, Vecht CJ (2007) Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 6:421–430. doi:10.1016/S1474-4422(07)70103-5
Göttlicher M, Minucci S, Zhu P, Krämer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T (2001) Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 20:6969–6978. doi:10.1093/emboj/20.24.6969
Gurvich N, Tsygankova OM, Meinkoth JL, Klein PS (2004) Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res 64:1079–1086
Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 276:36734–36741. doi:10.1074/jbc.M101287200
Acharya MR, Sparreboom A, Venitz J, Figg WD (2005) Rational development of histone deacetylase inhibitors as anticancer agents: a review. Mol Pharmacol 68:917–932. doi:10.1124/mol.105.014167
Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E, Herrera LA (2008) Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev 34:206–222. doi:10.1016/j.ctrv.2007.11.003
Barker CA, Bishop AJ, Chang M, Beal K, Chan TA (2013) Valproic acid use during radiation therapy for glioblastoma associated with improved survival. Int J Radiat Oncol Biol Phys 86:504–509. doi:10.1016/j.ijrobp.2013.02.012
Felix FH, Trompieri NM, de Araujo OL, da Trindade KM, Fontenele JB (2011) Potential role for valproate in the treatment of high–risk brain tumors of childhood-results from a retrospective observational cohort study. Pediatr Hematol Oncol 28:556–570. doi:10.3109/08880018.2011.563774
Guthrie GD, Eljamel S (2013) Impact of particular antiepileptic drugs on the survival of patients with glioblastoma multiforme. J Neurosurg 118:859–865. doi:10.3171/2012.10.JNS12169
Kerkhof M, Dielemans JC, van Breemen MS, Zwinkels H, Walchenbach R, Taphoorn MJ, Vecht CJ (2013) Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro Oncol 15:961–967. doi:10.1093/neuonc/not057
Weller M, Gorlia T, Cairncross JG, van den Bent MJ, Mason W, Belanger K, Brandes AA, Bogdahn U, Macdonald DR, Forsyth P, Rossetti AO, Lacombe D, Mirimanoff ROO, Vecht CJ, Stupp R (2011) Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology 77:1156–1164. doi:10.1212/WNL.0b013e31822f02e1
Bromberg JE, van den Bent MJ (2009) Oligodendrogliomas: molecular biology and treatment. Oncologist 14:155–163. doi:10.1634/theoncologist.2008-0248
Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, Silver JS, Stark PC, Macdonald DR, Ino Y, Ramsay DA, Louis DN (1998) Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 90:1473–1479
Hess-Stumpp H (2005) Histone deacetylase inhibitors and cancer: from cell biology to the clinic. Eur J Cell Biol 84:109–121. doi:10.1016/j.ejcb.2004.12.010
Walkinshaw DR, Yang XJ (2008) Histone deacetylase inhibitors as novel anticancer therapeutics. Curr Oncol 15:237–243
Blattmann C, Oertel S, Ehemann V, Thiemann M, Huber PE, Bischof M, Witt O, Deubzer HE, Kulozik AE, Debus J, Weber K-JJ (2010) Enhancement of radiation response in osteosarcoma and rhabdomyosarcoma cell lines by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys 78:237–245. doi:10.1016/j.ijrobp.2010.03.010
Camphausen K, Scott T, Sproull M, Tofilon PJ (2004) Enhancement of xenograft tumor radiosensitivity by the histone deacetylase inhibitor MS-275 and correlation with histone hyperacetylation. Clin Cancer Res 10:6066–6071. doi:10.1158/1078-0432.CCR-04-0537
Chinnaiyan P, Vallabhaneni G, Armstrong E, Huang S-MM, Harari PM (2005) Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys 62:223–229. doi:10.1016/j.ijrobp.2004.12.088
Entin-Meer M, Yang X, VandenBerg SR, Lamborn KR, Nudelman A, Rephaeli A, Haas-Kogan DA (2007) In vivo efficacy of a novel histone deacetylase inhibitor in combination with radiation for the treatment of gliomas. Neuro Oncol 9:82–88. doi:10.1215/15228517-2006-032
Camphausen K, Cerna D, Scott T, Sproull M, Burgan WE, Cerra MA, Fine H, Tofilon PJ (2005) Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int J Cancer 114:380–386. doi:10.1002/ijc.20774
Chinnaiyan P, Cerna D, Burgan WE, Beam K, Williams ES, Camphausen K, Tofilon PJ (2008) Postradiation sensitization of the histone deacetylase inhibitor valproic acid. Clin Cancer Res 14:5410–5415. doi:10.1158/1078-0432.CCR-08-0643
Detich N, Bovenzi V, Szyf M (2003) Valproate induces replication-independent active DNA demethylation. J Biol Chem 278:27586–27592. doi:10.1074/jbc.M303740200
Van Nifterik KA, Van den Berg J, Slotman BJ, Lafleur MV, Sminia P, Stalpers LJ (2012) Valproic acid sensitizes human glioma cells for temozolomide and γ-radiation. J Neurooncol 107:61–67. doi:10.1007/s11060-011-0725-z
Matheu A, Klatt P, Serrano M (2005) Regulation of the INK4a/ARF locus by histone deacetylase inhibitors. J Biol Chem 280:42433–42441
Idbaih A, Carvalho Silva R, Crinière E, Marie Y, Carpentier C, Boisselier B, Taillibert S, Rousseau A, Mokhtari K, Ducray F, Thillet J, Sanson M, Hoang-Xuan K, Delattre J-YY (2008) Genomic changes in progression of low-grade gliomas. J Neurooncol 90:133–140. doi:10.1007/s11060-008-9644-z
Watanabe T, Katayama Y, Yoshino A, Yachi K, Ohta T, Ogino A, Komine C, Fukushima T (2007) Aberrant hypermethylation of p14ARF and O6-methylguanine-DNA methyltransferase genes in astrocytoma progression. Brain Pathol 17:5–10
Kramer OH, Zhu P, Ostendorff HP, Golebiewski M, Tiefenbach J, Peters MA, Brill B, Groner B, Bach I, Heinzel T, Gottlicher M (2003) The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J 22:3411–3420
Lucio-Eterovic AK, Cortez MAA, Valera ET, Motta FJ, Queiroz RG, Machado HR, Carlotti CG, Neder L, Scrideli CA, Tone LG (2007) Differential expression of 12 histone deacetylase (HDAC) genes in astrocytomas and normal brain tissue: class II and IV are hypoexpressed in glioblastomas. BMC Cancer 8:243. doi:10.1186/1471-2407-8-243
Santoro F, Botrugno OA, Dal Zuffo R, Pallavicini I, Matthews GM, Cluse L, Barozzi I, Senese S, Fornasari L, Moretti S, Altucci L, Pelicci PG, Chiocca S, Johnstone RW, Minucci S (2013) A dual role for Hdac1: oncosuppressor in tumorigenesis, oncogene in tumor maintenance. Blood 121:3459–3468. doi:10.1182/blood-2012-10-461988
Acknowledgments
The authors confirm the originality of this work. The work was not submitted for publication to another journal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no financial or other conflict of interest in relation to this research and its publication.
Additional information
Navid Redjal and Clemens Reinshagen have Contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Redjal, N., Reinshagen, C., Le, A. et al. Valproic acid, compared to other antiepileptic drugs, is associated with improved overall and progression-free survival in glioblastoma but worse outcome in grade II/III gliomas treated with temozolomide. J Neurooncol 127, 505–514 (2016). https://doi.org/10.1007/s11060-016-2054-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2054-8