Abstract
We revisited a Pinus ponderosa planting 32 years after it was established with one-year-old seedlings grown in copper-treated containers that modified their root systems. This technique was intended to promote more root egress after outplanting from the entire length of the root plug with a goal of providing greater stem stability. After excavating and digitizing the root systems of five treated and five non-treated plants, we observed that regardless of treatment, all trees initiated more roots and accumulated more root volume in apparent response to mechanical stresses invoked by wind and slope, with more roots occurring windward and downslope. Few differences were noted between treatments for root length and volume for either the cage or the entire root system. Trees treated with copper were taller (8%) with stouter taproots (less taper) and less root volume in the lower soil profile than control trees. Although the copper treatment may have induced short-term changes to root system architecture, the long-term, plastic response of this species to mechanical stresses, and the time duration involved, was more critical to the observed expression of traits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wind-caused disturbance is a necessary agent in some forest ecosystems to maintain biological diversity and stand heterogeneity (Johnson and Miyanishi 2010), but this type of disturbance can also have enormous, deleterious economic impacts. More than half of the timber volume loss in European forests during the past two centuries can be linked to windstorms (Schelhaas et al. 2003). The number and intensity of storms affecting European forests increased during the past half century (Gregow et al. 2017) and wind disturbance has a synergistic effect on other disturbance agents, such as fire and insects (Seidl et al. 2017). Moreover, a tree’s susceptibility to windthrow increases with the steepness of the slope (Kenderes et al. 2007). Therefore, traits affecting tree stability are now under consideration in breeding programs (Telewski and Moore 2016) and adaptive silvicultural treatments (Cameron 2002). Recent literature has focused on understanding the contributions of fine and coarse roots, both independently and as part of the overall root system architecture, to stability in response to mechanical force associated with wind and slope conditions (e.g., Lombardi et al. 2017; Dumroese et al. 2019; Deljouei et al. 2020; Montagnoli et al. 2020). Furthermore, various authors have highlighted the importance of focusing on the coarse roots within the cylindrical zone surrounding the taproot (defined as the “cage”) because they provide important contributions to tree anchorage (Danjon et al. 2005; Yang et al. 2014, 2017; Montagnoli et al. 2020;). In particular, the taproot can provide up to 60% of the anchorage strength (Yang et al. 2017). A better understanding of the long-term mechanical stability of planted trees can be discerned by observing impacts of nursery treatments (Khuder et al. 2007).
Nursery production of reforestation seedlings may increase dramatically during the next decade. Many national, multi-national, and global forest restoration initiatives are in progress (Haase and Davis 2017) that often include a reforestation component with a stated goal of sequestering carbon to help mitigate changes to climate. Even modest assumptions of the number of nursery-produced seedlings necessary to meet these initiatives are staggering (Haase and Davis 2017). Seedling quality is paramount in achieving reforestation objectives, and roots have been an attribute of concern for more than 350 years. In the seventeenth century, Evelyn (1664) recommended protection of fine roots during outplanting because of their role in water and nutrient absorption and noted that coarse roots “signify little but to establish the stem.” During the past century, discussions have focused on nursery cultural practices that influence the abundance and distribution of fine and coarse roots and subsequent impacts on seedling survival, growth, and stem stability. Although wind is often a major contributor to the occurrence of stem instability (Chavasse 1978; Burdett et al. 1986), recently outplanted seedlings can present stem instability (i.e., topple; have stems leaning more than 15 degrees from vertical) for a variety of reasons, including characteristics seedlings develop during nursery production (Moore et al. 2008).
Toppling can occur with seedlings produced bareroot or in containers (Chavasse 1978; Watson and Tombleson 2002). In bareroot nurseries, various forms of root pruning and other cultural practices (e.g., seedbed density and fumigation) reduce taproot length and increase root fibrosity (i.e., more secondary and tertiary lateral roots). Toppling remains a concern (Moore et al. 2008) despite these seedlings often being considered “natural” in appearance because of their well-distributed (from a topological perspective) lateral roots, similar to those in naturally regenerated seedlings (Stein 1978; Mexal and South 1991). Container seedlings have also received attention concerning toppling. Widespread production of container seedlings for reforestation in the United States and Canada began in the 1970s. At the onset of this technology, root system development during nursery production sometimes translated to problems on the outplanting site. In particular, widespread toppling and reductions of growth of outplanted Pinus seedlings was attributed to container-induced spiraling of lateral roots (Burdett et al. 1986; Halter et al. 1993; Balisky et al. 1995; Lindström and Rune 1999). In the early 1980s, the addition of vertical ridges to the interior walls of containers reduced spiraling by deflecting lateral roots downward rather than allowing them to circle. These lateral roots grew, however, along the container wall‒medium interface, producing a cage, which also remained a concern for stem instability (Burdett 1978; Chavasse 1978; Balisky et al. 1995; Sayer et al. 2009). To address these observed unnatural root modifications, copper (Cu) compounds have been investigated for a variety of coniferous and broad-leaved tree species (e.g., Burdett and Martin 1982; Arnold and Struve 1989; Svenson et al. 1995; Tsakaldimi and Ganatsas 2006; Dumroese et al. 2013; Marler and Musser 2016). Lateral root growth is arrested when roots encounter container walls coated with copper. The result is seedlings producing more roots of higher root order (Burdett 1978). Copper root pruning is effective in reducing spiraling and the cage effect (Burdett 1978; Ruehle 1985; Wenny and Woollen 1989) and thereby generates a fibrous system; treated seedlings develop more lateral roots in the upper root plug profile (Wenny et al. 1988; Dumroese 2000; Sayer et al. 2009) with a lower incidence of juvenile stem instability (Krasowski 2003).
The topic of using copper during container production continues to receive attention in the literature, particularly on a species-by-species basis. Nearly all the literature is, however, focused on short term effects (1 to 3 years) related to survival, growth, and root system architecture whereas the few longer-term studies (5 to 8 years: Haywood et al. 2012; Regan et al. 2015; Sung et al. 2019) focus solely on survival and aboveground characteristics despite literature showing that the container imprint on root systems can be visible for decades (Halter et al. 1993).
Thus, to expand our knowledge on the potential long-term effects of pruning container seedling roots with copper, particularly whether the treatment establishes a trajectory of root system architecture development that may confer stem stability against mechanical forces rendered by wind and slope, we revisited a container root-pruning study outplanted in 1985 in the northern Rocky Mountains of the United States (USA). We first examined non-treated trees, analyzing the deployment of the coarse roots and found that prevailing wind and slope significantly affected root architecture; we observed substantial amounts of roots windward and downslope (Dumroese et al. 2019). We also found that trees were generating a range of cage shapes characterized by an inverse relationship between volume of first and second order roots with the volume of the taproot (Dumroese et al. 2019). Second, using a dendrochronological approach we noted that new lateral roots arose anywhere and at any time on the existing system in apparent response to mechanical forces (Montagnoli et al. 2019). Thus, our previous efforts based on the control trees showed a continual adjustment of their root spatial deployment in apparent response to environmental factors. In this study we further explore the root system architecture of seedlings produced in a container nursery with copper root pruning. Our null hypothesis was that copper treatment during nursery production would not influence root architecture three decades after outplanting.
Materials and methods
Background and site description
During 1985, an experiment to examine the effects of using copper to modify the root systems of container-grown seedlings was initiated at the University of Idaho nursery in Moscow, Idaho, USA (lat 46.725315, long -116.955836). The study evaluated combinations of three conifer species (using locally collected seeds of Pinus monticola, Pinus ponderosa, and Pseudotsuga menziesii var. glauca), two container types (Styroblock and Ray Leach Pine Cell containers), and five combinations of cupric carbonate (0 to 300 g L−1) delivered in a latex paint carrier to the interior surfaces of the containers. Seedlings were grown and overwintered in refrigerated storage following species-specific regimes (e.g., Wenny and Dumroese 1987). The nursery results were reported by Wenny and Woollen (1989). In March 1986, on a site that was clearcut harvested and broadcast burned the previous year, 10 seedlings of each species × container type × copper level were hand-planted in two replications. At about 1000 m elevation, the outplanting site is in the University of Idaho Experimental Forest in northern Idaho (lat 46.842240, long -116.871035) and is classified as a Clintonia uniflora phase within the Thuja plicata/Clintonia uniflora habitat type (Cooper et al. 1991) that supports mixed conifer forests. With a northeast aspect, slopes of 30 to 50%, and a deep (~ 1.5 m) Vassar series soil (Typic Udivitrands; Andisol) that formed in volcanic ash above weathered granite; see Dumroese et al. 2019, for full soil profile description), this site experiences an annual, average air temperature of 7.2 °C, about 100 frost-free days, and approximately 965 mm of annual precipitation with a seasonal summer (July through September) drought (Soil Survey Staff 2013). In 1986, the average bulk density, organic matter content, and pH in the top 25 cm of mineral soil was 0.94 g cm−3, 4.7%, and 5.9, respectively (Wenny et al. 1988). Winds during the growing season prevail from the west southwest (Western Regional Climate Center 2019).
Initially outplanted with 1-m spacing between seedlings within the row (a single copper treatment‒container combination) and 2-m spacing between rows, 5 seedlings from each species × container type × copper × replication combination were systematically excavated 6 months after outplanting (September 1986) to observe first-season shoot and root growth (Wenny et al. 1988), leaving residual trees on 2-m × 2-m spacing, the typical, initial planting density for northern Idaho at that time. Each seedling was marked with a metal stake. No irrigation, fertilization, weeding, or thinning was done after outplanting.
Excavation and architecture measurements
In July 2017, we revisited the experiment. Of the three species, we chose to sample Pinus ponderosa trees grown in the Styroblock 4A (313A) containers (60 ml volume, 14 cm depth, 936 cavities m−2; Beaver Plastics Ltd., Acheson, Alberta, Canada) because pines have had the most research work on copper root pruning, sufficient trees were available for sampling, and the Styroblock system of growing seedlings is still widely used in North America for production of reforestation stock.
An exhaustive description of our methods for assessing the site, felling the trees, excavating the roots, discretizing the root system, and analyzing the root data can be found in Dumroese et al. (2019). In summary, we revisited eight control trees (no copper) and seven treated (100 g L−1 cupric carbonate) trees located across the original two replications (Fig. 1). We sampled this treatment rate because it was closest to the concentrations reported by McDonald et al. (1981) and Wenny and Woollen (1989) to provide the maximum rooting response. Trees were measured for diameter at breast height (DBH; 137 cm above groundline; cross slope), height (after felling), and their position relative to neighbors (> 5 cm DBH) within a 5 m radius. The DBH of neighboring trees was measured to determine basal area.
Locations of the 15 (n = 8 control [C]; n = 7 treated [T]) 32-year-old Pinus ponderosa trees measured for height and DBH. The basal area of their neighbors was also determined. Of these, 10 trees (n = 5 control; n = 5 treated) were then randomly selected for root system excavation, digitization, and reconstruction using AMAPmod software
We then randomly selected ten trees (five from the control and five from the treatment) for excavation. After trees were felled, root systems were excavated to bedrock (approximately 1 to 1.5 m in depth) and to distances of approximately 1.5 m from the trunk using a high-pressure air lance and nozzle. After cutting roots that remained attached to soil, the root systems were carefully lifted and transported to the U.S. Department of Agriculture Forest Service, Rocky Mountain Research Station, Forestry Sciences Laboratory (Moscow, Idaho). We used a low magnetic field digitizer and AMAPmod software to discretize each root system (Godin and Caraglio 1998). Root topology was recorded using the “acropetal-development approach” (Danjon et al. 2005; Dumroese et al. 2019) with lateral roots emerging from the taproot designated first-order roots, with second-order roots originating from these first-order laterals, and so on (Zobel and Waisel 2010). Digitization allowed us to reconstruct each root system in three-dimensions using the AMAPmod and determine root order, length, and volume by depth and quadrant (Fig. 2).
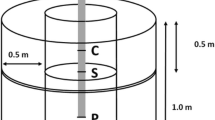
Four different three-dimensional views of a digitized root system from a 32-year-old Pinus ponderosa tree reconstructed using AMAPmod software. Root hierarchy was obtained using the “acropetal-development approach.” Different colors indicate differences in branching order: taproot, pink; first-order roots, green; second-order roots, blue; third-order roots, light blue; and fourth-order roots, yellow. The pink-shaded rectangles indicate the cage delimitation defined as all roots originating within a radial distance of 2.2 × DBH. The X+ axis is oriented downslope parallel to the slope direction
Length and volume data were analyzed using AMAPmod software (Godin et al. 1997). As part of the analysis, we examined the entire root system as well as the cage. The cage included all roots in a cylindrical region centered at the taproot (i.e., the largest vertical root originating directly from the stump) that were in the zone of rapid taper (i.e., characterized by an axis where the portion of the root proximate the taproot shows a very rapid taper) and to a depth corresponding to taproot length (Danjon et al. 2005; Montagnoli et al. 2020). Specifically, the cage zone was defined as all roots originating within a radial distance of 2.2 × DBH. We subsequently refined the AMAPmod data to identify three “types” of lateral roots: (1) entirely within the upper soil depth (< 30 cm); (2) sinker roots, which we classified as those initiated in the upper soil depth and subsequently growing downward into the lower soil depth (> 30 cm); and (3) entirely within the lower soil depth. For each root order and root type we measured root number and length, and estimated volume as a truncated cone (Montagnoli et al. 2019). Taproot taper (T) was calculated (Eq. 1) as the average taper for a series of frusta (n) moving from the soil surface (origin) downward, where for each frustrum, Dp is the proximal diameter, Dd is the distal diameter, and H is the distance between the two diameters (~ 15 cm; Dumroese et al. 2019).
Statistical analyses
Recognizing that we had a small sample size restricted by the number of original seedlings planted, compounded by the limited resources to excavate and analyze large trees, and that no other long-term studies of this type provide inference, we chose an alpha = 0.1 for all analyses. We employed Generalized Linear Mixed Models (GLMM) using PROC GLIMMIX (SAS Institute, Inc., Cary, NC, USA) for a complete randomized design with each of the five trees within a treatment serving as a replicate. To ensure the appropriateness of this approach given the 1986 experimental design (Fig. 1), we validated that replicates were not clustered within treatment as an artifact of the original design (i.e., no pseudoreplication; Hurlbert 1984). First, we used a “test of random labelling” (Baddeley et al. 2015) based on locations of the trees. Results of the test support the hypothesis that treatment levels assigned to each tree are random and independent of other trees, with fixed probability. Second, we computed stem-to-stem distances within three sets (control-to-control trees; treated-to-treated trees; control-to-treated trees) and tested for differences between the three sets of distances using a Kruskal–Wallis rank sum test (one-way rank-based ANOVA; Hollander and Wolfe 1973). We observed no significant difference (p = 0.4545) indicating that no subsampling occurred within clusters or groups of treatment levels. In our models described below, we further removed any potential influence of correlated observations because of spatial proximity and measures on the same tree by incorporating dependent residual error covariances in the models. This method ensured that standard errors of the treatment parameter estimates were unbiased and that appropriate error degrees of freedom were used to assess effects (Zuur et al. 2009, Sect. 5.4). The final error covariance used in each model was one of unstructured, Toeplitz or compound symmetry, depending upon the structure that provided a best fit while accounting for any residual correlation (Stroup 2016). For our test of random labelling, we used the R platform (R Core Team 2021) and library spatstat (Baddeley et al. 2015) with functions Jdot, Jest, and envelope. The Kruskal–Wallis rank sum test was performed using library stats (R Core Team 2021) with function kruskal.test.
The independent variables tree height, DBH, basal area of competitor trees, and taproot length and volume were modeled on treatment. Root length and volume for the entire root system and the cage were modeled on treatment and order. The number, length, volume, and initial and average diameter of cage roots were modeled on treatment, root order, and root type. We also modeled cage root length and volume on treatment, root order, quadrant, and depth. Although Dumroese et al. (2019) reported the data for the control trees with depths analyzed independently, here our goal was to investigate potential interactions of all independent variables. All models were a priori formulated and reported as factorial designs that include all second-order interaction terms.
Gamma distributions, appropriate for continuous, positive skewed distributions (Bolker 2008) as is characteristic of our data, were assumed in all models except for root number. We modeled root number using a negative binomial distribution because it is appropriate for discrete count data and has ability to model overdispersion, which is a feature of our data (White and Bennetts 1996; Bolker 2008; Lindén and Mäntyniemi 2011). As the gamma distribution is restricted to the open interval (0, ∞), we adjusted zero responses according to the method described in Stahel (2002). Our gamma and negative binomial models produced dispersion measures of one indicating adequate specification. Error degrees of freedom were properly calculated by assigning within-subject (tree) degrees of freedom to the treatment effect if treatment changes within a subject, and between-subject degrees of freedom otherwise (Schluchter and Elashoff 1990). For all least squares means generated by the above models, we calculated the lower and upper means (i.e., 90% confidence interval). Box plot visualizations were made using SigmaPlot 14 (Systat Software Inc., San Jose, CA, USA).
Results
For tree growth parameters and competition expressed as basal area, we observed that the most treatment variability was associated with basal area of neighboring (i.e., within 5 m) trees > 5 cm DBH followed by DBH and height (Table 1). Treated trees were about 1 m taller than their control cohorts but DBH was similar.
For length and volume of the entire root system and the cage, treatment was not significant (F1,8 < 1.14, p > 0.3161) but root order was (F2,8 > 31, p < 0.0002) (Table 2). Mean root length of second-order roots for the entire root system of control trees was about 50% greater than that of treated trees, contributing to a treatment × order interaction (Table 2). Root volume decreased as root order increased (Table 2). Taproot length and volume were unaffected by treatment (Table 2), but the taper of control tree taproots (23.2%, 90% confidence interval: 20.6–26.1) was greater (F1,8 = 3.62, p = 0.0935) than that of treated trees (19.6%, 90% confidence interval: 17.4–22.0).
Within the cage, lateral root number, length, volume, and diameter were affected by order, type, and the order × type interaction (Table 3). For root order and regardless of treatment, trees had fewer third order roots, which were shorter and thinner, and therefore had less volume, than their first and second order cohorts. Root volume decreased as root order increased. For root type and regardless of treatment, trees initiated more roots at soil depth > 30 cm and although their diameters were similar to roots initiated at soil depth < 30 cm, their high abundance translated into more length, and in the case of control trees, more volume, than roots that initiated and remained within the shallow soil profile or became sinker roots (Table 3).
Within the cage, when the model included treatment, root order, quadrant, and depth, we found that the role of treatment as an independent variable for length and volume was less pronounced (F1,8 < 0.46, p > 0.2642) when compared with the roles of the other independent variables: root order (F2,16 > 53, p < 0.0001), quadrant (F3,24 > 7.63, p < 0.0009), and depth (F1,8 > 11.28, p < 0.0100). The two-way interaction of depth × root order for root length (F2,18 = 3.79, p = 0.0423) showed that for all root orders, root length increased at the lower depth with second-order roots showing the highest values and the largest increase (Fig. 3a). The same interaction for root volume (F2,18 = 12.77, p = 0.0004) revealed that within each depth, volume decreased with increasing root order and generally decreased from the upper to the lower depth, with exception of the second-order root volume that instead slightly increased (Fig. 3b). Depth and quadrant interacted to affect root length (F3,27 = 35.83, p < 0.0001) and root volume (F3,27 = 28.72, p < 0.0001) and the interactions showed that in the shallow depth, most length and volume was associated with the downslope and windward quadrants. But in the lower depth, the upslope quadrant had the most length and volume with the other three quadrants having fairly similar values (Fig. 3c and d). Although the two-way interaction of root order × quadrant for root length was absent (F6,54 = 1.71, p = 0.1356) (Fig. 3e), it was present for volume (F6,54 = 3.21, p = 0.0091). Volume always decreased by order regardless of quadrant, but the magnitude of that decrease among orders within quadrants differed (Fig. 3f).
Interactions of root depth × root order (a and b), root depth × quadrant (c and d), and root order × quadrant (e and f) for root length (a, c, and e) and volume (b, d, and f). Vertical boxes represent 50% of the observations (25th to 75th percentiles) and error bars extending from each box are the upper (90th) and lower (10th) percentiles. Black circles represent values less than the 10th percentile or greater than the 90th percentile. The solid horizontal line in the center of each box is the median value and the dotted line is the mean
Discussion
At the conclusion of the growth cycle in the nursery, and after one growing season on the outplanting site, the root systems of the population of P. ponderosa seedlings that were the basis of our current study showed significant treatment effects when grown in Styroblock containers. Specifically, in a root growth potential test performed at the conclusion of the nursery cycle, Wenny and Woollen (1989) found that new root growth was about 6X greater in the upper two-thirds of the root plugs exposed to copper when compared to their non-treated controls. This was attributed to a resumption of lateral root growth in the upper plug that had been temporarily arrested by the presence of copper on the container wall. After one season on the outplanting site, the number of new roots emanating from the upper root plug was similar regardless of treatment, but the number of new roots growing from the bottom of the plug was reduced by half when copper was present (Wenny et al. 1988). These findings suggested that the number of lateral roots deflected downward along the side of the container wall was reduced by the presence of copper, and concur with numerous studies (e.g., Burdett 1978; Ruehle 1985; Svenson et al. 1995; Dumroese and Wenny 1997).
In this study, trees treated with copper as seedlings were taller than their non-treated cohorts three decades after outplanting, although no observed treatment effects were reported for height after nursery production (Wenny and Woollen 1989) or the first growing season (Wenny et al. 1988). Other studies with pines have reported that copper promoted taller seedlings during the nursery phase compared with control seedlings, and that this height differential persisted at least 6 growing seasons (e.g., Haywood et al. 2012; Regan et al. 2015). Given that larger seedlings generally remain larger after outplanting (e.g., Pinto et al. 2011; Sung et al. 2019), our results were not surprising.
For the entire root system, we observed no copper effects on total root length and volume. Upon outplanting, new roots emanating from the original root plug were affected by the nursery imprint as noted by Wenny et al. (1988), but undoubtedly were also influenced by genetics and environmental stimuli perceived by the seedlings (Gardiner et al. 2016; Rellán-Álvarez et al. 2016). Recently on a subset of these trees, we identified first-order lateral roots that originated during the first growing season on the outplanting site. These roots, along with other first-order lateral roots, have an innate ability, based on similar patterns of ring eccentricity (i.e., occurring in a non-circular pattern with an offset centroid), to respond to mechanical forces (Montagnoli et al. 2019). Our current results with copper treated trees confirm our earlier observations that slope and prevailing wind were important mechanical forces (Dumroese et al. 2019); such forces are common to other studies with other species (Chiatante et al. 2003; Danjon et al. 2005; Di Iorio et al. 2005; Lombardi et al. 2017). Toward improving stability, our control and copper-treated trees have, similar to the results of others (Chiatante et al. 2003; Scippa et al. 2006; Sun et al. 2008; Yang et al. 2014), partitioned more root resources downslope and windward in response to these mechanical forces. In addition to this coarse response, we demonstrated earlier that lateral roots of P. ponderosa can respond at a finer level, changing growth direction in response to mechanical forces as well as producing new lateral roots at any development stage and wherever along their axis. These findings suggest a high degree of plasticity in the entire root system and that trees respond to changes in environmental conditions by making ongoing spatial adjustments in root deployment (Montagnoli et al. 2019). Therefore, it is not too surprising that, for observations of the root traits length and volume for the entire root system after 32-years on the site, few significant copper treatment effects were discerned.
Critical to tree anchorage is the root cage. The root cage is described as the zone around the stump where the taproot and most of the sinker roots descend into the soil in a parallel pattern, as well as the portion of all the shallow roots that branch off from the taproot and undergo the most rapid decrease of diameter (Danjon et al. 2005). As with the entire root system, we observed no differences in root traits within the cage between treatments, suggesting that the response to external mechanical forces caused by the weight of the tree itself (self-loading), the slope, and the dominant wind were similar. Indeed, these forces are transmitted from the stem to the roots, which dissipates them into the soil to avoid tree uprooting, and this dissipation can be enhanced by shallow root quantity, size, distribution, and individual root structure (Danjon et al. 2005; Yang et al. 2014, 2017; Montagnoli et al. 2020). Within this zone of rapid taper, roots present high amounts of eccentricity and corresponding large cross-sectional areas, likely due to the formation of compression wood (Westing 1968; De Zio et al. 2020). The direction of this eccentricity changes from the top portion of the lateral root at the branching point with the taproot to the bottom portion at the cage edge (Montagnoli et al. 2019). In this regard, it is important to note that the stiffness of a root is proportional to its diameter to the fourth power (Coutts 1983); consequently, these roots provide significant mechanical stability. Our hypothesis put forth in Montagnoli et al. (2020), supported by a mathematical model, suggests these roots with high eccentricity are a response to the self-loading force of the trees’ aboveground biomass in concert with the variation in mechanical forces occurring in the various zones (i.e., downslope, upslope, windward, leeward) of the cage. Shallow lateral roots within the cage also present specialty root shapes (i.e., I-beam and T-beam; Dumroese et al. 2019) believed to increase further the trees’ stability against mechanical forces (Nicoll and Ray 1996; Stokes et al. 1996).
Within the cage, lateral roots along with sinker roots play a dominant role in tree anchorage, with the taproot being the first mechanical contributor to tree anchorage strength (Yang et al. 2014, 2017). After 32 years on the site, our data show that control trees had more taproot taper but more root volume in the lower soil profile than copper treated trees. The presence of more lateral roots in the lower soil profile of control trees may have offset the decreased contribution to stability afforded by their taproots (Di Iorio et al. 2005). Despite this difference, the high volume of first-order roots, regardless of nursery treatment, likely led to a smooth and rapid dissipation of force into the ground (Coutts 1983) that circumvents the root system being forced out of the soil or breaking (Wu 1976; Ennos 1990).
Finally, our values for root length and volume by quadrant and soil depth were unaffected by copper treatment. Examining potential treatment effects, we found that quadrant, root order, and depth were the most pronounced independent variables. As we previously concluded (Montagnoli et al. 2019, 2020), P. ponderosa responds to the mechanical forces of self-loading, slope, and wind through on-going adaptation of its root system that include an increase in root eccentricity and asymmetric root volume distribution.
Research continues to demonstrate that pine seedlings with some form of root pruning (either with copper or air pruning) initially have characteristics that promote more horizontal root orientation after outplanting (Chapman and Colombo 2007). This apparent short-term effect may be offset by site characteristics, especially slope and prevalent wind condition. Therefore, we concur with the conclusion of Jones et al. (2002) that conditions on the outplanting site influence seedling performance more than root modification by copper because of the rapid dissipation of nursery-induced changes to root systems.
Conclusions
Given that our comprehensive observations on the root traits within the entire root system and the cage likely reveal mostly genetic responses to mechanical forces, it is not too surprising that after three decades we noted few significant differences due to the original nursery treatment involving copper root pruning. We failed to completely reject our null hypothesis because we observed that tree height, taproot taper, and a few root characteristics appear to be related to nursery treatment, but overall long-term (32 years) measures of root order, length, and volume indicate little effect on trees that received a nursery treatment of copper that modified their root systems. All trees initiated more roots and accumulated more root volume in apparent response to mechanical stresses invoked by slope and wind, with more roots occurring downslope and windward. Given that recent work has revealed that P. ponderosa root system architecture is continually adapting in terms of root number, placement, volume, and eccentricity in response to apparent changes in mechanical forces, our inability to discern differences in location, length, and volume of roots based on original nursery treatments is reasonable. Long-term plasticity of P. ponderosa root system architecture off-sets any short-term changes caused by nursery treatments. Despite this conclusion, early alterations in the root system because of the copper treatment could have been functional (at least during the first few years after outplanting) in regard to tree stability (especially on slopes) and resource acquisition.
Data availability
Data are available at the USFS Research Data Archive (Montagnoli et al. 2021).
References
Arnold MA, Struve DK (1989) Growing green ash and red oak in CuCO3-treated containers increases root regeneration and shoot growth following transplant. J Am Soc Hort Sci 114:402–406
Baddeley A, Rubak E, Turner R (2015) Spatial point patterns: methodology and applications with R. Chapman and Hall, London, pp 608–609
Balisky AC, Salonius P, Walli C, Brinkman D (1995) Seedling roots and forest floor: misplaced and neglected aspects of British Columbia’s reforestation effort? Forest Chron 71:59–65. https://doi.org/10.5558/tfc71059-1
Bolker BM (2008) Ecological models and data in R. Princeton University Press, New York, p 408
Burdett AN (1978) Control of root morphogenesis for improved mechanical stability in container-grown lodgepole pine. Can J Forest Res 8:483–486. https://doi.org/10.1139/x78-072
Burdett AN, Martin PAF (1982) Chemical root pruning of coniferous seedlings. HortScience 17:622–624
Burdett AN, Coates H, Eremko R, Martin PAF (1986) Toppling in British Columbia’s lodgepole pine plantations: significance, cause and prevention. Forest Chron 62:433–439. https://doi.org/10.5558/tfc62433-5
Cameron AD (2002) Importance of early selective thinning in the development of long-term stand stability and improved log quality: a review. Forestry 75:25–35. https://doi.org/10.1093/forestry/75.1.25
Chapman KA, Colombo SJ (2007) Early root morphology of jack pine seedlings grown in different types of container. Scand J for Res 21:372–379. https://doi.org/10.1080/02827580600981888
Chavasse CGR (1978) The root form and stability of planted trees with special reference to nursery and establishment practice. In: Van Eerden E, Kinghorn JM (eds) Proceedings of the root form of planted trees symposium. Joint Report 8. British Columbia Ministry of Forests/Canadian Forest Service, Victoria, British Columbia, pp 54–64
Chiatante D, Scippa GS, Di Iorio A, Sarnataro M (2003) The influence of steep slopes on root system development. J Plant Growth Regul 21:247–260. https://doi.org/10.1007/s00344-003-0012-0
Cooper SV, Neiman KE, Roberts DW (1991) Forest habitat types of northern Idaho: a second approximation. General Technical Report INT–236. US Department of Agriculture, Forest Service, Intermountain Research Station, Ogden, Utah, pp 143. https://doi.org/10.2737/INT-GTR-236
Coutts MP (1983) Root architecture and tree stability. Plant Soil 71:171–188. https://doi.org/10.1007/BF02182653
Danjon F, Fourcaud T, Bert D (2005) Root architecture and wind-firmness of mature Pinus pinaster. New Phytol 168:387–400. https://doi.org/10.1111/j.1469-8137.2005.01497.x
De Zio E, Montagnoli A, Karady M, Terzaghi M, Sferra G, Antoniadi I, Scippa GS, Ljung K, Chiatante D, Trupiano D (2020) Reaction wood anatomical traits and hormonal profiles in poplar bent stem and root. Front Plant Sci 11:590985. https://doi.org/10.3389/fpls.2020.590985
Deljouei A, Abdi E, Schwarz M, Majnounian B, Sohrabi H, Dumroese RK (2020) Mechanical characteristics of the fine roots of two broadleaved tree species from the temperate Caspian Hyracanian Ecoregion. Forests 11:345. https://doi.org/10.3390/f11030345
Di Iorio A, Lasserre B, Scippa GS, Chiatante D (2005) Root system of Quercus pubescens trees growing on different sloping conditions. Ann Bot 95:351–361. https://doi.org/10.1093/aob/mci033
Dumroese RK (2000) Changes in interior Douglas-fir root development in containers after copper and auxin treatments. West J Appl for 15:213–216. https://doi.org/10.1093/wjaf/15.4.213
Dumroese RK, Wenny DL (1997) An assessment of ponderosa pine seedlings grown in copper-coated polybags. Tree Planters’ Notes 48(3–4):60–64
Dumroese RK, Sung S-JS, Pinto JR, Ross-Davis A, Scott AD (2013) Morphology, gas exchange, and chlorophyll content of longleaf pine seedlings in response to rooting volume, copper root pruning, and nitrogen supply in a container nursery. New For 44:881–897. https://doi.org/10.1007/s11056-013-9377-5
Dumroese RK, Terzaghi M, Chiatante D, Scippa GS, Lasserre B, Montagnoli A (2019) Functional traits of Pinus ponderosa coarse-roots in response to slope conditions. Front Plant Sci 10:947.
Ennos AR (1990) The anchorage of leek seedlings: the effect of root length and tensile strength. Ann Bot 65:409–416. https://doi.org/10.1093/oxfordjournals.aob.a087951
Evelyn J (1664) Sylva: or a discourse of forest trees & propagation of timber, vol 1. Reprinted by Arthur Doubleday & Co, Ltd, London
Gardiner B, Berry P, Moulia B (2016) Review: wind impacts on plant growth, mechanics and damage. Plant Sci 245:94–118. https://doi.org/10.1016/j.plantsci.2016.01.006
Godin C, Caraglio Y (1998) A multiscale model of plant topological structures. J Theor Biol 191:1–46. https://doi.org/10.1006/jtbi.1997.0561
Godin C, Costes E, Caraglio Y (1997) Exploring plant topological structure with the AMAPmod software: an outline. Silva Fenn 31:357–368
Gregow H, Laaksonen A, Alper ME (2017) Increasing large scale windstorm damage in western, central and northern European forests, 1951–2010. Sci Rep 7:46397. https://doi.org/10.1038/srep46397
Haase DL, Davis AS (2017) Developing and supporting quality nursery facilities and staff are necessary to meet global forest and landscape restoration needs. Reforesta 4:69–93
Halter MR, Chanway CP, Harper GJ (1993) Growth reduction and root deformation of containerized lodgepole pine saplings 11 years after planting. For Ecol Manag 56:131–146. https://doi.org/10.1016/0378-1127(93)90108-Y
Haywood JD, Sung S-JS, Sayer MAS (2012) Copper root pruning and container cavity size influence longleaf pine growth through five growing seasons. South J Appl For 36:146–151. https://doi.org/10.5849/sjaf.10-051
Hollander M, Wolfe DA (1973) Nonparametric statistical methods. Wiley, New York, pp 115–120
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211. https://doi.org/10.2307/1942661
Johnson EA, Miyanishi K (2010) Plant disturbance ecology: the process and the response. Elsevier/AP, Amsterdam, p 720
Jones MD, Kiiskila S, Flanagan A (2002) Field performance of pine stock types: two-year results of a trial on interior lodgepole pine seedlings grown in Styroblocks™, Copperblocks™, or AirBlocks™. BC J Ecosyst Manag 2:12
Kenderes K, Aszalós R, Ruff J, Barton Z, Standovár T (2007) Effects of topography and tree stand characteristics on susceptibility of forests to natural disturbances (ice and wind) in the Börzsöny Mountains (Hungary). Community Ecol 8:209–220. https://doi.org/10.1556/comec.8.2007.2.7
Khuder H, Stokes A, Danjon F, Gouskou K, Lagane F (2007) Is it possible to manipulate root anchorage in young trees? Plant Soil 294:87–102. https://doi.org/10.1007/s11104-007-9232-6
Krasowski MJ (2003) Root system modifications by nursery culture reflect on post-planting growth and development of coniferous seedlings. Forest Chron 79:882–891. https://doi.org/10.5558/tfc79882-5
Lindén A, Mäntyniemi S (2011) Using the negative binomial distribution to model overdispersion in ecological count data. Ecology 92:1414–1421. https://doi.org/10.1890/10-1831.1
Lindström A, Rune G (1999) Root deformation in plantations of container-grown Scots pine trees: effects on root growth, tree stability and stem straightness. Plant Soil 217:29–37. https://doi.org/10.1023/A:1004662127182
Lombardi F, Scippa GS, Lasserre B, Montagnoli A, Tognetti R, Marchetti M, Chiatante D (2017) The influence of slope on Spartium junceum root system: morphological, anatomical and biomechanical adaptation. J Plant Res 130:515–525. https://doi.org/10.1007/s10265-017-0919-3
Marler T, Musser C (2016) Chemical and air pruning of roots influence post-transplant root traits of the critically endangered Serianthes nelsonii. Plant Root 10:21–25. https://doi.org/10.3117/plantroot.10.21
McDonald SE, Tinus RW, Reid CPP (1981) Root morphology control in forest tree seedling containers. In: Proceedings of Intermountain Nurseryman's Association and Western Forest Nursery Association. General Technical Report INT-109. US Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station, Ogden, Utah, pp 40–45
Mexal JG, South DB (1991) Bareroot seedling culture. In: Duryea ML, Dougherty PM (eds) Forest regeneration manual. Kluwer Academic Publishers, The Netherlands, pp 89–115. https://doi.org/10.1007/978-94-011-3800-0_6
Montagnoli A, Terzaghi M, Chiatante D, Scippa GS, Lasserre B, Dumroese RK (2019) On-going modifications to root system architecture of Pinus ponderosa growing on a sloped site revealed by tree-ring analysis. Dendrochronologia 58:125650. https://doi.org/10.1016/j.dendro.2019.125650
Montagnoli A, Lasserre B, Sferra G, Chiatante D, Scippa GS, Terzaghi M, Dumroese RK (2020) The response of roots to mechanical stresses in Pinus ponderosa growing on slopes: formation of annual ring eccentricity within the root cage. Plants 9:181. https://doi.org/10.3390/plants9020181
Montagnoli A, Terzaghi M, Chiatante D, Dumroese RK (2021) Characteristics of 32-year-old Pinus ponderosa root systems in northern Idaho, USA. Fort Collins, CO: US Department of Agriculture, Forest Service, Research Data Archive. https://doi.org/10.2737/RDS-2021-0047
Moore JR, Tombleson JD, Turner JA, van der Colff M (2008) Wind effects on juvenile trees: a review with special reference to toppling of radiata pine growing in New Zealand. Forestry 81:377–387. https://doi.org/10.1093/forestry/cpn023
Nicoll BC, Ray D (1996) Adaptive growth of tree root systems in response to wind action and site conditions. Tree Physiol 16:891–898. https://doi.org/10.1093/treephys/16.11-12.891
Pinto JR, Marshall JD, Dumroese RK, Davis AS, Cobos DR (2011) Establishment and growth of container seedlings for reforestation: a function of stocktype and edaphic conditions. For Ecol Manag 261:1876–1884. https://doi.org/10.1016/j.foreco.2011.02.010
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 1 Aug 2021
Regan DJ, Apostol KG, Davis AS (2015) Stocktype influences western white pine seedling size 6 years after outplanting. Tree Planters’ Notes 58(1):37–41
Rellán-Álvarez R, Lobet G, Dinneny JR (2016) Environmental control of root system biology. Annu Rev Plant Biol 67:1–26. https://doi.org/10.1146/annurev-arplant-043015-111848
Ruehle JL (1985) The effect of cupric carbonate on root morphology of containerized mycorrhizal pine seedlings. Can J For Res 15:586–592. https://doi.org/10.1139/x85-095
Sayer MAS, Haywood JD, Sung S-JS (2009) Cavity size and copper root pruning affect production and establishment of container-grown longleaf pine seedlings. For Sci 55:377–389. https://doi.org/10.1093/forestscience/55.5.377
Schelhaas MJ, Nabuurs GJ, Schuck A (2003) Natural disturbances in the European forests in the 19th and 20th centuries. Global Change Biol 9:1620–1633. https://doi.org/10.1046/j.1365-2486.2003.00684.x
Scippa GS, Di Michele M, Di Iorio A, Costa A, Lasserre B, Chiatante D (2006) The response of Spartium junceum roots to slope: anchorage and gene factors. Ann Bot 97:857–866. https://doi.org/10.1093/aob/mcj603
Schluchter MD, Elashoff JD (1990) Small-sample adjustments to tests with unbalanced repeated measures assuming several covariance structures. J Stat Comput Sim 37:69–87. https://doi.org/10.1080/00949659008811295
Seidl R, Dominik T, Kautz M, Martin-Benito D, Peltoniemi M, Vacchiano G, Wild J, Ascoli D, Petr M, Honkaniemi J, Lexer MJ, Trotsiuk V, Mairota P, Svoboda M, Fabrika M, Nagel TA, Reyer CPO (2017) Forest disturbances under climate change. Nat Clim Change 7:395–402. https://doi.org/10.1038/nclimate3303
Soil Survey Staff, US Department of Agriculture, Natural Resources Conservation Service (2013) Official soil series descriptions. https://soilseries.sc.egov.usda.gov/OSD_Docs/V/VASSAR.html. Accessed 15 Nov 2021
Stahel WA (2002) Statistische datenanalyse: eine einführung für naturwissenschaftler. Springer Nature, Vieweg, Braunschweig, Germany
Stein WI (1978) Naturally developed seedling roots of five western conifers. In: Van Eerden E, Kinghorn JM (eds) Proceedings of the root form of planted trees symposium. Joint Report 8. British Columbia Ministry of Forests/Canadian Forest Service, Victoria, British Columbia, pp 28–35
Stokes A, Ball J, Fitter AH, Brain P, Coutts MP (1996) An experimental investigation of the resistance of model root systems to uprooting. Ann Bot 78:415–421. https://doi.org/10.1006/anbo.1996.0137
Stroup WW (2016) Generalized linear mixed models: modern concepts, methods and applications. CRC Press, London
Sun H-L, Lia S-C, Xiong W-L, Yang Z-R, Cui B-S, Yang T (2008) Influence of slope on root system anchorage of Pinus yunnanensis. Ecol Eng 32:60–67. https://doi.org/10.1016/j.ecoleng.2007.09.002
Sung S-JS, Dumroese RK, Pinto JR, Sayer MAS (2019) The persistence of container nursery treatments on the field performance and root system morphology of longleaf pine seedlings. Forests 10:807. https://doi.org/10.3390/f10090807
Svenson SE, Johnston DL, Coy BL (1995) Shoot and root responses of eight subtropical species grown in cupric hydroxide-treated containers. HortScience 30:249–251
Telewski FW, Moore JR (2016) Trait selection to improve windfirmness in trees. CAB Rev 11:1–10. https://doi.org/10.1079/PAVSNNR201611050
Tsakaldimi MN, Ganatsas PP (2006) Effect of chemical root pruning on stem growth, root morphology and field performance of the Mediterranean pine Pinus halepensis Mill. Sci Hortic-Amsterdam 109:183–189. https://doi.org/10.1016/j.scienta.2006.04.007
Watson AJ, Tombleson JD (2002) Toppling in juvenile pines: A comparison of the root system characteristics of direct-sown seedlings, and bare-root seedlings and cuttings. Plant Soil 239:187–196. https://doi.org/10.1023/A:1015036105630
Wenny DL, Dumroese RK (1987) A growing regime for containerized ponderosa pine seedlings. Bulletin 43. University of Idaho, Idaho Forest, Wildlife and Range Experiment Station, Moscow, Idaho, USA, 9 p
Wenny DL, Woollen RL (1989) Chemical root pruning improves the root system morphology of containerized seedlings. West J Appl For 4:15–17. https://doi.org/10.1093/wjaf/4.1.15
Wenny DL, Liu Y, Dumroese RK, Osborne HL (1988) First year field growth of chemically root pruned containerized seedlings. New For 2:111–118. https://doi.org/10.1007/BF00027762
Western Regional Climate Center (2019) Prevailing wind direction. https://wrcc.dri.edu/Climate/comp_table_show.php?stype=wind_dir_avg. Accessed 15 Nov 2021
Westing AH (1968) Formation and function of compression wood in Gymnosperms. Bot Rev 34:51–78. https://doi.org/10.1007/BF02858621
White GC, Bennetts RE (1996) Analysis of frequency count data using the negative binomial distribution. Ecology 77:2549–2557. https://doi.org/10.2307/2265753
Wu TH (1976) Investigation of landslides on Prince of Wales Island. Report 5. Civil Engineering Department, Geotechnical Engineering, Ohio State University, Columbus, Ohio, USA
Yang M, Défossez P, Danjon F, Fourcaud T (2014) Tree stability under wind: simulating uprooting with root breakage using a finite element method. Ann Bot 114:695–709. https://doi.org/10.1093/aob/mcu122
Yang M, Défossez P, Danjon F, Dupont S, Fourcaud T (2017) Which root architectural elements contribute the best to anchorage of Pinus species? Insights from in silico experiments. Plant Soil 411:275–291. https://doi.org/10.1007/s11104-016-2992-0
Zobel RW, Waisel Y (2010) A plant root system architectural taxonomy: a framework for root nomenclature. Plant Biosyst 144:507–512. https://doi.org/10.1080/11263501003764483
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
For their assistance with data collection, we thank Melissa Topping, and Dr. Robert Keefe and his crew at the University of Idaho Experimental Forest. Our gratitude to Dr. Frédéric Danjon of the French National Institute of Agronomic Research (INRA) at Bordeaux for help with digitalization and data processing. Our thanks to Jim Marin Graphics for assistance with visualizations, and to the reviewers and associate editor whose insightful comments helped improve the manuscript. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official U.S. Department of Agriculture or U.S. Government determination or policy.
Funding
This study was supported by the University of Insubria, the University of Molise, the U.S. Department of Agriculture Forest Service (USFS) Rocky Mountain Research Station, and the USFS National Center for Reforestation, Nurseries, and Genetic Resources.
Author information
Authors and Affiliations
Contributions
RKD and DC conceived the research project. RKD provided primary funding. AM, RKD, and DC developed the study plan. GSS provided important insights into the study plan and research process. AM was responsible for field excavations and data collection and analysis. AM and MT equally contributed to the field activities. AM performed the three-dimensional digitalization. BL performed the three-dimensional data arrangement. MA, LSB, and BL analyzed the data. RKD, AM, MA, and DC prepared the manuscript. RKD, LSB, and AM responded to reviewer comments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent for publication
All authors have read this manuscript and consent to its publication in New Forests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dumroese, R.K., Terzaghi, M., Acevedo, M. et al. Root system architecture of Pinus ponderosa three decades after copper root pruning in a container nursery. New Forests 53, 983–1001 (2022). https://doi.org/10.1007/s11056-022-09904-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-022-09904-2