Abstract
Primary glial brain tumors account for the majority of primary brain tumors in children. They are classified as low-grade gliomas (LGG) or high-grade gliomas (HGG), based on specific pathologic characteristics of the tumor, resulting in disparate clinical prognoses. Surgery is a mainstay of treatment for HGG, although it is not curative, and adjuvant therapy is required. Temozolomide, an oral imidazotetrazine prodrug, while considered standard of care for adult HGG, has not shown the same degree of benefit in the treatment of pediatric HGG. There are significant biologic differences that exist between adult and pediatric HGG, and targets specifically aimed at the biology in the pediatric population are required. Novel and specific therapies currently being investigated for pediatric HGG include small molecule inhibitors of epidermal growth factor receptor, platelet-derived growth factor receptor, histone deacetylase, the RAS/AKT pathway, telomerase, integrin, insulin-like growth factor receptor, and γ-secretase. Surgery is also the mainstay for LGG. There are defined front-line, multiagent chemotherapy regimens, but there are few proven second-line chemotherapy options for refractory patients. Approaches such as the inhibition of the mammalian target of rapamycin pathway, inhibition of MEK1 and 2, as well as BRAF, are discussed. Further research is required to understand the biology of pediatric gliomas as well as the use of molecularly targeted agents, especially in patients with surgically unresectable tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Primary brain tumors are the most common pediatric solid tumors and the second most common form of cancer occurring in children [1]. The overall incidence rate is approximately 4.8 per 100,000 person-years for children 0–19 years of age (4.7 per 100,000 person-years for children less than 15 years) [2]. The category of glioma accounts for 55 and 39 % of brain tumors in children aged 0–14 years, and adolescents aged 15–19 years, respectively [2]. In both age groups, gliomas account for over 70 % of malignant tumors [2]. Low-grade gliomas (LGG) are categorized by the WHO as either grade 1, which tend to have a low proliferative potential, or lesions designated as grade 2, which are generally infiltrative in nature. High-grade gliomas (HGG) are grade 3 or grade 4 and have anaplastic features, high mitotic activity with or without vascular proliferation, and necrosis [3]. Location, grade, and genetic predisposition all influence treatment strategies in managing gliomas. There is a large discrepancy in outcome between LGG and HGG as 5-year survival rates are 94 % for pilocytic astrocytomas (grade 1 astrocytoma) but less than 5 % for glioblastomas (grade 4 astrocytoma) [2, 4].
By reviewing the published literature and the rationale behind current trials (‘pediatric glioma’ used as a search term on http://www.clinicaltrials.gov), this review article will focus on the contemporary pharmacotherapeutic interventions available for the management of newly diagnosed and recurrent pediatric gliomas, as well as look at future research strategies.
2 High-Grade Gliomas
The cause of HGG in pediatrics is often unclear. Predisposing risk factors include previous exposure to ionizing radiation [5, 6] and inherited genetic defects such as Li-Fraumeni syndrome [7], neurofibromatosis type 1 (NF1) [8, 9], and Turcot syndrome [10]. However, the etiology of the majority of pediatric HGG cases is uncertain. A recent report on the lack of association between cell phone usage in children and the incidence of pediatric HGG was somewhat controversial and this question will likely require an appropriately powered prospective epidemiologic study to resolve [11]. Common presenting symptoms include increased intracranial pressure, which manifests as vomiting and headaches. Focal motor deficits, hemiplegia, ataxia, seizures, and dysmetria are also presenting signs depending on the location of the neoplasm.
2.1 Treatment Using Multiagent Chemotherapy
Treatment for HGG cases initially involves surgery. Surgical resection has consistently been shown to be one the most important prognostic factors in pediatric HGG in both North American and European studies irrespective of age, location, or histology [12–14]. However, surgery is not curative and adjuvant therapy is required in all cases. A number of treatment modalities, including radiotherapy (XRT) and cytotoxic chemotherapeutic agents, have been studied. One of the initial North American clinical trials that examined the addition of standard chemotherapy agents to a backbone of radiation was the Children’s Cancer Group (CCG)-943 study. Fifty-eight patients were randomized to receive either 5400 cGy alone or XRT combined with weekly vincristine during XRT followed by maintenance chemotherapy cycles consisting of prednisone, lomustine, and vincristine. After central histology review, there was a statistical survival benefit for patients with glioblastoma multiforme (GBM) who received XRT plus chemotherapy. The 5-year event-free survival (EFS) was 46 % for patients who received XRT plus chemotherapy compared with 18 % for patients who received XRT alone [15, 16].
In the subsequent CCG-945 study, 172 patients were randomized to receive a combination of eight agents in 1 day (vincristine, lomustine, procarbazine, hydroxyurea, cisplatin, cytosine arabinoside, cyclophosphamide, and methylprednisolone) versus prednisone, lomustine, and vincristine chemotherapy (the superior treatment regimen from CCG-943). XRT was given to all patients older than 2 years of age. There was no statistical difference between the two treatment regimens as the 5-year overall survival (OS) and PFS were 36 and 33 %, respectively [13]. The extent of resection was established as an important prognostic factor in this study in addition to the importance of central pathology review as many LGG were seen upon second review in this CCG-945 cohort [17, 18].
CCG-9933 evaluated the efficacy of three different chemotherapy regimens prior to XRT. Seventy-six evaluable patients were randomized to receive four courses of high-dose chemotherapy prior to XRT. The chemotherapy regimens included etoposide with carboplatin or ifosfamide or cyclophosphamide. Patients went on to receive XRT followed by lomustine and vincristine. Objective responses to the chemotherapy regimens ranged from 8 to 27 %; 5-year EFS was 8 ± 3 % with an OS of 24 ± 5 %. There was no difference among the response rates of the high-dose chemotherapy regimens; the relapse rate during high-dose chemotherapy was 30 %. Twenty-nine percent of patients experienced severe non-hematologic toxicities [19]. It was concluded that high-dose chemotherapy did not offer a survival benefit to pediatric HGG patients compared with radiation and standard-dose chemotherapy.
The experience of utilizing chemotherapy for pediatric HGG in Europe has been similar. Wolff et al. [20] recently reviewed data from the first three HIT-GBM (Hirntumor-Glioblastoma multiforme) protocols. The HIT-GBM concept was the first to start a cancer registry and to perform protocols with coherent inclusion criteria and then to compare the cohorts. The first protocol (HIT-GBM-A) treated 22 pediatric patients with GBM. Therapy consisted of either daily oral trofosfamide and etoposide or 21-day cycles of both agents interrupted by a 1-week rest. (Standard fractionated XRT was started with the first cycle.) The median OS was 12 months [21]. HIT-GBM-B treated 40 patients with XRT and concurrent chemotherapy (two cycles of chemotherapy consisting of cisplatin and etoposide in the first cycle, and cisplatin, etoposide, and ifosfamide in the second cycle). After induction therapy, immune stimulation therapy was initiated with interferon-γ given once daily. The median OS of 1 year was not significantly different from a historical control group [22].
The third protocol, HIT-GBM-C, consisted of newly diagnosed HGG patients treated with standard XRT, simultaneous chemotherapy (cisplatin, etoposide, vincristine, and ifosfamide) followed by maintenance chemotherapy, with further cycles of cisplatin, etoposide, and ifosfamide followed by oral valproic acid. When compared with previous protocols, there was no significant benefit for patients with residual tumor, but the 5-year OS rate for patients with complete resection treated on HIT-GBM-C was 63 % compared with 17 % for the historical control group (p = 0.003) [23]. The most recent protocol from this cooperative group (HIT-GBM-D) was a phase II study to evaluate the efficacy of methotrexate given as a single agent prior to XRT and chemotherapy for pediatric HGG. Patients received 24-h infusions of methotrexate on days 1 and 15. Subsequent XRT was administered with chemotherapy (cisplatin, etoposide, vincristine, and ifosfamide) followed by maintenance chemotherapy (vincristine, lomustine, and prednisone). Both response and EFS in the 30 patients treated were superior to the historical control group of 330 patients treated in various protocols of the same cooperative group [24]. The approach of giving two cycles of high-dose methotrexate prior to radiochemotherapy will be assessed in a randomized phase III trial.
2.2 Temozolomide
Progress in adult HGG treatment was made with the addition of adjuvant temozolomide. Temozolomide is an oral imidazotetrazine prodrug that undergoes spontaneous hydrolysis to the active metabolite 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide (MTIC), which methylates DNA at O6-guanine and other sites. Temozolomide has been shown to be effective against xenograft models of GBM and other aggressive central nervous system neoplasms [25]. In a randomized controlled clinical trial for adults with newly diagnosed GBM which evaluated radiation versus radiation with the addition of temozolomide, there was a statistically significant survival benefit when temozolomide was given with XRT (the 2-year survival rate was 26.5 % with XRT and temozolomide compared with 10.4 % with XRT alone). This is currently considered the standard of care for adult GBM [26]. Additional investigations have shown that patients with an unmethylated methylguanine methyltransferase (MGMT) promoter are resistant to temozolomide and subsequently have a worse prognosis [27, 28]. MGMT activity in neoplastic cells contributes to a resistant phenotype by blunting the therapeutic effect of alkylating agents such as temozolomide [28].
The Children’s Oncology Group (COG) study ACNS0126 treated newly diagnosed children with HGG with involved field XRT and concurrent temozolomide followed by temozolomide 200 mg/m2 daily for 5 days every 28 days for ten cycles. The 3-year EFS and OS rates were 11 ± 3 and 22 ± 5 %, respectively. There was no evidence that temozolomide given during XRT and as adjuvant therapy resulted in improved EFS compared with that found in CCG-945. The 3-year EFS rate for GBM was 7 ± 4 % compared with 15 ± 5 % in CCG-945 [29]. Although single-agent temozolomide has had modest activity at best in pediatric HGG [30–32], the chemoradiation therapy of XRT with concomitant and adjuvant temozolomide has been adopted by most pediatric neuro-oncology groups as the standard of care based on data in adults.
2.3 Angiogenesis Inhibitors
Bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), has been used in combination with irinotecan, a topoisomerase 1 inhibitor, for treating HGG. Vredenburgh et al. [33] first published phase II data using bevacizumab and irinotecan in adult patients with recurrent GBM. Additional studies have shown promising response rates in adults with recurrent GBM [34, 35]. Unfortunately, the response rates seen in adults with this combination have not been replicated in pediatric studies to date [36, 37]. These discrepancies may be due, in part, to the fact that VEGF is not the only mediator of angiogenesis, and other growth factors such as fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) may play a more prominent role in the pediatric setting [38]. Another hypothesis is that although bevacizumab may not be effective in the setting of recurrent HGG, it may prove more effective in the front-line setting, given the ability of antiangiogenics to normalize blood vessels and allow better delivery of cytotoxic therapy as well as enhancing XRT effectiveness [39–41]. Results from ongoing front-line studies are eagerly anticipated.
2.4 Molecularly Targeted Agents
Increasing evidence indicates that while the histology of adult and pediatric HGG is similar, significant biologic differences may exist between these tumors. This suggests that therapies found to be effective in adult patients may not be of benefit to children with HGG, as noted in the cases of temozolomide and bevacizumab. Therefore, current efforts to develop therapies for pediatric HGG will likely require an understanding of the biology involved and clinical trials designed specifically for this population. Multiple cell signaling pathways have been elucidated in HGG biology and multiple investigators have demonstrated differences between HGG in adult patients and HGG in pediatric patients. Comprehensive studies utilizing copy number, gene expression and mutation analyses have reported that the majority of adult GBM have disrupted p53, receptor tyrosine kinase/phosphatidylinositol-3-kinase (PI3K), and retinoblastoma (RB) pathways [42, 43]. By comparison, pediatric HGG has not been as well studied and genetic alterations for the most part have been defined by directed analyses of genes that are mutated in adult HGG. TP53, CDKN2A, and PIK3CA mutations are common in both adult and pediatric HGG, while phosphatase and tensin homolog (PTEN) mutations and epidermal growth factor receptor (EGFR) amplifications, which are seen in adult HGG, are less common in pediatric HGG [44–47]. Potential molecular targets that lie within dysregulated cellular pathways in pediatric HGG have led to the investigation of a number of novel and specific therapies. These agents include small molecule inhibitors, which are discussed below and listed in Table 1.
2.4.1 Epidermal Growth Factor Receptor Inhibitors
Deletions of the gene encoding PTEN and amplification with or without mutation of the EGFR gene are commonly detected in adult malignant gliomas [48, 49]. Analysis of 62 pediatric glioma samples showed that mutation at both the PTEN and EGFR loci occur infrequently [47]. This was confirmed by Wong et al. [50] in 2006 who used single nucleotide polymorphism (SNP) analysis to map chromosomal aberrations in 14 pediatric HGG samples. They noted that only 2 of the 13 pediatric GBMs studied showed amplification of EGFR. Liang et al. [51] noted differences in protein expression in pediatric HGG when they examined tumor samples by immunohistochemistry. While HGG from children and adults exhibited a similar degree of immunopositivity for wild-type EGFR, pediatric samples had a low rate of expression of the constitutively activated mutant, EGFRvIII. Additionally, pediatric HGG were noted to have overexpression of EGFR when compared with pediatric LGG [52].
While there are differences between pediatric and adult HGG with regard to expression of EGFR, and the precise role of EGFR in pediatric HGG remains to be defined, multiple clinical trials of EGFR inhibition have been undertaken. Erlotinib and gefitinib, two small molecule EGFR inhibitors, have yielded different results in single-agent trials. Erlotinib plus XRT was examined in a pediatric phase I study and no relationship was observed between dose and exposure to the drug [53]. However, gefitinib was also studied in combination with XRT and activity was found in proportion to EGFR expression, and gefitinib is now being advanced to phase II trials [54]. Nimotuzumab is a monoclonal anti-EGFR antibody that was studied in a phase II trial in 47 patients with refractory or relapsed pediatric HGG. A partial response in four patients and stable disease in ten patients was observed with the median OS being extended for responders (10 months) compared with non-responders (4 months) [55].
2.4.2 RAS and AKT Pathways
The Cancer Genome Atlas Project’s genomic analysis of 206 adult glioblastomas found frequent genetic alterations in PI3K/AKT/PTEN network with AKT amplification in 2 %, PI3K mutations in 15 %, NF1 mutations or homozygous deletions in 18 %, and PTEN mutations or homozygous deletions in 36 %. Based on data from human studies as well as from animal models of GBM that activation of the RAS and AKT pathways play a role in controlling cell growth, differentiation, and survival [56–59], Faury et al. [60] compared pediatric and adult GBM samples for activation of these pathways. Pathway activation was assessed through the phosphorylation of downstream effectors, and gene expression profiles were generated using the University Health Network Human 19K cDNA array platform. One subset of pediatric GBM was associated with RAS and AKT pathway activation and had a very poor prognosis, exhibiting increased expression of genes related to proliferation and the adoption of a neural stem-cell phenotype, similar to findings in aggressive adult GBM. However, this subset was still molecularly distinguishable from adult GBM using either unsupervised or supervised analyses of expression profiles. There was also a clear distinction between the RAS/AKT activated tumors and a second subset that conferred a better prognosis for patients and was not associated with activation of RAS/AKT pathways. Both subsets of pediatric GBM in this study showed overexpression of Y-box-protein-1 that may assist in driving oncogenesis in these tumors [60]. The study by Faury et al. provided valuable insight into active pathways of pediatric GBM, reinforces that pediatric GBM cannot be understood exclusively through studies of adult GBM, and suggests new targetable pathways in a cancer with poor survival.
Numerous small molecule candidates that target the RAS/AKT pathways are in the clinical pipeline but relatively few have been tested in pediatric HGG patients. MK2206 is a highly selective non-adenosine triphosphate competitive allosteric AKT inhibitor that is currently being studied in a phase I trial by the COG [61]. Enzastaurin is a selective inhibitor of protein kinase C (PKC)-β. PKC-β mediates VEGF receptor 2 (VEGFR2) signaling through MEK and MAP kinase activation [62]. PKC-β also interacts with the PTEN/PI3K/AKT pathway. The Pediatric Brain Tumor Consortium (PBTC) has recently completed a phase I trial of enzastaurin in refractory pediatric HGG [63].
An additional downstream target in the RAS/AKT pathway is the mammalian target of rapamycin (mTOR). mTOR is a signaling protein that regulates cell growth, angiogenesis, proliferation, and cellular survival pathways [64, 65]. mTOR inhibitors have significant anti-tumor activity in pediatric cancer xenograft models [66]. In malignant glioma cells, silencing mTOR with small interfering ribonucleic acid increases autophagy, which mediates the growth inhibitory effect of rapamycin [67]. mTOR inhibition has also led to growth inhibition of xenografts derived from malignant glioma cells [66]. These effects have led to investigators evaluating mTOR inhibitors in pediatric HGG. A study of temsirolimus (an ester of sirolimus and an inhibitor of mTOR) administered weekly showed disease stabilization in children with HGG although the study did not meet the primary objective efficacy threshold [68].
The insulin-like growth factor (IGF) receptors are cell-surface tyrosine kinase signaling molecules that, when bound by the ligands IGF-1 or IGF-2, lead to activation of intracellular proliferation and survival signaling pathways such as PI3K, AKT, and mTOR [69]. Overexpression of the IGF-1 receptor (IGF-1R) has been reported in GBM cells [70] and inhibition of IGF-1R suppressed glioma proliferation in vitro and in vivo [71]. Clinical evidence that IGF-1R may play a role in HGG biology was provided by Andrews et al. [72], who treated 12 patients with recurrent HGG using an antisense oligonucleotide directed against IGF-1R. Two complete responses and four partial responses were seen (overall objective response rate of 50 %), although treatment was associated with venous thrombosis (noted in 33 % of patients). Currently, a phase I study of cixutumumab, a recombinant monoclonal antibody to IGF-1R, in combination with temsirolimus, an mTOR inhibitor, is under investigation by the COG [73].
2.4.3 Platelet-Derived Growth Factor Receptor Inhibitors
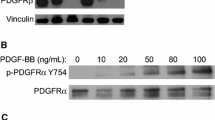
The first report of analyses of genomic imbalances and gene expression signatures in a large collection of pediatric HGG was performed by Paugh et al. [74] in 2010. They conducted a high-resolution analysis of genomic imbalances in 78 pediatric HGG using SNP microarrays. Gene expression was analyzed with gene expression microarrays on a subset of 53 tumors. They found significant differences in copy number alterations that distinguished pediatric and adult GBM. The gene encoding PDGF receptor-α (PDGFRA) was the predominant target of focal amplification in pediatric HGG, and gene expression analyses supported a critical role for deregulated PDGFR-α signaling. Contrary to adult GBM studies, no isocitrate dehydrogenase 1 (IDH1) mutations were found in pediatric tumors, further highlighting the molecular differences with adult secondary GBM. Pediatric and adult GBM were also distinguished by the frequency of chromosome 1q gains (30 vs 9 %, respectively), chromosome 7 gains (13 vs 74 %, respectively) and chromosome 10q losses (35 vs 80 %, respectively). PDGFRA amplification and 1q gain occurred at significantly higher frequency in irradiation-induced tumors, suggesting that these are initiating events in childhood glioma formation. This study showed substantial differences between pediatric and adult GBM at the molecular level and suggested that PDGFR-α may be a useful target for pediatric HGG. Imatinib targets PDGFR-α and was investigated in a phase I trial as a molecular agent for pediatric malignant gliomas. A phase II dose was determined; however, efficacy studies have yet to be reported [75]. Several other small molecule inhibitors (e.g. dasatinib, crenolanib) target PDGFR-α and clinical trials are underway (see Sect. 3.2 below).
2.4.4 Histone Deacetylase Inhibitors
Epigenetic alterations, including changes in structure of chromatin by histone modification, play an important role in tumorigenesis by altering gene expression and subsequently effecting viability and growth of neoplastic cells [76]. Enzymes that modify the core histones, H3 and H4, by phosphorylation, methylation, or acetylation are therefore potential targets for cancer therapy. Excessive deacetylation by histone deacetylases (HDAC) and the subsequent silencing of gene expression can be seen in malignancies [77–79]. HDAC inhibitors have been shown to induce cell-cycle arrest in glioma cells with an associated increase in p21 and reduced Cyclin B1 levels, and can inhibit the growth of glioblastoma cell lines [80, 81]. Multiple oral HDAC inhibitors, including valproic acid and suberoylanilide hydroxamic acid, have been studied in single-agent, phase I trials in pediatric patients, and have been shown to be well tolerated [82, 83]. Combination trials using HDAC inhibitors with either radiation [84] or other agents are being investigated. A phase I trial of suberoylanilide hydroxamic acid in combination with temozolomide for recurrent CNS malignancies has just been completed by the COG and the combination was well tolerated [85].
2.4.5 Other Agents
The role of telomerase in the development of pediatric CNS tumors has been the subject of recent research. Telomerase is a specialized reverse transcriptase that adds nucleotide repeats to telomeres, and compensates for the progressive DNA loss that occurs as a result of cell division [86, 87]. Because telomerase is expressed in the vast majority of malignant gliomas but not in normal brain tissues, it is a potential target for glioma-specific therapy [88–90]. Imetelstat, a telomerase inhibitor, is now being investigated within the confines of a pediatric phase I trial through the COG [91].
Integrins are cellular adhesion receptors that link the cytoskeleton to the extraceullar matrix and are regulators of tissue structure and cell motility [92]. Cilengitide is an inhibitor of avb3 and avb5 integrins [93], and demonstrated antitumor activity when used in combination with standard chemotherapy in adults with newly diagnosed GBM [93]. In the pediatric phase I trial of cilengitide, one patient with GBM had a complete response, and two patients with HGG had stable disease [94]. Future trials within the COG and within Europe will utilize cilengitide with XRT and are currently being developed [95, 96].
NOTCH signaling plays a major role in tissue development, proliferation, and survival [97]. Ligand binding activates proteolytic cleavage mediated by γ-secretase. Cleavage of the NOTCH receptor releases the receptor intracellular domain, allowing it to pass to the nucleus where it binds to the transcriptional regulator CSL and activates gene transcription [98]. NOTCH signaling has been described as crucial for GBM cell survival [99]. MK-0752 is a γ-secretase inhibitor that was investigated in a phase I trial by the PBTC where it was found to be well tolerated but no objective responses were seen [100]. RO4929097, another γ-secretase inhibitor, is currently under investigation in a phase I trial by the COG [101].
Recently, two groups have reported the presence of highly recurrent point mutations in the gene encoding Histone H3.3 and, to a lesser extent, in a related histone gene (encoding Histone H3.1) in one-third of pediatric HGG [102, 103]. While the functional mechanism of these alterations is currently not understood, their relevance to pediatric HGG biology is underscored by the finding that the Histone H3.3 associated proteins, ATRX and DAXX, are also mutated in a subset of pediatric HGG. Furthermore, these mutations were nearly absent in adult gliomas and in all other pediatric malignancies. Therefore, these mutations may regulate a pathway that is highly specific for pediatric HGG, and elucidation of its mechanism may lead to novel therapeutic opportunities.
3 Diffuse Intrinsic Pontine Gliomas
Gliomas located within the brainstem represent approximately 10–15 % of pediatric brain tumors [104] and the majority are diffusely infiltrating lesions called diffuse intrinsic pontine gliomas (DIPG). DIPG are aggressive lesions that are not amenable to surgical resection and are diagnosed based on magnetic resonance imaging (MRI) characteristics and clinical presentation, including cranial nerve deficits, long tract signs, and/or ataxia [105]. Given the characteristic appearance on MRI combined with classic signs, most neurooncologists agree that a biopsy is not warranted to establish a diagnosis. When the diagnosis has been less certain, most biopsies reveal that these lesions are glial in nature.
Prognosis for these tumors has remained dismal with a median survival of less than 1 year and less than 20 % surviving to 2 years after diagnosis [106]. The current standard of care is focal XRT. Indeed, XRT has been the only treatment thus far that has led to clinical improvement, albeit temporary, in a large percentage of patients [107].
3.1 Multiagent Chemotherapy
Chemotherapeutic interventions in this population have not been well defined and multiple trials involving various regimens given with radiation therapy have not demonstrated any improvement in outcome [108]. The CCG conducted a trial between 1977 and 1980 to assess the potential benefit of adding chemotherapy to XRT for children with newly diagnosed brainstem gliomas. Patients were randomized to receive either involved field XRT or XRT with concomitant vincristine followed by cycles of procarbazine, vincristine, and lomustine. No difference between the two arms was observed and 5-year survival was about 20 % [16]. The follow-up CCG-9941 study randomized patients between the pre-XRT use of cyclophosphamide and cisplatin versus ifosfamide and carboplatin. Both regimens led to few objective responses and high progression rates prior to XRT with no improvement in OS [109].
Korones et al. [110] reported the results of the Pediatric Oncology Group (POG) 9836 study using a combination of XRT at 54 Gy with two 28-day cycles of vincristine and oral etoposide starting concurrently with XRT and then continuing post-XRT for ten cycles. OS at 1 and 2 years was approximately 27 and 3 %, respectively, with a median survival of 9 months. Other studies that have similarly demonstrated no survival benefit over XRT alone for patients with DIPG include marrow ablative chemotherapy consisting of thiotepa and busulfan, thiotepa and etoposide with carmustine or carboplatin, or thiotepa and cyclophosphamide [111–113].
3.2 Molecularly Targeted Agents
Most newly diagnosed patients are treated with focal XRT combined with a biologic or novel chemotherapeutic agent, usually within the confines of a phase I or II clinical trial. One such example is Geoerger et al. [53], using erlotinib in addition to XRT in a European multicenter, phase I trial which treated 26 patients with DIPG but with limited efficacy. Current trials by the COG and the PBTC include suberoylanilide hydroxamic acid (HDAC inhibitor) given during and after XRT [84], capecitabine (anti-metabolite) in combination with XRT [114], bevacizumab (anti-VEGF) with XRT followed by bevacizumab/irinotecan maintenance chemotherapy [115], a combination of vandetanib (VEGFR/EGFR inhibitor) and dasatinib (BCR-ABL/PDGFR-α/SRC inhibitor) given during and after XRT [116], as well as lenalidomide (angiogenesis inhibitor) with concurrent XRT [117].
Postmortem DIPG tissue has been analyzed in multiple studies and the results have led to targeted agents in clinical trials. A study at St Jude Children’s Research Hospital looked at 28 DIPG tumor samples taken either by biopsy or autopsy and found increasing expression of EGFR that correlated with increasing WHO tumor grade, suggesting that the EGFR signaling pathway could be a therapeutic target for DIPG [118]. A trial studying radiation therapy combined with cetuximab (an EGFR monoclonal antibody) followed by irinotecan and cetuximab is ongoing [119]. Zarghooni et al. [120] described the genomic analysis of 11 pediatric DIPG samples and found involvement of the PDGFR-α pathway as well as gain of poly [ADP-Ribose] polyermase-1 (PARP-1), highlighting two potential therapeutic targets. A phase I trial is underway evaluating the oral PDGF receptor inhibitor crenolanib (CP-868596) in children with DIPG and HGG [121]. Additional genomic analysis of DIPG using single-nucleotide polymorphism arrays suggested that targeted inhibition of receptor tyrosine kinases and RB regulatory proteins may be useful therapies for DIPG [122]. Monje et al. [123] utilized postmortem DIPG tumor tissue and established in vitro cell culture and xenograft models. They found that the Hedgehog signaling pathway (implicated in many developmental and oncogenic processes) is active in DIPG tumor cells and could represent a potential therapeutic target in DIPG. Additionally, investigators have identified highly recurrent mutations of the genes encoding Histone H3.3 or Histone H3.1 affecting 78 % of DIPG tumors [103]. This points to a novel and specific mechanism of gliomagenesis for DIPG which may ultimately lead to novel therapeutic options. The need for future innovative therapies in DIPG is apparent; however, their development is hampered by the paucity of our understanding of the genetic and molecular biology of this disease due to the lack of tumor tissue from newly diagnosed patients. Biopsies of the brainstem had previously been thought to be dangerous and unnecessary. This paradigm is being challenged and proven inaccurate, as evidenced in studies by Geoerger et al. [53] and Roujeau et al. [124]. Overall, 26 and 24 patients, respectively, safely underwent a stereotactic biopsy of their DIPG. The concept of biopsy at diagnosis is further reinforced by the recent publication of Grill et al. [125] in which 20 patients newly diagnosed with DIPG were biopsied and were then analyzed for mutations in oncogenes and tumor suppressor genes. Oncogenic mutations in TP53, PI3KCA, and ATM/MPL were identified. Routine biopsies in newly diagnosed patients can potentially lead to a better understanding of the biology of DIPG, and the development of newly targeted agents and innovative therapies for patients with this poor prognosis tumor.
4 Low-Grade Gliomas
Pediatric LGG account for approximately 30–50 % of central nervous system tumors in children and comprise a heterogeneous set of tumors encompassing multiple histologic variants. The etiology of pediatric LGG remains unknown although there are syndromes such as NF1 and tuberous sclerosis that predispose an individual to LGG [126]. The majority of pediatric LGG do not exhibit aggressive clinical behavior nor undergo malignant transformation [127, 128]. Surgery, specifically a gross total resection, is the most consistent prognostic factor for prolonged PFS and OS in pediatric LGG [129–131]. When LGG are not amenable to resection, adjuvant therapy is usually recommended. XRT has been used for older patients with doses from 45 to 54 Gy [132–134], and subsequent studies have revealed improved survival associated with administration of conventional XRT [132, 135, 136]. A major concern in adult LGG is the possibility of malignant transformation, which can occur in 50–90 % of these cases [137, 138]. Broniscer et al. [139] noted that the long-term risk of malignant transformation in histologically comparable pediatric LGG is less than 10 % underscoring the differences in the biology between LGG in children and adults.
4.1 Multiagent Chemotherapy
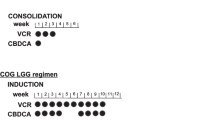
In infants and young children with progressive/refractory LGG, chemotherapy is usually the front-line adjuvant therapy offered. Carboplatin-containing regimens with vincristine are typically the first regimen offered; one study demonstrated a decrease in tumor burden or stable disease with a 3-year PFS of 68 % [140]. However, nearly 40 % of patients can experience hypersensitivity reactions to carboplatin, particularly with an increasing number of doses [141, 142]. An alternative regimen utilizing tioguanine, procarbazine, lomustine (CCNU), and vincristine was also shown to be effective in LGG with a median time to treatment failure of 132 weeks [143]. The COG protocol, A9952, then directly compared the combination of carboplatin and vincristine with the tioguanine/procarbazine/CCNU/vincristine (TPCV) regimen. Preliminary results presented at the 2008 International Symposium on Pediatric Neuro-Oncology showed a non-significant trend toward improved EFS for the TPCV regimen [144].
There are few proven second-line chemotherapy options for refractory pediatric LGG patients. Cisplatin plus etoposide has been evaluated in unresectable pediatric LGG with a 3-year progression-free survival (PFS) of 78 %, although 28 % of patients did exhibit high frequency ototoxicity [145]. Vinblastine, a vinca alkaloid that binds tubulin and inhibits the assembly of microtubules, given weekly has shown promise in initial studies in pediatric LGG [146]. Temozolomide has also shown to be active in pediatric LGG. Gururangan et al. [147] observed that the 2-year PFS and OS in 32 patients with progressive LGG who received temozolomide was 49 %. Additionally, Khaw et al. [148] evaluated 13 patients with progressive LGG and noted the EFS rate at 3 years was 57 %. Finally, the COG phase II trial of temozolomide noted that 41 % of patients with LGG had stable disease after 12 courses of therapy [149]. Additionally, bevacizumab and irinotecan, a regimen described in Sect. 2.3 in the treatment of HGG, has been shown by Packer et al. [150] to yield durable responses in seven of ten children with multiple recurrent LGG (nine of whom had progressed after three or more chemotherapy regimens).
4.2 Molecularly Targeted Agents
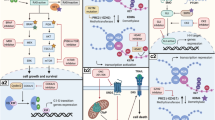
Discovering targeted agents in LGG has been difficult as tumorigenesis of pediatric LGG is not well understood. Common molecular abnormalities in tumor specimens are difficult to identify and karyotype analysis has been unrevealing in multiple studies [151, 152]. However, recently there has been evidence of a small non-random duplication in the 7q34 region in the majority of pilocytic astrocytomas [151, 153–155]. This duplication involves a known oncogene, BRAF, and appears to result in upregulation of the RAS/RAF/MEK pathway (Fig. 1). Many agents are under development to target this pathway. One such agent, selumetinib, is an oral small molecule inhibitor of MEK1 and 2 that is currently being studied in a multicenter, phase I and pharmacokinetic trial by the PBTC for pediatric LGG [156]. Sorafenib, an inhibitor of the BRAF kinase, is also being studied for recurrent or progressive pediatric LGG [157].
Multiple targeted pathways currently being studied in pediatric glioma. EGFR epithelial growth factor receptor, FdUMP fluorodeoxyuridine monophosphate, FTUP fluorouridine triphosphate, FU fluorouracil, HDAC histone deacetylase, IGFR insulin-like growth factor receptor, mTOR mammalian target of rapamycin, NIC notch intracellular domain, PDGFR platelet-derived growth factor receptor, PI3K phosphatidylinositol-3-kinase, PKC protein kinase C, PLC phospholipase C, TS thymidylate synthase, VEGFR vascular endothelial growth factor receptor
One pathway that may lead to targeted therapies for pediatric LGG is the mTOR pathway. TSC1, located on chromosome 9q34, encodes the protein hamartin; TSC2, located on chromosome 16p13.3, encodes tuberin. The tuberous sclerosis complex (TSC)-1 and TSC2 proteins form a complex in cells that functions as a GTPase activating protein towards Rheb (Ras homolog enriched in brain) [158–160]. Through Rheb, this TSC1/TSC2 protein complex, also known as the hamartin-tuberin complex, limits the activation of mTOR. The intracellular serine-threonine kinase mTOR is a central regulator that senses changes in growth factors, nutrients, and fuel/energy, and also regulates angiogenesis, cell growth, nutrient uptake and utilization, and metabolism [161, 162]. mTOR has many functions, but its primary activity is to regulate protein synthesis and influence cell growth, proliferation, and angiogenesis [163]. Therefore, patients with TSC (TSC1/TSC2-deficient genes) have a constitutive activation of mTOR, which leads to hyperactive mTOR signaling and abnormal cell division. In the central nervous system, these events can lead to the development of subependymal giant cell astrocytomas (SEGAs).
No large, prospective studies have been conducted but individual case reports have demonstrated activity of rapamycin, an mTOR inhibitor, in reducing tumor volume in TSC patients with bilateral SEGAs [164, 165]. In four patients aged 3–21 years, three with SEGAs and one with pilocytic astrocytoma, oral rapamycin resulted in a reduction in tumor volume by 50–75 %, but did not result in elimination of the tumors [166]. In another small series of three pediatric patients from the Hospital for Sick Children (Toronto, ON, Canada), SEGA volume was reduced by 50–65 % after 3 months of rapamycin therapy [167]. A phase II, prospective, single-center, open-label trial evaluated the efficacy and safety of a related orally administrated mTOR inhibitor, everolimus, in 28 pediatric patients with TSC-related SEGAs [168]. This study demonstrated a clinically meaningful and statistically significant median reduction in primary SEGA volume (p < 0.001) at a primary endpoint of 6 months. Of 28 evaluable patients, 21 (75 %) experienced a volume reduction of at least 30 % and 9 (32 %) experienced a volume reduction of at least 50 %. None required surgical resection or other treatment. The median duration of treatment for everolimus was 21.5 months (range 4.7–34.4 months) and the majority of these patients (25/28; 89.3 %) are still on treatment [168]. This demonstration of mTOR inhibition by everolimus led to the recent approval of the drug by the US FDA for this indication.
Similarly, children who have NF1 have upregulation of the RAS family of proteins due to the inactivation of neurofibromin. This upregulation leads to abnormalities in multiple oncogenic signal transduction pathways in glial cells [169, 170]. NF1-associated tumors have also been shown to have increased mTOR activity [171]. Trials utilizing mTOR inhibitors are currently being considered for refractory LGG in patients with NF1.
5 Conclusions
The biology that is understood to play a crucial role in pediatric gliomas differs considerably from gliomas seen in adults. Clearly, more research is required to elucidate further targets in pediatric HGG and to define the use of combining molecular targeted agents. Obtaining tissue for molecular and genetic analysis, especially in DIPG, will be crucial for the development and success of future novel pharmacotherapeutic interventions in pediatric HGG and DIPG. Recent clarifications of the molecular pathology of pediatric LGG may lead to improved treatment options for surgically unresectable patients.
References
Altekruse SF, Kosary CL, Krapcho M, et al. editors. SEER cancer statistics review, 1975–2007, National Cancer Institute. Bethesda (MD) (based on November 2009 SEER data submission, posted to the SEER web site, 2010). http://seer.cancer.gov/csr/1975_2007/. Accessed 8 Nov 2012.
Central Brain Tumor Registry of the United States (CBTRUS). CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2007. February 2011: Hinsdale (IL). http://www.CBTRUS.org. Accessed 8 Nov 2012.
Louis DN, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109.
Bondy ML, et al. Brain tumor epidemiology: consensus from the brain tumor epidemiology consortium. Cancer. 2008;113(7 suppl):1953–68.
Neglia JP, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98(21):1528–37.
Salvati M, et al. Radio-induced gliomas: 20-year experience and critical review of the pathology. J Neurooncol. 2008;89(2):169–77.
Varley JM. Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat. 2003;21(3):313–20.
Leonard JR, et al. The role of surgical biopsy in the diagnosis of glioma in individuals with neurofibromatosis-1. Neurology. 2006;67(8):1509–12.
Guillamo JS, et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain. 2003;126(Pt 1):152–60.
Melean G, et al. Genetic insights into familial tumors of the nervous system. Am J Med Genet C Semin Med Genet. 2004;129C(1):74–84.
Aydin D, et al. Mobile phone use and brain tumors in children and adolescents: a multicenter case-control study. J Natl Cancer Inst. 2011;103(16):1264–76.
Wolff JE, et al. Preradiation chemotherapy for pediatric patients with high-grade glioma. Cancer. 2002;94(1):264–71.
Finlay JL, et al. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen. Childrens Cancer Group. J Clin Oncol. 1995;13(1):112–23.
Kramm CM, et al. Improved survival after gross total resection of malignant gliomas in pediatric patients from the HIT-GBM studies. Anticancer Res. 2006;26(5B):3773–9.
Sposto R, et al. The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial. A report from the Childrens Cancer Study Group. J Neurooncol. 1989;7(2):165–77.
Finlay JL, Zacharoulis S. The treatment of high grade gliomas and diffuse intrinsic pontine tumors of childhood and adolescence: a historical-and futuristic-perspective. J Neurooncol. 2005;75(3):253–66.
Fouladi M, et al. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on children’s cancer group high-grade glioma study CCG-945. Cancer. 2003;98(6):1243–52.
Wisoff JH, et al. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the children’s cancer group trial no. CCG-945. J Neurosurg. 1998;89(1):52–9.
MacDonald TJ, et al. Phase II study of high-dose chemotherapy before radiation in children with newly diagnosed high-grade astrocytoma: final analysis of children’s cancer group study 9933. Cancer. 2005;104(12):2862–71.
Wolff JE, et al. Subpopulations of malignant gliomas in pediatric patients: analysis of the HIT-GBM database. J Neurooncol. 2008;87(2):155–64.
Wolff JE, et al. Oral trofosfamide and etoposide in pediatric patients with glioblastoma multiforme. Cancer. 2000;89(10):2131–7.
Wolff JE, et al. Maintenance treatment with interferon-gamma and low-dose cyclophosphamide for pediatric high-grade glioma. J Neurooncol. 2006;79(3):315–21.
Wolff JE, et al. Valproic acid was well tolerated in heavily pretreated pediatric patients with high-grade glioma. J Neurooncol. 2008;90(3):309–14.
Wolff JE, et al. High dose methotrexate for pediatric high grade glioma: results of the HIT-GBM-D pilot study. J Neurooncol. 2011;102(3):433–42.
Friedman HS, et al. Activity of temozolomide in the treatment of central nervous system tumor xenografts. Cancer Res. 1995;55(13):2853–7.
Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Hegi ME, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003.
Stupp R, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66.
Cohen KJ, et al. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro Oncol. 2011;13(3):317–23.
Broniscer A, et al. Temozolomide after radiotherapy for newly diagnosed high-grade glioma and unfavorable low-grade glioma in children. J Neurooncol. 2006;76(3):313–9.
Cohen KJ, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol. 2011;13(4):410–6.
Lashford LS, et al. Temozolomide in malignant gliomas of childhood: a United Kingdom Children’s Cancer Study Group and French Society for Pediatric Oncology Intergroup Study. J Clin Oncol. 2002;20(24):4684–91.
Vredenburgh JJ, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–9.
Ali SA, et al. Bevacizumab and irinotecan therapy in glioblastoma multiforme: a series of 13 cases. J Neurosurg. 2008;109(2):268–72.
Bokstein F, Shpigel S, Blumenthal DT. Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer. 2008;112(10):2267–73.
Gururangan S, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a pediatric brain tumor consortium study. J Clin Oncol. 2010;28(18):3069–75.
Narayana A, et al. Bevacizumab in recurrent high-grade pediatric gliomas. Neuro Oncol. 2010;12(9):985–90.
Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14(20):6371–5.
Gorski DH, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59(14):3374–8.
Lee CG, et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60(19):5565–70.
Yuan F, et al. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA. 1996;93(25):14765–70.
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8.
Parsons DW, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–12.
Gallia GL, et al. PIK3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol Cancer Res. 2006;4(10):709–14.
Newcomb EW, et al. Incidence of p14ARF gene deletion in high-grade adult and pediatric astrocytomas. Hum Pathol. 2000;31(1):115–9.
Pollack IF, et al. Age and TP53 mutation frequency in childhood malignant gliomas: results in a multi-institutional cohort. Cancer Res. 2001;61(20):7404–7.
Pollack IF, et al. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children’s Cancer Group 945 cohort. J Neurosurg. 2006;105(5 Suppl.):418–24.
Voldborg BR, et al. Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann Oncol. 1997;8(12):1197–206.
Weiss WA. Genetics of brain tumors. Curr Opin Pediatr. 2000;12(6):543–8.
Wong KK, et al. Genome-wide allelic imbalance analysis of pediatric gliomas by single nucleotide polymorphic allele array. Cancer Res. 2006;66(23):11172–8.
Liang ML, et al. Tyrosine kinase expression in pediatric high grade astrocytoma. J Neurooncol. 2008;87(3):247–53.
Khatua S, et al. Overexpression of the EGFR/FKBP12/HIF-2alpha pathway identified in childhood astrocytomas by angiogenesis gene profiling. Cancer Res. 2003;63(8):1865–70.
Geoerger B, et al. Innovative therapies for children with cancer pediatric phase I study of erlotinib in brainstem glioma and relapsing/refractory brain tumors. Neuro Oncol. 2011;13(1):109–18.
Daw NC, et al. Phase I and pharmacokinetic study of gefitinib in children with refractory solid tumors: a Children’s Oncology Group Study. J Clin Oncol. 2005;23(25):6172–80.
Bode U, Buchen M, Warmuth-Metz M, et al. Final report of a phase II trial of nimotuzumab in the treatment of refractory and relapsed high-grade gliomas in children and adolescents. J Clin Oncol. 2006;24(Suppl. 18):1522.
Maher EA, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15(11):1311–33.
Zhu Y, Parada LF. The molecular and genetic basis of neurological tumours. Nat Rev Cancer. 2002;2(8):616–26.
Holland EC, et al. Signaling control of mRNA translation in cancer pathogenesis. Oncogene. 2004;23(18):3138–44.
Holland EC, et al. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25(1):55–7.
Faury D, et al. Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB-1 expression in tumors. J Clin Oncol. 2007;25(10):1196–208.
Children’s Oncology Group. MK2206 in treating younger patients with recurrent or refractory solid tumors or leukemia [ClinicalTrials.gov identifier NCT01231919]. US National Institutes of Health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
Yoshiji H, et al. Protein kinase C lies on the signaling pathway for vascular endothelial growth factor-mediated tumor development and angiogenesis. Cancer Res. 1999;59(17):4413–8.
Pediatric Brain Tumor Consortium. Enzastaurin in treating young patients with refractory primary CNS tumors [ClinicalTrials.gov identifier NCT00503724]. US National Institutes of Health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4(5):335–48.
Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12(1):9–22.
Geoerger B, et al. Antitumor activity of the rapamycin analog CCI-779 in human primitive neuroectodermal tumor/medulloblastoma models as single agent and in combination chemotherapy. Cancer Res. 2001;61(4):1527–32.
Iwamaru A, et al. Silencing mammalian target of rapamycin signaling by small interfering RNA enhances rapamycin-induced autophagy in malignant glioma cells. Oncogene. 2007;26(13):1840–51.
Geoerger B, et al. Phase II trial of temsirolimus in children with high-grade glioma, neuroblastoma and rhabdomyosarcoma. Eur J Cancer. 2012;48(2):253–62.
Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4(7):505–18.
Naidu KA, et al. Antiproliferative and apoptotic effect of ascorbyl stearate in human glioblastoma multiforme cells: modulation of insulin-like growth factor-I receptor (IGF-IR) expression. J Neurooncol. 2001;54(1):15–22.
Liu TJ, et al. Inhibition of both focal adhesion kinase and insulin-like growth factor-I receptor kinase suppresses glioma proliferation in vitro and in vivo. Mol Cancer Ther. 2007;6(4):1357–67.
Andrews DW, et al. Results of a pilot study involving the use of an antisense oligodeoxynucleotide directed against the insulin-like growth factor type I receptor in malignant astrocytomas. J Clin Oncol. 2001;19(8):2189–200.
Children’s Oncology Group. Cixutumumab and temsirolimus in treating young patients with solid tumors that have recurred or not responded to treatment [ClinicalTrials.gov identifier NCT00880282]. US National Institutes of Health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
Paugh BS, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28(18):3061–8.
Pollack IF, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a pediatric brain tumor consortium report. Neuro Oncol. 2007;9(2):145–60.
Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–92.
Marks P, et al. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1(3):194–202.
Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26(9):1351–6.
Weidle UH, Grossmann A. Inhibition of histone deacetylases: a new strategy to target epigenetic modifications for anticancer treatment. Anticancer Res. 2000;20(3A):1471–85.
Xu J, et al. Vorinostat modulates cell cycle regulatory proteins in glioma cells and human glioma slice cultures. J Neurooncol. 2011;105(2):241–51.
Yin D, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res. 2007;13(3):1045–52.
Fouladi M, et al. Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children’s Oncology Group phase I consortium report. J Clin Oncol. 2010;28(22):3623–9.
Su JM, et al. Phase 1 study of valproic acid in pediatric patients with refractory solid or CNS tumors: a Children’s Oncology Group report. Clin Cancer Res. 2011;17(3):589–97.
Children’s Oncology Group. Vorinostat and radiation therapy followed by maintenance therapy with vorinostat in treating younger patients with newly diagnosed pontine glioma [ClinicalTrials.gov identifier NCT01189266]. US National Institutes of Health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 1 Sep 2011.
Hummel TR, Wagner LM, Ahern CH, et al. A pediatric phase I trial of vorinostat and temozolomide in relapsed or refractory primary brain or spinal cord tumors: a Children’s Oncology Group Phase I Consortium Study [abstract no. 9579]. J Clin Oncol 2011; 29Suppl.
Greider CW. Telomeres do D-loop-T-loop. Cell. 1999;97(4):419–22.
de Lange T. How telomeres solve the end-protection problem. Science. 2009;326(5955):948–52.
Boldrini L, et al. Telomerase activity and hTERT mRNA expression in glial tumors. Int J Oncol. 2006;28(6):1555–60.
Huang F, et al. Correlation of clinical features and telomerase activity in human gliomas. J Neurooncol. 1999;43(2):137–42.
Maes L, et al. Relation between telomerase activity, hTERT and telomere length for intracranial tumours. Oncol Rep. 2007;18(6):1571–6.
Children’s Oncology Group. Imetelstat sodium in treating young patients with refractory or recurrent solid tumors or lymphoma [ClinicalTrials.gov identifier NCT01273090]. US National Institutes of Health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
Tabatabai G, et al. Targeting integrins in malignant glioma. Target Oncol. 2010;5(3):175–81.
Reardon DA, et al. Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs. 2008;17(8):1225–35.
MacDonald TJ, et al. Phase I clinical trial of cilengitide in children with refractory brain tumors: pediatric brain tumor consortium study PBTC-012. J Clin Oncol. 2008;26(6):919–24.
Lambret CO. Cilengitide in combination with irradiation in children with diffuse intrinsic pontine glioma (CILENT-0902) [ClinicalTrials.gov identifier NCT01165333]. US National Institutes of Health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
Martin-Luther-Universität Halle-Wittenberg. Cilengitide and metronomic temozolomide for relapsed or refractory high grade gliomas or diffuse intrinsic pontine gliomas in children and adolescents (HGG-CilMetro) [ClinicalTrials.gov identifier NCT01517776]. US National Institutes of Health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3(10):756–67.
Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194(3):237–55.
Purow BW, et al. Expression of notch-1 and its ligands, delta-like-1 and jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65(6):2353–63.
Fouladi M, et al. Phase I trial of MK-0752 in children with refractory CNS malignancies: a pediatric brain tumor consortium study. J Clin Oncol. 2011;29(26):3529–34.
Children’s Oncology Group. Gamma-secretase inhibitor RO4929097 in treating young patients with relapsed or refractory solid tumors, CNS tumors, lymphoma, or T-cell leukemia [ClinicalTrials.gov identifier NCT01088763]. US National Institutes of Health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
Schwartzentruber J, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–31.
Wu G, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44(3):251–3.
Fangusaro J. Pediatric high-grade gliomas and diffuse intrinsic pontine gliomas. J Child Neurol. 2009;24(11):1409–17.
Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7(3):241–8.
Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys. 1998;40(2):265–71.
Mandell LR, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a pediatric oncology group phase III trial comparing conventional vs. hyperfractionated radiotherapy. Int J Radiat Oncol Biol Phys. 1999;43(5):959–64.
Broniscer A, Gajjar A. Supratentorial high-grade astrocytoma and diffuse brainstem glioma: two challenges for the pediatric oncologist. Oncologist. 2004;9(2):197–206.
Jennings MT, et al. Preradiation chemotherapy in primary high-risk brainstem tumors: phase II study CCG-9941 of the Children’s Cancer Group. J Clin Oncol. 2002;20(16):3431–7.
Korones DN, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children’s Oncology Group phase II study. Pediatr Blood Cancer. 2008;50(2):227–30.
Bouffet E, et al. Radiotherapy followed by high dose busulfan and thiotepa: a prospective assessment of high dose chemotherapy in children with diffuse pontine gliomas. Cancer. 2000;88(3):685–92.
Dunkel IJ, O’Malley B, Finlay JL. Is there a role for high-dose chemotherapy with stem cell rescue for brain stem tumors of childhood? Pediatr Neurosurg. 1996;24(5):263–6.
Finlay JL, et al. High-dose multi-agent chemotherapy followed by bone marrow ‘rescue’ for malignant astrocytomas of childhood and adolescence. J Neurooncol. 1990;9(3):239–48.
Pediatric Brain Tumor Consortium. Capecitabine and radiation therapy in treating young patients with newly diagnosed, nonmetastatic brain stem glioma or high-grade glioma [ClinicalTrials.gov identifier NCT00357253]. US National Institutes of Health, ClinicalTrials. Gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
Children’s Hospital Medical Center. A study of bevacizumab therapy in patients with newly diagnosed high-grade gliomas and diffuse intrinsic pontine gliomas [ClinialTrials.gov identifier NCT00890786]. US National Institutes of Health [online]. http://clinicaltrials.gov. Accessed 8 Nov 2012.
St Jude Children’s Research Hospital. Clinical trial evaluating the combination of vandetanib and dasatinib during and after radiation therapy (RT) in children with newly diagnosed diffuse intrinsic pontine glioma (DIPG) [ClinicalTrials.gov identifier NCT00996723]. US National Institutes of Health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
National Cancer Institute. Lenalidomide and radiation therapy in high grade gliomas or diffuse intrinsic pontine gliomas [ClinicalTrials.gov identifier NCT01222754]. US National Institutes of Health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
Gilbertson RJ, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res. 2003;9(10 Pt 1):3620–4.
Memorial Sloan-Kettering Cancer Center. External beam radiation therapy and cetuximab followed by irinotecan and cetuximab for children and young adults with newly diagnosed diffuse pontine tumors and high-grade astrocytomas [ClinicalTrials.gov identifier NCT01012609]. US National Institutes of health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
Zarghooni M, et al. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol. 2010;28(8):1337–44.
St Jude Children’s Research Hospital. PDGFR inhibitor crenolanib in children/young adults with diffuse intrinsic pontine glioma or recurrent high-grade glioma [ClinicalTrials.gov identifier NCT01393912]. US National Institutes of Health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
Paugh BS, et al. Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol. 2011;29(30):3999–4006.
Monje M, et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc Natl Acad Sci USA. 2011;108(11):4453–8.
Roujeau T, et al. Stereotactic biopsy of diffuse pontine lesions in children. J Neurosurg. 2007;107(1 Suppl.):1–4.
Grill J, et al. Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr Blood Cancer. 2012;58(4):489–91.
Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24(11):1397–408.
Freeman CR, Farmer JP, Montes J. Low-grade astrocytomas in children: evolving management strategies. Int J Radiat Oncol Biol Phys. 1998;41(5):979–87.
Pollack IF. The role of surgery in pediatric gliomas. J Neurooncol. 1999;42(3):271–88.
Desai KI, et al. Prognostic factors for cerebellar astrocytomas in children: a study of 102 cases. Pediatr Neurosurg. 2001;35(6):311–7.
Fisher PG, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51(2):245–50.
Pollack IF, et al. Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J Neurosurg. 1995;82(4):536–47.
Grabenbauer GG, et al. Radiation therapy of optico-hypothalamic gliomas (OHG): radiographic response, vision and late toxicity. Radiother Oncol. 2000;54(3):239–45.
Karim AB, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36(3):549–56.
Kortmann RD, et al. Current and future strategies in radiotherapy of childhood low-grade glioma of the brain. Part I: treatment modalities of radiation therapy. Strahlenther Onkol. 2003;179(8):509–20.
Laws ER Jr, et al. Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. J Neurosurg. 1984;61(4):665–73.
Shibamoto Y, et al. Supratentorial low-grade astrocytoma: correlation of computed tomography findings with effect of radiation therapy and prognostic variables. Cancer. 1993;72(1):190–5.
Vertosick FT Jr, Selker RG, Arena VC. Survival of patients with well-differentiated astrocytomas diagnosed in the era of computed tomography. Neurosurgery. 1991;28(4):496–501.
McCormack BM, et al. Treatment and survival of low-grade astrocytoma in adults: 1977–1988. Neurosurgery. 1992;31(4):636–42. (discussion 642).
Broniscer A, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25(6):682–9.
Packer RJ, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747–54.
Lafay-Cousin L, et al. Carboplatin hypersensitivity reaction in pediatric patients with low-grade glioma: a Canadian Pediatric Brain Tumor Consortium experience. Cancer. 2008;112(4):892–9.
Yu DY, et al. Weekly dosing of carboplatin increases risk of allergy in children. J Pediatr Hematol Oncol. 2001;23(6):349–52.
Prados MD, et al. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32(3):235–41.
Ater J, Holmes E, Zhou T, et al. Abstracts from the thirteenth international symposium on pediatric neuro-oncology: results of COG protocol A9952: a randomized phase 3 study of two chemotherapy regimens for incompletely resected low-grade glioma in young children. Neuro Oncol. 2008;10:451.
Massimino M, et al. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20(20):4209–16.
Lafay-Cousin L, et al. Weekly vinblastine in pediatric low-grade glioma patients with carboplatin allergic reaction. Cancer. 2005;103(12):2636–42.
Gururangan S, et al. Temozolomide in children with progressive low-grade glioma. Neuro Oncol. 2007;9(2):161–8.
Khaw SL, et al. Temozolomide in pediatric low-grade glioma. Pediatr Blood Cancer. 2007;49(6):808–11.
Nicholson HS, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children’s Oncology Group. Cancer. 2007;110(7):1542–50.
Packer RJ, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52(7):791–5.
Orr LC, et al. Cytogenetics in pediatric low-grade astrocytomas. Med Pediatr Oncol. 2002;38(3):173–7.
White FV, et al. Nonrandom chromosomal gains in pilocytic astrocytomas of childhood. Hum Pathol. 1995;26(9):979–86.
Bar EE, et al. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67(9):878–87.
Jones DT, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–7.
Sievert AJ, et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single-nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 2009;19(3):449–58.
Pediatric Brain Tumor Consortium. MEK inhibitor AZD6244 in treating young patients with recurrent or refractory low grade glioma [ClinicalTrials.gov identifier NCT01089101]. US National Institutes of Health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
New York University School of Medicine. Sorafenib in children and young adults with recurrent or progressive low-grade astrocytomas [ClinicalTrials.gov identifier NCT01338857]. US National Institutes of Health, ClinicalTrials.gov [online]. http://www.clinicaltrials.gov. Accessed 8 Nov 2012.
Dowling RJ, et al. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804(3):433–9.
Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14 Spec No. 2:R251–8.
Napolioni V, Curatolo P. Genetics and molecular biology of tuberous sclerosis complex. Curr Genomics. 2008;9(7):475–87.
Inoki K, et al. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4(9):648–57.
Orlova KA, Crino PB. The tuberous sclerosis complex. Ann NY Acad Sci. 2010;1184:87–105.
Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355(13):1345–56.
Koenig MK, Butler IJ, Northrup H. Regression of subependymal giant cell astrocytoma with rapamycin in tuberous sclerosis complex. J Child Neurol. 2008;23(10):1238–9.
Birca A, Mercier C, Major P. Rapamycin as an alternative to surgical treatment of subependymal giant cell astrocytomas in a patient with tuberous sclerosis complex. J Neurosurg Pediatr. 2010;6(4):381–4.
Franz DN, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59(3):490–8.
Lam C, et al. Rapamycin (sirolimus) in tuberous sclerosis associated pediatric central nervous system tumors. Pediatr Blood Cancer. 2010;54(3):476–9.
Krueger DA, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363(19):1801–11.
Gutmann DH. Using neurofibromatosis-1 to better understand and treat pediatric low-grade glioma. J Child Neurol. 2008;23(10):1186–94.
Listernick R, et al. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic Pathway Glioma Task Force. Ann Neurol. 1997;41(2):143–9.
Dasgupta B, et al. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65(7):2755–60.
Acknowledgments
No sources of funding were used to conduct this study or prepare this manuscript. Lionel M. Chow is a St Baldrick’s Foundation Scholar and is supported by a Distinguished Scientist Award from the Sontag Foundation. Maryam Fouladi has received research funding from Merck and Genentech for the conduct of studies partially funded by these companies, and has conducted a Lily-sponsored study at the Cincinnati Children’s Hospital Medical Center. Novartis has paid David Franz’s employer for the performance of clinical trials he is involved in and for consulting work he has done. They have also paid ApotheCom to assist in the preparation of this review and travel costs for David Franz to attend meetings of investigators participating in the trial by Krueger et al. [168]. Novartis provided the drug for the clinical trials David Franz has conducted, while Novartis and UCB Pharma have paid him for lectures. Various attorneys have paid David Franz to review medical legal cases.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hummel, T.R., Chow, L.M., Fouladi, M. et al. Pharmacotherapeutic Management of Pediatric Gliomas. Pediatr Drugs 15, 29–42 (2013). https://doi.org/10.1007/s40272-012-0002-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-012-0002-4