Abstract
Although bevacizumab has not proven effective in adults with newly diagnosed high-grade gliomas (HGG), feasibility in newly diagnosed children with diffuse intrinsic pontine gliomas (DIPG) or HGG has not been reported in a prospective study. In a safety and feasibility study, children and young adults with newly diagnosed HGG received radiotherapy (RT) with bevacizumab (10 mg/kg: days 22, 36) and temozolomide (75–90 mg/m2/day for 42 days) followed by bevacizumab (10 mg/kg, days 1, 15), irinotecan (125 mg/m2, days 1, 15) and temozolomide (150 mg/m2/day days 1–5). DIPG patients did not receive temozolomide. Telomerase activity, quality of life (QOL), and functional outcomes were assessed. Among 27 eligible patients (15 DIPG, 12 HGG), median age 10 years (range 3–29 years), 6 discontinued therapy for toxicity: 2 during RT (grade 4 thrombocytopenia, grade 3 hepatotoxicity) and 4 during maintenance therapy (grade 3: thrombosis, hypertension, skin ulceration, and wound dehiscence). Commonest ≥grade 3 toxicities included lymphopenia, neutropenia and leukopenia. Grade 3 hypertension occurred in 2 patients. No intracranial hemorrhages occurred. For DIPG patients, median overall survival (OS) was 10.4 months. For HGG patients, 3-year progression free survival and OS were 33 % (SE ± 14 %) and 50 % (SE ± 14 %), respectively. All 3 tested tumor samples, demonstrated histone H3.3K27M (n = 2 DIPG) or G34R (n = 1 HGG) mutations. QOL scores improved over the course of therapy. A bevacizumab-based regimen is feasible and tolerable in newly diagnosed children and young adults with HGG and DIPG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite aggressive multi-modal therapy, prognosis for children with high-grade gliomas (HGG) and diffuse intrinsic pontine gliomas (DIPG) remains poor [1, 2]. While radiotherapy (RT) prolongs survival in HGG [3] and DIPG [4] patients, adjuvant chemotherapy has had little impact on survival [5–8], highlighting the need for novel therapeutic approaches.
Bevacizumab (BVZ; Avastin; Genentech, San Francisco, CA) is a VEGF-specific recombinant, humanized monoclonal antibody that binds to and inhibits vascular endothelial growth factor (VEGF) from binding to its receptors. Endothelial proliferation is a hallmark of glioblastoma multiforme (GBM) [9, 10]. Gorski et al. demonstrated that irradiation of tumor cells induced increased VEGF expression [11]. Pre-treatment of U87 GBM xenografts with anti-VEGF therapy, had a greater than additive effect when combined with subsequent radiation [11]. Using the same model, Lee et al. demonstrated that anti-VEGF mAB treatment with radiation yielded greater than additive tumor growth delay [12]. Furthermore, elevated expression of VEGF and its receptors, VEGFR-1 and VEGFR-2, reported in GBM [13, 14], have been shown to correlate with a worse prognosis [15].
BVZ received accelerated FDA approval for second-line treatment of adult GBM in 2009, based on durable objective responses in phase II trials. Friedman et al. [16] reported a 6-month progression-free survival (PFS) of 42.6 %, and a median overall survival (OS) of 9.2 months in recurrent HGG patients receiving BVZ. Kreisl et al. reported a 6-month PFS of 29 % in recurrent GBM patients who received BVZ plus irinotecan (IRO) [17]. Despite these promising results, randomized phase III trials of BVZ in newly diagnosed adults with GBM have recently failed to demonstrate a survival advantage [18, 19].
Although BVZ was well tolerated at doses of 5, 10 or 15 mg/kg every 2 weeks in children with recurrent solid tumors [20], phase II studies of BVZ plus IRO in children with recurrent HGG or DIPG, showed little efficacy [21].
To date, results of prospective pediatric studies using BVZ in newly diagnosed HGG and DIPG patients have not been published. This study sought to determine the feasibility of BVZ with concurrent RT and temozolomide (TEM) followed by BVZ, IRO ± TEM in patients with newly diagnosed HGG or DIPG.
Methods
Study design
The study’s primary objectives were to determine the toxicities and feasibility of the proposed treatment regimen. Secondary objectives were to: determine the one-year event-free-survival (EFS), median PFS and OS, document changes in magnetic resonance (MR) perfusion and diffusion after BVZ during RT, correlate functional changes in tumor with response to treatment using MR diffusion/perfusion imaging, assess telomerase activity, hTERT and hTERC expression, and telomere length in HGG, and assess the health-related quality-of-life and functional abilities of patients during and following treatment.
Eligibility criteria
Patients >36 months and ≤30 years with newly-diagnosed HGG or DIPG and a performance score ≥50, were eligible. HGG patients required histologic verification of a GBM, anaplastic astrocytoma or gliosarcoma by central review (LM). Biopsy was not required for patients with DIPG. The imaging diagnosis of DIPG required that the tumor be centered in, and involve, at least 2/3 of the pons with diffuse involvement [22, 23]. Patients with NF associated brainstem enlargement were not eligible. All patients had to have adequate bone marrow, liver, and renal function, an INR and aPTT <2× upper limit of normal and a fibrinogen <0.75× lower limit of normal. Patients with controlled seizures on non-enzyme inducing anticonvulsants were eligible.
The institutional review boards of participating institutions approved the protocol; continuing approval was maintained throughout the study. Patients or their legal guardians gave written informed consent, and assent was obtained as appropriate at the time of enrollment.
Patients were excluded if pregnant, had metastatic disease, a new intracranial hemorrhage on MRI within 14 days prior to enrollment, chronic non-healing wounds, a history of a deep venous thrombosis, a known thrombophilic condition, or arterial thromboembolic events within 1 year prior to enrollment. Patients on systemic anticoagulants, non-steroidal anti-inflammatory medications, potent CYP3A4 inducers/inhibitors, anti-hypertensives or medications known to inhibit platelet function were excluded.
Treatment plan
This pilot study was conducted at Cincinnati Children’s Hospital Medical Center and Ann and Robert H Lurie Children’s Hospital. HGG patients (stratum 1), started RT within 30 days of surgery and TEM by day 5 of RT. For planning target volumes (PTV) <200 cc, 59.4 Gray (Gy) was delivered in 1.8 Gy daily fractions. For PTV >200 cc, 50.4 Gy was delivered in 1.8 Gy daily fractions to the initial PTV followed immediately by an additional 9.0 Gy in 1.8 daily fractions to the PTVboost. Patients with HGG received RT with concomitant TEM at 90 mg/m2/dose daily (75 mg/m2/day PO daily × 42 days for patients ≥ 19 years of age) [24] for a total of 42 days and BVZ (10 mg/kg) on days 22 and 36 of RT to prevent surgical complications post-surgical resection or biopsy. In DIPG patients (stratum 2), the PTV received 54.0 in 1.8 Gy daily fractions with no boost. Concomitant BVZ (10 mg/kg) was administered on days 1, 15, 29, and 43 of RT.
Maintenance chemotherapy began 4 weeks after RT completion. Each course was 28 days with 12 planned courses. HGG patients (stratum 1) received TEM at 150 mg/m2/day PO on days 1–5, BVZ at 10 mg/kg and IRO, 125 mg/m2/day IV, on days 1 and 15 of each 28-day course. DIPG patients (stratum 2) received the same maintenance chemotherapy without TEM.
Toxicity and dose modifications
Dose modifying toxicities (DMT) were defined as any of the following events at least possibly attributable to therapy: grade 3 or 4 non-hematological toxicity, (except grade 3 nausea and vomiting <5 days, grade 3 transaminase elevation that returned to baseline within 7 days of therapy interruption and did not recur upon re-challenge, grade 3 fever or infection of <5 days, grade 3 electrolyte abnormality which resolves to ≤grade 2 ± within 7 days of interrupting therapy, grade 3 hypertension well-controlled with oral medication prior to next BVZ course, or grade 3 diarrhea responsive to anti-diarrheals), any toxicities that required holding BVZ for >7 days, grade 4 neutropenia for >7 days, or grade 4 thrombocytopenia on two separate days over 7 days, grade 3 thrombocytopenia that required transfusion on >two occasions either during RT or over 7 days.
Statistical methods
The primary objective of the study was to assess the regimen’s safety and feasibility. The stopping rules for the primary feasibility endpoints were: the incidence of >2 week delay in (a) the completion of RT or removal from protocol therapy during RT or (b) >2 two week delay in the start of any course of maintenance or removal from therapy during maintenance therapy after appropriate dose modifications. The study regimen would not be considered feasible if 5 or more significant delays/removals were observed during RT or 5 or more significant delays/removals were observed during maintenance therapy among the target number of 35 patients (20 HGG and 15 DIPGs) estimated to be accrued at the two institutions over a 40–48 month period.
Median and frequencies were used to describe continuous and categorical variables, respectively. PFS and OS curves and estimates were computed using the Kaplan–Meier method. All computations were done in R version 3.1.3.
Response criteria
MRIs were obtained prior to enrollment, within 72 h of surgery, prior to starting maintenance therapy, and every 8 weeks thereafter. Tumor response was defined on non-contrast MRI sequences as: complete response (CR), disappearance of all target lesions; partial response (PR), ≥50 % decrease in tumor size by bi-dimensional measurement compared to baseline; stable disease (SD), MRI response not meeting the criteria for other categories, with stable neurological examination; progressive disease (PD) >25 % increase in the bi-dimensional measurement, or appearance of new lesions. Furthermore, if MRI showed >25 and <50 % increase in size in the first 3 months after completion of RT, patients were not considered to have PD.
Correlative studies
All correlative studies were optional. Telomerase activity, hTERT, hTERC expression, ALT, H3.3 mutation and telomere length in tumor and blood specimens were obtained prior to therapy. Tumor samples were analyzed for hTERT and hTERC subunits expression using quantitative real-time PCR. Telomerase enzyme activity was assessed using the TRAP assay. Telomere length was assessed by Southern blot. H3.3 mutations were determined by Sanger sequencing on isolated genomic DNA from tumor samples. To determine ALT status, one µg of genomic DNA was digested by Rsa1 and Hinf1 and separated by gel electrophoresis. Telomeres were detected using telomere specific probes by Southern blot (TeloTAGGG, Roche). Heterogeneous lengths, including large telomeres of >20 kb, were considered consistent with ALT on TRF analysis.
Functional outcomes were assessed using the Bruininks–Oseretsky Test of Motor Proficiency, 2nd Edition (BOT-2) and the Functional Rehabilitation Evaluation of Sensori-Neurologic Outcomes (FRESNO), prior to therapy, within 2 weeks of completing RT, and at completion of therapy. The FRESNO measures functional performance in self-care, motor, communication, cognition and socialization. The BOT-2 assesses fine and gross motor performance of children ages 4–21 years.
Quality of life (QOL) assessments were administered prior to therapy, after completing RT, prior to course three maintenance, then every other course, at completion of therapy, and 90 days thereafter. Patients 5–18 years and their parent/guardian were assessed using: Pediatric Quality of Life (PedsQL) Brain Tumor Model, Parent and Self Reports; Peds QL Multidimensional Fatigue Scale, Parent Report; and the general PedsQL Parent and Self Reports. QOL for patients >18 years were assessed with Functional Assessment of Cancer Therapy [Brain (FACT-Br)], a health-related QOL measure validated for adult brain tumor patients. Domains assessed were comparable to those being assessed by the PedsQL.
Results
Patient characteristics
From May 2009 to October 2013, 27 (15 DIPG and 12 HGG) eligible and evaluable patients, median age 10 years (range 3–29 years) were enrolled. The 27 patients received 170 courses of maintenance therapy. Because of slow accrual to the high-grade glioma stratum, the Data Safety Monitoring Committee (DSMC) of the study recommended closure of the study once the DIPG stratum had completed accrual with 15 patients in May 2013. Five HGG and two DIPG patients were >18 years of age. Table 1 summarizes the characteristics of eligible patients.
Toxicity and safety
Two patients discontinued therapy during RT due to grade 4 thrombocytopenia and grade 3 hepatotoxicity, and 4 during maintenance therapy due to grade 3 central line thrombosis (n = 1), uncontrolled hypertension (n = 1), skin ulceration on chronic steroids (n = 1), or wound dehiscence at the port site (n = 1) among 27 eligible patients. Only 2 of 27 patients (7 %) discontinued therapy during RT and 4 of 27 patients (14 %) who received 170 courses of maintenance therapy discontinued therapy during maintenance. Based on these results, the DSMC declared the regimen feasible. During RT, fatigue and lymphopenia were the most common grade 2 and 3 toxicities (Table 2). Grade 4 toxicities included neutropenia (n = 2), lymphopenia (n = 2), leukopenia (n = 1) and thrombocytopenia (n = 1).
During maintenance therapy, anorexia, nausea, vomiting, pain, lymphopenia, and neutropenia were the most common grade 2/3 toxicities. Grade 4 toxicities included leukopenia (n = 1), lymphopenia (n = 1), and thrombocytopenia (n = 1). Nasal septum perforation (without need for medical intervention) occurred in one patient. Epistaxis (grade 2) with normal platelet counts occurred in two patients (7 %) (one each during RT and maintenance). There were no intracranial hemorrhages, cerebral ischemia, or toxic deaths.
Outcome
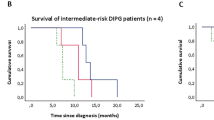
Median PFS and OS for DIPG patients were 8.2 and 10.4 months, respectively. Median PFS and OS for HGG patients were 15.2 and 25.4 months, respectively, with a median follow-up of 45.6 months. The 1-year PFS and OS for HGG patients were both 92 % (95 % CI 77–100) with a 3-year PFS and OS of 33 % (95 % CI 15–74) and 55 % (95 % CI 28–88) respectively (Figs. 1, 2).
Radiological evaluation and response
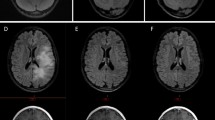
Eleven of 27 (DIPG 7, HGG 4) patients had minimal punctate and/or linear intra-tumoral hemorrhage at baseline; two DIPG patients developed new punctuate hemorrhages during treatment. Four DIPG patients without baseline tumoral hemorrhage developed new punctate hemorrhage, and three foci of hemorrhage slightly enlarged, without any clinical significance.
Baseline tumoral enhancement was noted in 16 patients (12 DIPG); three developed new areas of enhancement during therapy. For this study, enhancement was used only if it allowed us to identify the tumor margins more accurately. Disappearance of contrast enhancement, in the setting of tumor signal on other sequences was not considered a response. In general, tumor response was based on non-contrast imaging appearance.
Correlative studies
Six patients had adequate tissue for analysis of expression levels of hTERT mRNA and TERC RNA: 2 demonstrated elevated levels of hTERT and four had elevated levels of TERC. All patients with adequate tumor tissue showed evidence of ALT activity by TRF Southern Analysis (n = 3) while none showed positive telomerase activity by TRAP (n = 5). Partial sequencing of H3F3A gene was performed on two DIPG and one GBM specimens. Both DIPG specimens demonstrated the K27M mutation. The GBM specimen harboured the G34R mutation (Table 3).
All four patients who participated in functional evaluation during and after RT demonstrated below average scores at enrollment in motor (2/2) and body coordination areas (4/4). Only 1 patient demonstrated improved body coordination scores at follow-up. Two patients completed all motor and coordination studies. Manual coordination scores declined after RT, all patients functioning below, to well below average at follow-up.
The greatest deficits in functional independence at enrollment were in mobility/transfers and locomotion, but three of four patients demonstrated maintenance or improvement following RT. No patients completed functional outcome assessment at therapy completion; thus, changes in functional capabilities after maintenance chemotherapy were not assessed.
QOL reports were available for a subset of patients. For patients under 18 years (n = 10), patient-and parent-reported PedsQL Brain Tumor subscale scores were stable or improved over time. For patients over 18 years (n = 7) QOL remained stable or improved over time as assessed by the FACT-BR.
Discussion
This prospective study demonstrates the feasibility of a BVZ-based regimen in children with newly diagnosed HGG and DIPG. A total of six patients (two during RT and four during maintenance) discontinued therapy due to BVZ-related toxicities.
No grade 3 or 4 hemorrhages were observed. Four of 27 patients (14.8 %) developed asymptomatic new punctate hemorrhages and 2/27 patients (7 %) developed Grade 2 epistaxis after receiving therapy, comparable to adult and pediatric CNS trials where the incidence of new intracranial hemorrhage attributable to BVZ was very low [16, 17, 21]. Fangusaro et al. reported on the toxicities of 92 children with recurrent brain tumors who received BVZ and IRO. Grade 1 or 2 epistaxis occurred in 24 % of patients, while 10.8 % developed grade 1 CNS hemorrhages. A grade 3 subdural hematoma occurred in 1 patient (1.1 %) [25]. Although 8–25 % of adults with recurrent HGG treated with BVZ, developed thromboembolic events [26]; only one (3 %) of 27 patients developed a grade 3 thrombosis in our study.
Inhibition of angiogenesis may impair or delay wound healing [27]. Fangusaro et al. reported no delayed wound healing in patients on treatment; however, three (3 %) were removed from therapy for delayed wound healing due to “other complicating diseases”, physician discretion and presence of skin ulcers [25]. In our study, the two patients with grade 3 skin toxicities attributed to BVZ had additional factors including prolonged steroid use and trauma, which may have exacerbated the risk for ulceration/wound dehiscence.
Although our HGG cohort is small and the median age is 16 years, the outcomes are similar to previously reported pediatric HGG studies. Median PFS and OS for 12 HGG patients on our study were 15.2 and 25.4 months, respectively, with a 3-year PFS and OS of 33 % (95 % CI 15–74) and 55 % (95 % CI 28–88) respectively. Because of the small numbers of patients, we could not correlate prognostic parameters such as tumor grade, extent of resection, and molecular characteristics of patients with PFS or OS. Factors such as isocitrate dehydrogenase (IDH) mutations, which rarely occur in younger patients with HGG, and were beginning to emerge as a good prognostic factor [28, 29], at the time of this study’s development, were not assessed.
Friedman et al. reported favorable results in 3 children with newly-diagnosed GBM treated with BVZ, TEM and RT followed by maintenance bevacizumab [30]: with two remaining disease-free 38 and 49 months from diagnosis, and one recurring 14 months off therapy. By comparison, in ACNS0126, a Children’s Oncology Group study utilizing TEM with concurrent radiation followed by maintenance TEM, the 3-year EFS and OS for 90 children with HGG were 11 ± 3 and 22 ± 5 %, respectively [31]. In CCG-945, the median PFS and OS were approximately 16 and 26 months, respectively with a 5-year EFS and OS of 33 ± 5, and 36 ± 6 %, respectively [5].
By contrast, two recent randomized phase III trials in adults with newly diagnosed GBM by Chinot et al. [18] and Gilbert et al. [19] failed to demonstrate an improvement in OS with BVZ. Chinot et al. noted that OS did not differ significantly between BVZ (72.4 % at 1 year, 33.9 % at 2 years) and placebo (66.3 % at 1 year and 30 % at 2 years) arms. Gilbert et al. similarly found no significant difference in OS between the BVZ and the placebo groups (15.7 and 16.1 months, respectively) with more adverse events reported with BVZ.
BVZ had no significant impact on median OS in newly-diagnosed DIPG patients on this trial By contrast, two smaller retrospective reports have demonstrated promising activity of BVZ-based therapy in children with newly-diagnosed DIPG. Zaky et al. reported an OS of 14.6 ± 3.55 months in six patients who received different chemoradiotherapy followed by maintenance IRO, TEM, and BVZ [32], while MacDonald et al. reported a PFS of 37 and 42 months in two children treated with RT followed by TEM and BVZ [33]. Our median OS of 10.4 months is very similar to previous large reviews of frontline DIPG studies by Hargrave [2] and Jansen [34], who reported median OS of 8–11 and 7–14 months, respectively.
H3.3K27M mutation has been shown to define a clinically and biologically distinct subgroup of DIPG associated with shorter survival [35]. Two DIPG specimens exhibited the H3.3K27M mutations and one GBM specimen harboured the G34R mutation, yet no conclusions can be drawn due to limited samples.
Telomerase activation (telomere length, telomerase activity, and hTERT expression) predicts aggressive tumor behavior in adults with GBM and higher telomerase activity has been associated with shorter OS in adults [36, 37]. We previously reported that children with HGG, particularly DIPG, have increased hTERT and TERC levels compared to normal controls [38]. More importantly, increased hTERT mRNA and TERC RNA expression are associated with worse OS in children with non-brainstem HGG. In the present study, two patients demonstrated elevated levels of hTERT and 4 had elevated levels of TERC. However, no clear correlation could be demonstrated as the OS ranged from 9.6 to 25 months among these 6 patients.
Both general fatigue and brain-tumor-specific quality-of-life measures remained stable or improved over time, similar to Chinot et al.’s study, which showed maintenance of baseline QOL and performance status with BVZ [18]. Future studies with larger sample sizes are needed to assess the impact of this regimen on QOL.
In conclusion, a BVZ-containing regimen is feasible and safe in children and young adults with newly diagnosed HGG and DIPG, but confers no survival benefit to patients with DIPG. Based on the preliminary reports of feasibility of this study, the Children’s Oncology Group has recently conducted ACNS0822, including BVZ and RT in one of three randomized radiosensitization arms followed by maintenance BVZ and TEM for all patients. The final results of ACNS0822 will better determine the efficacy of this regimen in children with newly diagnosed HGG.
References
Cage TA, Mueller S, Haas-Kogan D, Gupta N (2012) High-grade gliomas in children. Neurosurg Clin N Am 23(3):515–523. doi:10.1016/j.nec.2012.04.007
Hargrave D, Bartels U, Bouffet E (2006) Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7(3):241–248. doi:10.1016/S1470-2045(06)70615-5
Laperriere N, Zuraw L, Cairncross G, Cancer Care Ontario Practice Guidelines Initiative Neuro-Oncology Disease Site G (2002) Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol 64(3):259–273
Donaldson SS, Laningham F, Fisher PG (2006) Advances toward an understanding of brainstem gliomas. J Clin Oncol 24(8):1266–1272. doi:10.1200/JCO.2005.04.6599
Finlay JL, Boyett JM, Yates AJ, Wisoff JH, Milstein JM, Geyer JR, Bertolone SJ, McGuire P, Cherlow JM, Tefft M et al (1995) Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Childrens Cancer Group. J Clin Oncol 13(1):112–123
Hummel TR, Chow LM, Fouladi M, Franz D (2013) Pharmacotherapeutic management of pediatric gliomas: current and upcoming strategies. Paediatr Drugs 15(1):29–42. doi:10.1007/s40272-012-0002-4
MacDonald TJ, Arenson EB, Ater J, Sposto R, Bevan HE, Bruner J, Deutsch M, Kurczynski E, Luerssen T, McGuire-Cullen P, O’Brien R, Shah N, Steinbok P, Strain J, Thomson J, Holmes E, Vezina G, Yates A, Phillips P, Packer R (2005) Phase II study of high-dose chemotherapy before radiation in children with newly diagnosed high-grade astrocytoma: final analysis of Children’s Cancer Group Study 9933. Cancer 104(12):2862–2871. doi:10.1002/cncr.21593
Sposto R, Ertel IJ, Jenkin RD, Boesel CP, Venes JL, Ortega JA, Evans AE, Wara W, Hammond D (1989) The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial. A report from the Childrens Cancer Study Group. J Neurooncol 7(2):165–177
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8(8):610–622. doi:10.1038/nrn2175
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109. doi:10.1007/s00401-007-0243-4
Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR (1999) Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res 59(14):3374–3378
Lee CG, Heijn M, di TE, Griffon-Etienne G, Ancukiewicz M, Koike C, Park KR, Ferrara N, Jain RK, Suit HD, Boucher Y (2000) Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res 60(19):5565–5570
Clara CA, Marie SK, de Almeida JR, Wakamatsu A, Oba-Shinjo SM, Uno M, Neville M, Rosemberg S (2014) Angiogenesis and expression of PDGF-C, VEGF, CD105 and HIF-1alpha in human glioblastoma. Neuropathology. doi:10.1111/neup.12111
Huang H, Held-Feindt J, Buhl R, Mehdorn HM, Mentlein R (2005) Expression of VEGF and its receptors in different brain tumors. Neurol Res 27(4):371–377. doi:10.1179/016164105X39833
Zhou YH, Hess KR, Liu L, Linskey ME, Yung WK (2005) Modeling prognosis for patients with malignant astrocytic gliomas: quantifying the expression of multiple genetic markers and clinical variables. Neuro-oncology 7(4):485–494. doi:10.1215/S1152851704000730
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740. doi:10.1200/JCO.2008.19.8721
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27(5):740–745. doi:10.1200/JCO.2008.16.3055
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722. doi:10.1056/NEJMoa1308345
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ Jr, Mehta MP (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708. doi:10.1056/NEJMoa1308573
Glade Bender JL, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, Kerbel RS, Cooney-Qualter EM, Stempak D, Chen HX, Nelson MD, Krailo MD, Ingle AM, Blaney SM, Kandel JJ, Yamashiro DJ, Children’s Oncology Group S (2008) Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol 26(3):399–405. doi:10.1200/JCO.2007.11.9230
Gururangan S, Chi SN, Young Poussaint T, Onar-Thomas A, Gilbertson RJ, Vajapeyam S, Friedman HS, Packer RJ, Rood BN, Boyett JM, Kun LE (2010) Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J Clin Oncol 28(18):3069–3075. doi:10.1200/JCO.2009.26.8789
Garzon M, Garcia-Fructuoso G, Guillen A, Sunol M, Mora J, Cruz O (2013) Brain stem tumors in children and adolescents: single institutional experience. Child’s Nerv Syst 29(8):1321–1331. doi:10.1007/s00381-013-2137-1
Poussaint TY, Kocak M, Vajapeyam S, Packer RI, Robertson RL, Geyer R, Haas-Kogan D, Pollack IF, Vezina G, Zimmerman R, Cha S, Patay Z, Boyett JM, Kun LE (2011) MRI as a central component of clinical trials analysis in brainstem glioma: a report from the Pediatric Brain Tumor Consortium (PBTC). Neuro-oncology 13(4):417–427. doi:10.1093/neuonc/noq200
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996. doi:10.1056/NEJMoa043330
Fangusaro J, Gururangan S, Poussaint TY, McLendon RE, Onar-Thomas A, Warren KE, Wu S, Packer RJ, Banerjee A, Gilbertson RJ, Jakacki R, Gajjar A, Goldman S, Pollack IF, Friedman HS, Boyett JM, Kun LE, Fouladi M (2013) Bevacizumab (BVZ)-associated toxicities in children with recurrent central nervous system tumors treated with BVZ and irinotecan (CPT-11): a Pediatric Brain Tumor Consortium Study (PBTC-022). Cancer 119(23):4180–4187. doi:10.1002/cncr.28343
Gordon MS, Cunningham D (2005) Managing patients treated with bevacizumab combination therapy. Oncology 69(Suppl 3):25–33. doi:10.1159/000088481
Scappaticci FA, Fehrenbacher L, Cartwright T, Hainsworth JD, Heim W, Berlin J, Kabbinavar F, Novotny W, Sarkar S, Hurwitz H (2005) Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol 91(3):173–180. doi:10.1002/jso.20301
Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA Jr, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321(5897):1807–1812. doi:10.1126/science.1164382
Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El Hallani S, Boisselier B, Mokhtari K, Hoang-Xuan K, Delattre JY (2009) Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 27(25):4150–4154. doi:10.1200/JCO.2009.21.9832
Friedman GK, Spiller SE, Harrison DK, Fiveash JB, Reddy AT (2013) Treatment of children with glioblastoma with conformal radiation, temozolomide, and bevacizumab as adjuncts to surgical resection. J Pediatr Hematol Oncol 35(3):e123–e126. doi:10.1097/MPH.0b013e318282cd7f
Cohen KJ, Pollack IF, Zhou T, Buxton A, Holmes EJ, Burger PC, Brat DJ, Rosenblum MK, Hamilton RL, Lavey RS, Heideman RL (2011) Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro-oncology 13(3):317–323. doi:10.1093/neuonc/noq191
Zaky W, Wellner M, Brown RJ, Bluml S, Finlay JL, Dhall G (2013) Treatment of children with diffuse intrinsic pontine gliomas with chemoradiotherapy followed by a combination of temozolomide, irinotecan, and bevacizumab. Pediatr Hematol Oncol 30(7):623–632. doi:10.3109/08880018.2013.829895
Aguilera DG, Mazewski C, Hayes L, Jordan C, Esiashivilli N, Janns A, Macdonald TJ (2013) Prolonged survival after treatment of diffuse intrinsic pontine glioma with radiation, temozolamide, and bevacizumab: report of 2 cases. J Pediatr Hematol Oncol 35(1):e42–e46. doi:10.1097/MPH.0b013e318279aed8
Jansen MH, van Vuurden DG, Vandertop WP, Kaspers GJ (2012) Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev 38(1):27–35. doi:10.1016/j.ctrv.2011.06.007
Schwartzentruber J, Korshunov A, Liu XY, Jones DT, Pfaff E, Jacob K, Sturm D, Fontebasso AM, Quang DA, Tonjes M, Hovestadt V, Albrecht S, Kool M, Nantel A, Konermann C, Lindroth A, Jager N, Rausch T, Ryzhova M, Korbel JO, Hielscher T, Hauser P, Garami M, Klekner A, Bognar L, Ebinger M, Schuhmann MU, Scheurlen W, Pekrun A, Fruhwald MC, Roggendorf W, Kramm C, Durken M, Atkinson J, Lepage P, Montpetit A, Zakrzewska M, Zakrzewski K, Liberski PP, Dong Z, Siegel P, Kulozik AE, Zapatka M, Guha A, Malkin D, Felsberg J, Reifenberger G, von Deimling A, Ichimura K, Collins VP, Witt H, Milde T, Witt O, Zhang C, Castelo-Branco P, Lichter P, Faury D, Tabori U, Plass C, Majewski J, Pfister SM, Jabado N (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482(7384):226–231. doi:10.1038/nature10833
Boldrini L, Pistolesi S, Gisfredi S, Ursino S, Ali G, Pieracci N, Basolo F, Parenti G, Fontanini G (2006) Telomerase activity and hTERT mRNA expression in glial tumors. Int J Oncol 28(6):1555–1560
Huang F, Kanno H, Yamamoto I, Lin Y, Kubota Y (1999) Correlation of clinical features and telomerase activity in human gliomas. J Neurooncol 43(2):137–142
Dorris K, Sobo M, Onar-Thomas A, Panditharatna E, Stevenson CB, Gardner SL, Dewire MD, Pierson CR, Olshefski R, Rempel SA, Goldman S, Miles L, Fouladi M, Drissi R (2014) Prognostic significance of telomere maintenance mechanisms in pediatric high-grade gliomas. J Neurooncol 117(1):67–76. doi:10.1007/s11060-014-1374-9
Acknowledgments
We thank Jan Englehart for outstanding administrative and data management throughout the development and conduct of this trial. We are also grateful to Elizabeth Gilger, Maureen Gallagher, Heather Ward and Kristine Feld for outstanding patient care and conduct on this study. Grants from The Cure Starts Now Foundation and support from Genentech, in part, funded this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Genentech funded, in part, a portion of this study.
Rights and permissions
About this article
Cite this article
Hummel, T.R., Salloum, R., Drissi, R. et al. A pilot study of bevacizumab-based therapy in patients with newly diagnosed high-grade gliomas and diffuse intrinsic pontine gliomas. J Neurooncol 127, 53–61 (2016). https://doi.org/10.1007/s11060-015-2008-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-2008-6