Abstract
Glioblastoma is a devastating cancer with universally poor outcomes in spite of current standard multimodal therapy. Immunotherapy is an attractive new treatment modality given its potential for exquisite specificity and its favorable side effect profile; however, clinical trials of immunotherapy in GBM have thus far shown modest benefit. Optimally combining radiation with immunotherapy may be the key to unlocking the potential of both therapies given the evidence that radiation can enhance anti-tumor immunity. Here we review this evidence and discuss considerations for combined therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM) is the most common and deadly primary brain tumor in humans. It is unforgivingly aggressive and invasive, rendering it impossible to completely eradicate with current standard treatments. Survival of patients with GBM averages less than 2 years from the time of diagnosis even with maximal therapy, which includes maximal safe surgical resection, temozolomide chemotherapy concurrent with radiotherapy, followed by adjuvant temozolomide [1]. Thus, new therapies and treatment strategies are needed.
Immunotherapy is a promising treatment modality with potential for eliciting broad antitumor effects by activating the various effector functions of immune cells. However, clinical trials of immunotherapy for GBM have to date demonstrated fairly modest results [2, 3]. Tumor immunosuppression and poor integration of immunotherapy with standard therapy may account for suboptimal responses.
Temozolomide remains the standard chemotherapeutic agent for the treatment of GBM. Most chemotherapies, including temozolomide, can cause lymphopenia and non-inflammatory cell death and therefore was previously regarded as antagonistic to immunotherapy. However, depletion of immunosuppressive lymphocytes with chemotherapy can actually result in a more favorable host immune profile and enhance the effectiveness of subsequent immunotherapy [4]. The synergy of combining chemotherapy with immunotherapy for GBM is more thoroughly reviewed elsewhere [5].
Radiation is a mainstay in the treatment of GBM and is typically delivered to the tumor or tumor cavity with a boost to the margins, which contain invasive glioma cells. Radiation induces DNA damage, which preferentially leads to the death of the rapidly growing and dividing cancer cells. In addition to this direct effect on cancer cells, radiation has also been shown to activate the immune system and augment antigen presentation, results that would enhance the anti-tumor effects of immunotherapy [6, 7]. Here we review the evidence supporting the combination of immunotherapy and radiation for the treatment of GBM.
Immunotherapy and GBM-induced immunosuppression
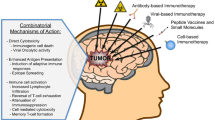
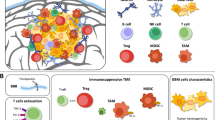
Several types of immunotherapy are being investigated for GBM including cytokine therapy, antibodies, vaccines, and modified T cells [2, 3, 8]. The efficacy of these strategies depends on the susceptibility of the tumor to immune-mediated killing. T cells must be able to migrate to the site of the tumor, recognize the antigens that are expressed by the tumor, and initiate a tumor-specific immune response in order to achieve the desired anti-tumor effect. GBM is notoriously immunosuppressive and uses a variety of mechanisms to impede these steps [9, 10]. GBMs are known to upregulate expression of signal transducer and activator of transcription 3 (STAT3), a key regulator of tumorigenesis and GBM-induced immunosuppression, and downregulate the expression of major histocompatibility complex (MHC) class I, molecules which are critical for antigen presentation [9, 10]. While GBMs have been shown to express a number of tumor-specific antigens including cancer testis antigens, EGFRvIII, IL13Rα2, and EphA2, which can be targeted with immunotherapy [11–14], GBMs also express surface markers such as Fas ligand [15–17] and programmed death ligand 1 (PD-L1) [18, 19] that impede cellular immunity by suppressing the function of tumor-infiltrating lymphocytes or inducing apoptosis. Thus, despite the ability of T cells to enter the brain and traffic to the tumor microenvironment, GBMs are equipped to prevent them from mounting an adequate anti-tumor immune response [20]. Additionally, GBMs are known to express soluble factors, such as interleukin 1 and transforming growth factor-β (TGF-β) that impair activation of T cells, and have been associated with the generation of immunosuppressive T regulatory cells [21–24]. TGF-β has also been implicated in the downregulation of NKG2D on NK and CD8 T cells of glioma patients thus hindering NKG2D-ligand mediated tumor killing [25]. Overcoming these mechanisms of immunosuppression will be a key component to the success of any immunotherapeutic intervention.
Radiation therapy for GBM
Radiation has long been used to treat GBM and remains a major component of standard therapy for the disease. Adjuvant radiation therapy has been shown to improve local control and survival by targeting the microscopic and gross residual disease that remains following surgical resection. The first studies demonstrating this benefit utilized whole brain radiation therapy (WBRT) [26, 27]. The benefit of radiation therapy is dose-dependent with total doses between 50 and 60 Gy delivered in multiple fractions being optimal for improving survival [28, 29]. The neurotoxic effects of WBRT, however, prompted the move from whole brain to involved-field radiation therapy (IFRT), which is the current standard radiation approach. The use of IFRT is supported by the fact that GBM recurrence after WBRT is usually within 2 cm of the original tumor site [30, 31]. A margin of radiographically normal-appearing brain tissue is included in IFRT to cover infiltrating tumor cells. Other radiotherapy techniques that may be used to limit radiation exposure to normal brain structures include three dimensional conformal radiation therapy and intensity modulated radiation therapy, however these techniques have not been shown to impact survival [32, 33].
In contrast to fractionated radiation therapy, stereotactic radiosurgery (SRS) is a method of radiation delivery which involves focusing multiple beams from different directions onto the tumor tissue to achieve a high dose of radiation delivered to the target in one fraction while limiting radiation delivered to normal tissues. While observation studies have shown mixed results, a phase III trial showed no added benefit of including SRS to a regimen of IFRT and carmustine therapy [34].
The anti-tumor effect of radiation therapy is based on the increased susceptibility of tumor cells versus normal cells to repeated exposure to sublethal damage caused by the radiation. Normal tissues are more effective at repairing the radiation-induced DNA damage, while tumor cells are more sensitive to DNA damage given their higher rates of proliferation and cell turnover. Accumulation of sufficient DNA damage in tumor cells causes cell death. While radiation is used for its direct effects in killing cancer cells, there is much evidence suggesting that there is also an indirect effect of radiation in stimulating a more effective anti-tumor immune response.
Radiation enhances anti-tumor immunity
Effective anti-tumor immunity depends on the interplay of several mechanisms including antigen presentation, activation of innate and adaptive immune responses, upregulation of costimulatory molecules, and release of proinflammatory cytokines and chemokines. Antigen presentation is mediated by major histocompatibility complex (MHC) class I molecules, which bind endogenous peptides and traffic to the cell surface. Cytotoxic T cells are then able to survey these cells and eliminate those presenting foreign material. Radiation has been shown to enhance antigen presentation to the immune system via several mechanisms. It induces MHC class I expression in brain tumors in a dose-dependent fashion [7, 35]. This upregulation in MHC class I expression has been shown to be mediated by radiation-induced production of interferon-γ (IFN-γ) [36]. IFN-γ has also been implicated in the upregulation of chemokines important for T cell infiltration [36]. In addition to upregulation of MHC class I, radiation also enhances antigen presentation by augmenting the quantity and diversity of intracellular peptide pools. This is accomplished via increased protein degradation and peptide production through activation of the mammalian target of rapamycin (MTOR) pathway and generation of novel proteins [7]. This results in the presentation of a larger repertoire of antigens and increases the likelihood of triggering an effective immune response.
Radiation of tumor cells can alter their surface proteins making them more susceptible to immune-mediated anti-tumor activity. In addition to MHC class I, radiation increases the expression of Fas (CD95) and intercellular adhesion molecule-1 (ICAM-1) on tumor cells, which increases T-cell infiltration of the tumor and makes them more susceptible to cytotoxic T-cell mediated killing [37]. Radiation has also been shown to increase the expression of NKG2D ligands, cell surface proteins which are part of the body’s stress response and play an integral role in NK-mediated tumor killing [38]. Adding radiation to a vaccine against one tumor associated antigen (TAA) has also been found to increase T-cell responses to other TAAs that were not originally targeted by the vaccine suggesting that radiation promotes the donation of TAAs by the tumor itself [39] resulting in a broader, more robust anti-tumor response.
While the direct anti-tumor effect of radiation is generally accepted to be due to the cytotoxicity caused by lethal DNA damage, one group showed that the effects of radiation are absent in type I interferon nonresponsive hosts [6]. Radiation increases the expression of interferon-β by tumor cells that increase priming of tumor-infiltrating dendritic cells, which leads to the expansion of antigen-specific T cells and an anti-tumor response. Another group demonstrated the importance of CD8+ T cells in mediating the anti-tumor effect of radiation and showed that local immunotherapy can enhance the effect of suboptimal doses of radiation [40]. These studies suggest that the effect of radiation on the activation of the immune system may indeed be a fundamental mechanism explaining the effectiveness of radiation therapy for cancer.
Abscopal effect
The abscopal effect is a phenomenon seen in the treatment of metastatic cancer where local irradiation to one tumor site leads to regression of tumor at a distant non-irradiated site. The effect is ascribed to the activation of the immune response by radiation and has been seen in patients treated for melanoma, lymphoma, and renal-cell cancer [41–43]. Surrogate markers for increased anti-tumor immunity, including increased followed by decreased tumor-antigen-specific antibody titers and increased CD4+ ICOShigh subset of T-cells (inducible costimulator), were seen following radiotherapy in patients who received ipilimumab for metastatic melanoma [41].
The abscopal effect suggests that radiation therapy can be used to generate an ‘in situ’ vaccine [44]. Radiation promotes immunogenic tumor cell death turning the tumor into an “immunogenic hub” which then activates effector immune cells which can propagate and produce distant antitumor effects [44, 45]. Radiation enhances immunogenicity via a number of mechanisms including promoting TAA presentation from APCs to T-cells, release of chemokines which attract effector cells to the tumor, and upregulation of molecules such as MHC and NKG2D ligands [46]. The fact that the abscopal effect is only rarely observed in patients, however, might lead one to surmise that an effective response depends on the optimal coalescence of several factors including the overall host immune status, the degree of radiosensitivity of the tumor, the type of tumor cell death triggered by radiation, and the breadth and specificity of the immunogenic mechanisms that are triggered.
Radiation and immunotherapy in preclinical glioma models
The effectiveness of combining radiation and immunotherapy for treatment of brain tumors has been demonstrated in several preclinical studies. Radiation plus vaccination with irradiated glioma cells significantly increased the survival of mice with intracranial gliomas compared with radiation or vaccination alone [47]. This effect was associated with an increase in MHC class I expression by glioma cells and increased CD4 and CD8 T-cell infiltration [47]. Similarly, the combination of stereotactic radiation and PD-1 blockade was more effective for treating mice with intracranial gliomas than either therapy alone [48]. The rationale for using single fraction SRS in this study was to minimize leukopenia and other potential immunologic and neurologic side effects while still gaining the effect of enhanced anti-tumor immunity. This combined with PD-1 blockade, which prevents GBM-induced T-cell apoptosis and exhaustion, proved to have a synergistic effect [48]. Similarly, combining radiation therapy with cytotoxic T lymphocyte antigen-4 blockade and/or 4-1BB (CD137) activation showed a greater anti-tumor effect than either radiation therapy or immunotherapy alone [49, 50].
Radiation-induced immunosuppression?
One group demonstrated that radiation for GBM is associated with decreased CD4 counts suggesting that radiation itself may also cause immunosuppression [51]. Indeed radiation for patients with genitourinary, head and neck, and breast cancer has been previously shown to result in absolute decreases in total number of T cells, relative decreases in T helper/inducer cell populations and relative increases in T suppressor/cytotoxic cell populations [52]. Unfortunately, the study in GBM patients only looked at CD4 count and did not subclassify the various T cell subtypes. It is known, for instance, that regulatory CD4 T cells (Tregs) are associated with GBM and induce immunosuppression; a decrease in tumor burden after GBM resection results in a decrease in Tregs, an effect which would improve antitumor immunity [53]. Interestingly, patients with lower CD4 counts following radiation and temozolomide had poorer survival from earlier tumor progression, not more opportunistic infection [51]. It is difficult, however, to ascertain the relative contribution of radiation effect versus tumor burden on immunosuppression in these patients who have received steroids and chemotherapy as well as varying degrees of tumor resection versus biopsy, particularly without subclassification of the patients’ T cells. It seems possible that radiation-induced lymphopenia may allow for better uptake of adoptive cellular immunotherapy by the host, as was seen with temozolomide therapy [4]. Ultimately, more studies are needed to better characterize the effect of radiation on the overall host immune status so that immunosuppressive effects can be minimized in favor of immunoactivating responses.
Combining radiation and immunotherapy in the clinic
The effect of radiation on the tumor and the host immune response, particularly in the context of other therapies like steroids and chemotherapy, are complex and involve an interplay of immunoactivating and immunosuppressive effects. While more preclinical studies will help to clarify some of the competing mechanisms, overall, the existing preclinical and observational data seem to demonstrate a net positive antitumor effect of radiation and immunotherapy on GBM and warrant moving forward with clinical trials of combined therapy. However, several questions remain in terms of how best to implement combined radioimmunotherapy for GBM. Should immunotherapy be combined with fractionated radiotherapy or SRS? Currently, the lack of survival benefit of SRS for GBM may argue in favor of fractionated radiation therapy, which is, however, delivered over the course of several weeks, making the timing of delivering the immunotherapeutic agent a more complicated one. Dewan et al. showed in two different mouse carcinoma models that the combination of anti-CTLA-4 antibody with fractionated but not single-dose radiotherapy induces a distant antitumor response [54]. Hypofractionated radiotherapy consisting of 5 days of radiosurgery treatment is currently being tested at Stanford and aims to achieve equivalent survival to conventional fractionated radiotherapy with higher quality of life (NCT01120639). This middle ground approach may be an ideal option for combining with immunotherapy.
Should the immunotherapeutic agent be delivered before, concurrently, or after radiation therapy? This also begs the question of what type of immunotherapy ought to be combined with radiation therapy. In regards to timing, the delivery of immunotherapy concurrently or after radiation therapy seems the most logical as the initial radiation “prepares” the tumor for attack by immune cells. Delaying immunotherapy until the completion of radiation therapy, however, may unnecessarily delay any potential therapeutic benefit of the immunotherapy. Different schedules for delivery of immunotherapy and radiation in one animal study, however, did not show a significant effect of relative timing on anti-tumor response [49]. Bouquet el al, showed that the administration of a TGF-β inhibitor prior to radiation increased clonogenic cell death and promoted tumor growth delay in a breast cancer cell model [55] suggesting that immunotherapy can be used as a radiosensitizer. Ultimately, the relative timing of delivery of the immunotherapeutic agent and radiation therapy will depend on the type of immunotherapy being used.
In regards to what type of immunotherapy should be combined with radiation therapy, the discussion of the pros and cons of the various immunotherapeutic modalities tested against GBM are beyond the scope of this review, but it is likely that the enhanced immunogenicity of the tumor that is triggered by radiation exposure stands to benefit vaccine, T cell, cytokine, and antibody therapies alike. While specifics of implementing combined immunotherapy and radiation in GBM clinical trials still need to be worked out, it is likely that combined therapy will be beneficial and more research towards this endeavor is warranted.
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Xu LW, Chow KKH, Lim M, Li G (2014) Current vaccine trials in glioblastoma: a review. J Immunol Res 2014:796856
Chow KH, Gottschalk S (2011) Cellular immunotherapy for high-grade glioma. Immunotherapy 3:423–434
Heimberger AB, Sun W, Hussain SF et al (2008) Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro Oncol 10:98–103
Patel MA, Kim JE, Ruzevick J, Li G, Lim M (2014) The future of glioblastoma therapy: synergism of standard of care and immunotherapy. Cancers (Basel) 6:1953–1985
Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, Fu Y-X, Auh SL (2011) The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res 71:2488–2496
Reits EA, Hodge JW, Herberts CA et al (2006) Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 203:1259–1271
Nicholas S, Mathios D, Ruzevick J, Jackson C, Yang I, Lim M (2013) Current trends in glioblastoma multiforme treatment: radiation therapy and immune checkpoint inhibitors. Brain Tumor Res Treat 1:2–8
Waziri A (2010) Glioblastoma-derived mechanisms of systemic immunosuppression. Neurosurg Clin N Am 21:31–42
Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M (2011) Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol 2011:732413
Liu G, Ying H, Zeng G, Wheeler C, Black K, John S (2004) HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res 64:4980–4986
Saikali S, Avril T, Collet B, Hamlat A, Bansard J-Y, Drenou B, Guegan Y, Quillien V (2007) Expression of nine tumour antigens in a series of human glioblastoma multiforme: interest of EGFRvIII, IL-13Ralpha2, gp100 and TRP-2 for immunotherapy. J Neurooncol 81:139–148
Camara-Quintana JQ, Nitta RT, Li G (2012) Pathology: commonly monitored glioblastoma markers: EFGR, EGFRvIII, PTEN, and MGMT. Neurosurg Clin N Am 23:237–246
Chow KKH, Naik S, Kakarla S et al (2013) T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther 21:629–637
Didenko VV, Ngo HN, Minchew C, Baskin DS (2002) Apoptosis of T lymphocytes invading glioblastomas multiforme: a possible tumor defense mechanism. J Neurosurg 96:580–584
Ichinose M, Masuoka J, Shiraishi T, Mineta T, Tabuchi K (2001) Fas ligand expression and depletion of T-cell infiltration in astrocytic tumors. Brain Tumor Pathol 18:37–42
Saas P, Walker PR, Hahne M et al (1997) Fas ligand expression by astrocytoma in vivo : Maintaining immune privilege in the brain? J Clin Invest 99:1173–1178
Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H (2003) Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res 63:7462–7467
Parsa AT, Waldron JS, Panner A et al (2007) Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 13:84–88
Dunn GP, Dunn IF, Curry WT (2007) Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun 7:12
Fontana A, Hengartner H, de Tribolet N, Weber E (1984) Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol 132:1837–1844
Bodmer S, Strommer K, Frei K, Siepl C, de Tribolet N, Heid I, Fontana A (1989) Immunosuppression and transforming growth factor-beta in glioblastoma. Preferential production of transforming growth factor-beta 2. J Immunol 143:3222–3229
Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE, Bigner DD, Dranoff G, Sampson JH (2006) Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res 66:3294–3302
El Andaloussi A, Lesniak MS (2006) An increase in CD4+ CD25+ FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol 8:234–243
Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT (2010) TGF-b downregulates the activating receptor NKG2D on NK cells and CD8 + T cells in glioma patients. Neuro Oncol 12:7–13
Walker M, Green S, Byar D et al (1980) Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 303:1323–1329
Walker M, Alexander E Jr, Hunt W et al (1978) Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: a cooperative clinical trial. J Neurosurg 49:333–343
Coffey R, Lunsford D, Taylor F (1988) Survival after stereotactic biopsy of malignant gliomas. Neurosurgery 22:465–473
Chang C, Horton J, Schoenfeld D, Salazer O, Perez-Tamayo R, Kramer S, Weinstein A, Nelson J, Tsukada Y (1983) Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas: a joint radiation therapy oncology group and eastern cooperative oncology group study. Cancer 52:997–1007
Wallner K, Galicich J, Krol G, Arbit E, Malkin M (1989) Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys 16:1405–1409
Choucair A, Levin V, Gutin P, Davis R, Silver P, Edwards M, Wilson C (1986) Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J Neurosurg 65:654–658
Narayana A, Yamada J, Berry S, Shah P, Hunt M, Gutin PH, Leibel SA (2006) Intensity-modulated radiotherapy in high-grade gliomas: clinical and dosimetric results. Int J Radiat Oncol Biol Phys 64:892–897
Chan J, Lee S, Fraass B, Normolle D, Greenberg H, Junck L, Gebarski S, Sandler H (2002) Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol 20:1635–1642
Souhami L, Seiferheld W, Brachman D et al (2004) Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys 60:853–860
Klein B, Loven D, Lurie H, Rakowsky E, Nyska A, Levin I, Klein T (1994) The effect of irradiation on expression of HLA class I antigens in human brain tumors in culture. J Neurosurg 80:1074–1077
Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM (2008) Radiation-Induced IFN-production within the tumor microenvironment influences antitumor immunity. J Immunol 180:3132–3139
Garnett CT, Palena C, Chakarborty M, Tsang K, Schlom J, Hodge JW (2004) Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 64:7985–7994
Kim J-Y, Son Y-O, Park S-W, Bae J-H, Chung JS, Kim HH, Chung B-S, Kim S-H, Kang C-D (2006) Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med 38:474–484
Chakraborty M, Abrams S, Norman Coleman C, Camphausen K, Schlom J, Hodge J (2004) External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res 64:4328–4337
Lee Y, Auh SL, Wang Y et al (2009) Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114:589–595
Postow MA, Callahan MK, Barker CA et al (2012) Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 366:925–931
Robin HI, AuBuchon J, Varanasi VR, Weinstein AB (1981) The abscopal effect: demonstration in lymphomatous involvement of kidneys. Med Pediatr Oncol 9:473–476
Wersäll PJ, Blomgren H, Pisa P, Lax I, Kälkner K-M, Svedman C (2006) Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol 45:493–497
Formenti SC, Demaria S (2012) Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys 84:879–880
Formenti SC, Demaria S (2013) Combining radiotherapy and cancer immunotherapy: a paradigm shift. JNCI J Natl Cancer Inst 105:256–265
Demaria S, Pilones KA, Vanpouille-Box C, Golden EB, Formenti SC (2014) The optimal partnership of radiation and immunotherapy: from preclinical studies to clinical translation. Radiat Res 182:170–181
Newcomb EW, Demaria S, Lukyanov Y et al (2006) The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clin Cancer Res 12:4730–4737
Zeng J, See AP, Phallen J et al (2013) Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys 86:343–349
Belcaid Z, Phallen JA, Zeng J et al (2014) Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS ONE 9:e101764
Newcomb EW, Lukyanov Y, Kawashima N, Alonso-Basanta M, Wang S-C, Liu M, Jure-Kunkel M, Zagzag D, Demaria S, Formenti SC (2010) Radiotherapy enhances antitumor effect of anti-CD137 therapy in a mouse Glioma model. Radiat Res 173:426–432
Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S (2011) Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res 17:5473–5480
Yang S, Rafla S, Youssef E, Selim H, Salloum N, Chuang J (1988) Changes in T-cell subsets after radiation therapy. Radiology 168:537–540
Crane CA, Ahn BJ, Han SJ, Parsa AT (2012) Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro Oncol 14:584–595
Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S (2009) Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 15:5379–5388
Bouquet F, Pal A, Pilones KA et al (2011) TGFβ1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res 17:6754–6765
Conflict of interest
None of the authors have any conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chow, K.K.H., Hara, W., Lim, M. et al. Combining immunotherapy with radiation for the treatment of glioblastoma. J Neurooncol 123, 459–464 (2015). https://doi.org/10.1007/s11060-015-1762-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1762-9