Abstract
The East Indian sandalwood (Santalum album L), one among the valued timber species in the global market due to its sweet-scented heartwood, is facing a drastic decline of its natural populations over the past three decades. The major constraint in the regeneration of sandal is the slow, staggered, and poor germination of the seeds. The germination may continue even up to one year. The present investigation focused on the impact of biopriming on the germination and seedling performance in sandal. Fresh mature seeds of sandal were procured from the Nachivayal Reserve Forest, Marayur Sandal Division. The seeds were subjected to 16 biopriming treatments using Pseudomonas fluorescens at 4 concentrations (25, 50, 75 and 100%) and 4 durations (2, 4, 6, and 8 days). The results revealed that the biopriming at 100% for 8 days recorded the highest germination percentage (88%) within 21 days with lowest energy period (15 days) and highest germination energy (62.98%). Biopriming at 50% concentration for 8 days was the next best treatment which improved the germination of sandal seeds and the lowest germination percentage was recorded in control seeds (46%). With regard to the seedling growth, the biopriming treatment contributed a significant increase in growth attributes of the seedlings. We recommend the biopriming with Pseudomonas fluorescens at 100% for 8 days as a potential technique to enhance seed germination and seedling growth in sandal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Santalum album L. (sandalwood) belonging to family Santalaceae is a valuable tropical tree species, native to Indian subcontinent (Rai 1990). It is an evergreen tree attaining 12–15 m height and 1–2.4 m girth with slender drooping as well as erect branching. It is valued for the heartwood, posing a delightful aroma and fixative property for ages and it is one of the finest natural materials for carving. Besides, the sweet aromatic essential oil obtained from heartwood is widely used to cure skin diseases and in soap making and perfumery. In India, the sandal is mainly distributed in the Deccan plateau with a total extent of 9600 km2 of which Karnataka and Tamil Nadu cover 8200 km2 (Srinivasan et al. 1992). In Kerala, it occurs sporadically in the deciduous forest up to 900 m elevation and fairly common at Marayur. The tree burgeons well from the sea level up to 1800 m altitude.
The estimated global demand for sandalwood is about 5000–6000 tyear−1 and that of its oil is 100 t year−1. However, the production of sandalwood had declined from 3176 t year−1 in 1960–1965 to 1500 t year−1 in 1997–1998 and eventually to 500 t year−1 in 2007 and 100 t year−1 in 2011–2012 (Joshi and Arun Kumar 2007). Sandal heartwood prices have increased from 6 $ t−1 in 1900 to $ 1050 t1 in 2000 and to $ 60,000 t−1 in 2007 (Joshi and Arun Kumar 2007). The global demand of sandalwood is outstripping its supply resulting in the overexploitation of the sandal populations. The natural populations of sandal is dwindling over the past three decades due to overexploitation, illegal trading, poor regeneration and the spike disease causing an 80% reduction in the population in its natural habitat. This led to the categorization of species as ‘Vulnerable’ in the IUCN red list, 2010. Economically viable sandal trees are reported to be commercially extinct due to illegal harvesting and overexploitation in India.
Major constraints in raising large scale plantations of sandal are the poor germination rate and prolonged germination period of the seeds and slow rate of establishment of the seedlings in the field. The hard seed coat makes the seed difficult to germinate and the dormancy of the seeds is a major cause of the poor germination of sandal. Baskin and Baskin (1988) concluded that the sandal seeds possess physiological dormancy or morpho-physiological dormancy i.e. seeds contain a miniscule embryo which elongates inside the seed before, during or after the loss of physiological dormancy. Das and Tah (2013) stated that the imposed dormancy of sandal seed is possibly due to the presence of chemical inhibitors in the seed coat which are impervious to water and gases. Dileepa et al. (2015) confirmed the occurrence of morpho-physiological dormancy in S. album, and suggested that the level is non-deep simple. The artificial propagation methods have been unsuccessful in sandal, hitherto. Different pretreatments like soaking in cow dung, acid scarification, hot water soaking, and gibberellic acid have been practiced in sandal till date and currently, gibberellic acid 500 mg l−1 is the best pretreatment for sandal seeds (Sutheesh et al. 2016).
Seed priming is one of the simple and key technologies to achieve seed enhancement through rapid germination and optimizing the seedling establishment in the field (Ma et al. 2003). Seed biopriming is one of the most suitable methods for the application and subsequent establishment of bacterial antagonists in the spermospheres and rhizospheres (El Zain 2006). Plant growth-promoting rhizobacteria (PGPR) are free-living, soil-borne bacteria, which enhance the growth of the plant either directly or indirectly (Glick and Ibid 1995). There is an increased interest in the study of PGPR recently due to their ability to improve the growth and yield of various crops. Pseudomonas spp. forms the most studied PGPR, particularly Pseudomonas fluorescens strains. Beneficial effects of these rhizobacteria have been attributed to their ability to produce compounds including phytohormones, organic acids, siderophores, fixation of atmospheric nitrogen, phosphate solubilization, antibiotics and some other unidentified mechanisms (Glick and Ibid 1995). The increased growth and biomass production due to application of biopriming methods has been reported in many crops (Asha et al. 2011, Rozier et al. 2019). Rodriguez et al. (2015) studied the effect of both hydropriming and biopriming on seed germination and seedling vigor of two Mexican fir tree species, Abies hickelii and A. religiosa. They suggested that biopriming in combination with hydropriming has become a viable treatment for increasing seed germination rate and seedling vigour. With this background, present study was formulated to assess the impact of biopriming techniques on the germination and seedling growth in sandal.
Materials and methods
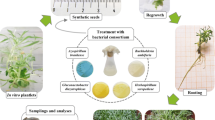
Present study was carried out at Department of Silviculture & Agroforestry, College of Forestry, Kerala Agricultural University, India. Seeds of sandal were collected from the selected trees in the Nachivayal Reserve Forest I and II of Marayoor Sandal Division by the Pallanadu Vana Samrakshana Samithi, Idukki district. Sandal seeds were made available to the College of Forestry. The seeds were fresh and mature, collected during February to March 2018. Physical examination of the seeds indicated that they are globose to spherical with an average weight of 0.1 g and having an average diameter of 0.74 cm. Collected seeds were cleaned and thoroughly mixed to improve the homogeneity.
Talc-based culture of Pseudomonas fluorescens was @ 108 c.f.u ml−1 obtained from the Department of Microbiology, College of Horticulture, Kerala Agricultural University. Prior to biopriming, seeds were surface sterilized in 1% mercuric chloride solution for 3 minutes and thoroughly washed before subjecting to biopriming. The experiment was conducted in two factorial Completely Randomized Design with four concentrations of the powder formulations viz., 25, 50, 75 and 100% as first factor and 2, 4, 6 and 8 days duration as the second. Powder formulation of 20 g was estimated as 100% of P. fluorescens for 50 seeds. sandal seeds were mixed with suspension culture in the sterilized glass bottles of 200 ml capacity and covered with aluminum foil. During treatment period, distilled water was added to make up the volume of priming solution sufficient to suspend seeds within it. Seeds were stirred at regular intervals to prevent hardening of powder formulation. non-primed seeds were taken as control. Table 1 depicts various priming treatments adopted for the study.
The seeds were thoroughly washed with distilled water after the completion of priming and placed on a piece of Whatman filter paper, allowing dehydration under the shade at 25 °C till seeds retrieved the original moisture level as that of pre-priming stage. Seeds in the priming treatments and control were pretreated with 0.05% (w/v) gibberellic acid (GA3) solution for overnight before sowing.
The seeds were sown in germination trays filled with sand medium and were watered regularly until germination was completed. Daily germination counts were recorded for a period of 60 days by the time germination was completed. From these observations, germination percentage, germination energy and emergence rate index (ERI) were calculated. Emergence rate index (ERI), an indicator of speed of germination was calculated using the formula suggested by Evetts and Burnside (1972):
where, G1 – the percentage of seeds germinated at T1, G2- percentage of seeds germinated at T2, G3- percentage of seeds germinated at T3, Gn- percentage of seeds germinated at Tn and the T1 – weeks from sowing to the first count, T2- weeks from sowing to the second count, T3-weeks from sowing to the third count, Tn-weeks from sowing to nth count.
Electrical conductivity of the seed leachates was determined using a direct reading conductivity meter (CDC 40101) and was expressed as dS cm−1. To measure electrical conductivity, leachate was filtered to a conical flask when each biopriming treatment was completed. Five ml of the leachate was then transferred to a 25 ml standard flask and the final volume was made to 25 ml by adding distilled water. The total carbohydrate content of the seeds was estimated by the Anthrone reagent method as suggested by Yemm and Wills (1954), protein by Lowry’s method (Lowry et al. 1951) and the crude fat content by Soxhlet extraction (Kennedy 1949) and was expressed in %.
Each treatment was replicated thrice with 50 seeds constituting one replication in the germination studies. The emerged seedlings at 4 to 5 leaf stage were transplanted to polythene bag filled with soil: sand: FYM in the ratio of 3:1:1. Growth attributes like shoot length, collar girth, and root length and biomass production were recorded at 90th and 180th days after transplanting (DAT). Twelve seedlings from each treatment were randomly selected for destructive sampling. No host plants were used during the study period to support the sandal seedlings.
Statistical analysis
The analysis of variance was carried out using R package – Agricolae taking the concentration and duration as the factors and Tukey’s HSD (Honestly Significant Difference) at 5% probability for the post-hoc test (Mendiburu 2015). Data were submitted to normality by the Levene's test and homoscedasticity by the Bartlett test and arc-sine transformed and analysed wherever appropriate.
Results
Seed germination attributes as influenced by biopriming
Marked differences were observed in the germination percentage of the sandal seeds due to interaction effect of seed treatments (p = 0.05). Seed germination ranged from 66.67 to 88.00%. Non-primed seeds recorded the lowest germination (46.67%) which was spread over a period of 56 days (Table 1). All the priming treatments were statistically on par. Seed biopriming at 100% concentration for 8 days (T16) recorded the highest germination percentage (88%) which was 1.88 times more compared to control, followed by T8 (biopriming at 50% for 8 days, 86.67%) and both with shortest germination period (21 days) and imbibition period (7 days). Although specific trend was not observed in germination of seeds due to duration and concentration of the treatments, the biopriming treatments at higher durations exhibited the highest germination with shortest imbibition and germination period. Highest imbibition period was recorded by seeds of control (T17, 13 days). Germination period of T3, T5, T7, and T9 were almost on par with control. Energy period, an indicator of speed of germination was lowest in T16 (15 days) recording highest value of germination energy (62.98%). T8 was identified as the next best treatment in the case of germination energy. The energy period of the non-primed seeds was the highest (34 days) indicating lowest germination energy of 26.80%. Emergence rate index which is also considered as an indicator of germination speed also followed a similar trend as that of germination energy (Table 1).
Changes in electrical conductivity and biochemical composition of primed seeds
A sharp decline in the electrical conductivity of the seed leachates was observed in primed seeds compared to control (p = 0.05). However, any specific trend could not be deduced in the variation of EC with either the duration or concentration of biopriming agent. Biopriming treatment could impart a 97.7% reduction in the loss of leachates compared to control indicating that biopriming invariably plays an important role in improving the seed quality. The reduction in EC was justified with an increase in the total carbohydrate and protein content of the seeds. Increase or decrease in the quantity of these constituents was affected by neither durations nor concentrations of biopriming treatments. Meanwhile, crude fat content reduced considerably in the bioprimed seeds. An exception to this was observed in T4 (57.01%) and T11 (55.80%) recording a higher percentage of crude fat over control (55.35%). Total carbohydrate was increased by 1.7 times in T5 followed by 1.68 times in T9 and the lowest was recorded by seeds of control (1.03 mg g−1). Also, the increment in protein content in seeds of T2 was 1.4 times that of control (Table 2). Analysis of variance revealed significant difference in electrical conductivity, carbohydrate and protein content at 5% level significant and fat at 1% level (edf-=15).
Seedling growth and biomass production
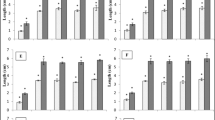
Growth attributes of the sandal seedlings obtained from the seeds subjected to biopriming at 90 and 180 days after transplanting exhibited significant variation between the biopriming treatments and control (Table 3). Analysis of variance revealed significant differences (p = 0.01) in shoot height due to duration at 90 days and concentration x duration at 180 DAT and collar girth varied significantly at due to interaction effect of concentration and duration (p = 0.01) at both period whereas difference in root length was significant (p = 0.01) only at 180 days after transplanting. The results indicated that the biopriming treatments resulted in an increment of 33.2% in shoot height, 46.4% in collar girth, and 31.4% in root length. The highest increment was observed in the seedlings from T16.
The effect of biopriming was apparent on the biomass production of the sandal seedlings (Table 3). Analysis of variance revealed a significant difference in shoot (p = 0.05) and total (p = 0.01) biomass due to interaction effect of concentration x duration at 90 DAT. The root dry weight at 90 DAT varied only due to duration. At 180 DAT only the effect of concentration was significant (p = 0.01) in all parameters. Similar to the shoot and root growth attributes, the biomass production of the seedlings in terms of dry weight was highest in seedlings of T16. The highest value obtained seedling and root dry weight due to biopriming were 1.53 times higher than that of the control whereas the shoot dry weight was 1.15 times higher than the control.
Discussion
Results of present study indicated that biopriming with PGPR- Pseudomonas fluorescens could improve the germination and seedling growth attributes in sandal. Perusal of literature on the seed germination and subsequent seedling growth of sandal helped to explain the constraints of sandal regeneration. Sandal seeds require 4–12 weeks (Srimathi et al. 1995) and 2–22 weeks (Sutheesh et al. 2016) to complete germination. With the adoption of biopriming, in the present study the total germination period of sandal seeds was reduced to 3 weeks from 8 weeks (control). However, germination in control seeds was only 46%. The biopriming treatments could also reduce the days to initiate germination to 7 days compared to the 14 days reported by Sutheesh et al (2016). The longest imbibition period recorded in the present study was only 10 days. The biopriming treatments could also increase the germination energy, thereby increasing the speed of germination greatly over control. In the present study, the increase in germination of the sandal seeds ranged from 44.93 to 91.30% which was a significant improvement in germination through biopriming and the results are in conformity with the findings of Rodriguez et al. (2015). They stated that biopriming of seeds of Abies hickelii with Pseudomonas fluorescens could increase germination rate up of 91%. From findings of the present study, it can be concluded that biopriming along with GA3 pre-treatment might have helped to overcome seed dormancy in sandal seeds resulting in greater and uniform germination rate of sandal seeds. Since more than 80% of seeds could complete germination within a period of three weeks, the problem of scattered germination which is a major constraint in the forest nurseries also can be overcome resulting in uniform planting stock production.
Generally, the increase in the electrical conductivity of the seed leachates is associated with loss of seed viability and vigour (Perry and Harrison 1977). Lower electrical conductivity of seeds indicates the precise integrity of cellular membranes during priming which reduces the leakage from the cells (Copeland and Mc Donald 2001). Lower values of EC of leachates of seeds subjected to biopriming in present study indicate a reduction in seed leakage leading to better membrane integrity of sandal seeds. This can be ascribed to the enzyme activity induced cell repair mechanism. This is line with finding of Rinku et al. (2017) where biopriming resulted in a low electrical conductivity value compared to hydropriming and control. Reduction in electrical conductivity is a direct indicator of the recorded increase in total carbohydrate and protein content of bioprimed sandal seeds. This further indicates improved membrane integrity of the cells resulting in better utilization of the mobilized seed reserves and hence enhanced germination and fastened subsequent seedling growth. This can also be attributed to the reduction in crude fat content of the primed seeds, which might have hydrolyzed to soluble sugars during priming. Biopriming has reported to increase the carbohydrate, protein, and oil content in safflower (Sharifi 2012) and soybean (Silva et al. 2013). The reduction in crude fat content of bioprimed sandal seeds contradictory to previous studies can be due to increased protein and carbohydrate content of sandal seeds. Protein and carbohydrates greatly influence the germination percentage of seeds (Soriano et al. 2014). In the present study, seeds bioprimed for 4 days recorded a considerable increase in carbohydrate and protein content compared to other treatments. These treatments also recorded a very good germination percentage which was comparable to that of the best treatments. In fact, the best treatment identified in terms of germination was found to have less increment in the carbohydrate and protein content. The seedlings of these treatments were also found to exhibit comparable seedling growth performance with that of those seeds primed for 8 days. Jijeesh and Sudhakara (2016) obtained a high positive correlation for vigor index and biochemical constituents with crude oil and soluble and total carbohydrates in Tectona grandis. Collar diameter, number of lateral roots and seedling dry weight were also correlated with vigor index and biochemical constituents. The results of the study were also following these findings.
Corresponding to the enhanced seed germination due to biopriming, sandal seedlings obtained from seeds subjected to biopriming treatments also showed enhanced growth and biomass production over control. In addition to increased rate and uniformity of germination, biopriming protects seeds against the soil and seed-borne pathogens. Moreover, some bacteria used as biocontrol agents can colonize the rhizosphere and support plants in both direct and indirect ways after the germination stage (Callan et al. 1997). With regard to shoot and root growth parameters of seedlings, it can be concluded that benefits of priming had been accumulated on seedlings that emerged from seeds subjected to biopriming with Pseudomonas fluorescens for longer durations (8 days). Biopriming with Pseudomonas fluorescens at 100% for 8 days will be the best priming treatment for the sandal to improve the biometric growth as well as biomass based on dry weight. These results conformed the findings in sunflower (Moeinzadeh et al. 2010), pea (Negi et al. 2008) and Abies hickelii (Rodriguez et al. 2015) where the priming of seeds with bio-agents increased mineral levels (N, P and K), chlorophyll biosynthesis and photosynthetic activity. Yadav et al. (2010) reported that the increased shoot height of bioprimed seedlings is due to early emergence of seedlings, which was similar to present study. Studies revealed that P. fluorescens is capable of increased nitrogen fixing and phosphate solubalisation and production of growth-promoting substances increasing the dry matter production of the seedlings (Nezarat and Golami 2009). Results were also indicating that the seeds of sandal subjected to biopriming for 4 days also exhibit germination and seedling growth comparable to that of seedlings bioprimed for 8 days. Hence, these treatments can also be adopted as better priming treatments to avoid long duration soaking. Further, some of the possible mechanisms behind the increased germination and seedling growth due to P. fluorescens had been asserted as the provision of plant and tree hormones in the rhizosphere, increase in the availability of nutrients (N, P, K), biological protection due to antibiotics or siderophores production (Duda et al. 2004; Deepa et al. 2010) which were identified in some pines (Mafia et al. 2009).
Conclusion
Overall, it can be concluded that the biopriming of sandal seeds with PGPR Pseudomonas fluorescence significantly influenced seed germination and seedling growth of sandal resulting in enhanced germination and seedling performance. The sandal seeds primed at 100 and 50% concentration of 108 cfu of P. fluorescence for 8 days were superior to other treatments. Generally, biopriming can be recommended as a viable technology to enhance seed germination and seedling growth of sandals to produce quality plant stock in nurseries.
References
Asha BB, Chandra NS, Shankar UAC, Srinivas C, Niranjana SR (2011) Biological control of Fusarium oxysporum f. sp. lycopersici causing wilt of tomato by Pseudomonas fluorescens. Int J Microbiol Res 3:79–84
Baskin CC, Baskin JM (1988) Germination ecophysiology of herbaceous plant species in a temperate region. Am J Bot 75(2):286–305
Callan NW, Mathre DE, Miller JB, Vavrina CS (1997) Biological seed treatments: factors involved in efficacy. Hortic Sci 32:179–183
Copeland LO, Mc Donald MB (2001) Principles of Seed Science and Technology, 4th edn. Springer, New York, p 333
Das SC, Tah J (2013) Effect of GA3 on seed germination of Sandal (Santalum album L.). Int J Curr Sci 8:79–84
Deepa CK, Dastager SG, Pandey A (2010) Plant growth-promoting activity in newly isolated Bacillus thioparus (NII-0902) from Western Ghat forest, India. World J Microbiol Biotechnol 26:2277–2283
Dileepa MJM, Jayasuriya KG, Walck JL (2015) Confirmation of morphophysiological dormancy in sandalwood (Santalum album, Santalaceae) seeds. J Natn Sci Found Sri Lanka 43(3):209–215
Duda B, Orlikowski LB (2004) Rhizoctonia solani on coniferous seedlings in forest nurseries. J Plant Prot Res 44:175–180
Evetts LL, Burnside OC (1972) Germination and seedling development of common milkweed and other species. Weed Sci 20(4):371–378
Glick B, Ibid R (1995) Genotyping of antifungal compounds producing PGPR pseudomonas. Can J Microbiol 41:107–109
Jijeesh CM, Sudhakara K (2016) Relationship of biochemical composition and drupe size to seedling performance of teak (Tectona grandis). Seed Technol 37(1):65–71
Joshi G, Arun Kumar AN (2007) Standardization of optimum conditions for storage of Santalum album L. seeds for ex situ germplasm conservation. In: Gairola S et al. (eds) Proceedings of the National Seminar on Conservation, Improvement, Cultivation and Management of Sandal (Santalum album L.). 12–13 December 2007, Institute of Wood Science and Technology, Malleswaram
Kennedy WK (1949) Rapid method for determining the oil content of safflower and sunflower seeds. Agris 41(2):93–95
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin-phenol reagent. J Biol Chem 193:265–275
Ma Y, Feurtado JA, Kermode AR (2003) Effect of solid matrix priming during moist chilling on dormancy breakage and germination of seeds of four fir species. New Forests 25:67–81. https://doi.org/10.1023/A:1022353831661
Mafia RG, Alfenas AC, Ferreira EM, Binoti DHB, Mafia GMV, Mounteer AH (2009) Root colonization and interaction among growth promoting rhizobacteria isolates and eucalypts species. Revista Árvore 33:1–9
Mendiburu FD (2015) Agricola: Statistical Procedures for Agricultural Research. R Package Version 1.2-3. Available at http://CRAN.R-project.org/package=agricolae
Moeinzadeh A, Sharif-Zadeh F, Ahmadzadeh M, Tajabadi FH (2010) Biopriming of sunflower (Helianthus annuus L.) seed with Pseudomonas fluorescens for improvement of seed invigoration and seedling growth. Aus J Crop Sci 4:564–570
Negi K, Garg SK, Kumar J (2008) Plant growth promoting and biocontrol activities of cold-tolerant Pseudomonas fluorescens isolates against root rot in pea. Ind Phytopathol 61(4):461–470
Nezarat S, Gholami A (2009) Screening plant growth promoting rhizobacteria for improving seed germination, seedling growth and yield of maize. Pak J BiolSci 1(12):26–32
Perry DA, Harrison JG (1977) Effects of seed deterioration and seed-bed environment on emergence and yield of spring-sown barley. Ann Appl Biol 86:291–300
Rai SN (1990) Status and cultivation of sandalwood in India. In Hamilton L, Conrad CE (eds) Proceedings of a Symposium on Sandalwood in the Pacific. Honolulu, Hawaii. Pacific Southwest Research Station, USDA Forest Service, Berkeley
Rinku VP, Krishna YP, Jasrai RT, Nayana B (2017) Effect of hydropriming and biopriming on seed germination of brinjal and tomato seed. Res J Agri For Sci 5(6):1–14
Rodríguez RZ, Luis GHM, Bernardo MA, Edgar ORL, Lara CE, Troyo D, Matson MVC (2015) Effect of hydropriming and biopriming on seed germination and growth of two Mexican fir tree species in danger of extinction. Forests 6(9):3109–3122
Rozier C, Gerin F, Czarnes S, Legendre L (2019) Biopriming of maize germination by the plant growth-promoting rhizobacterium Azospirillum lipoferum CRT1. J Plant Physiol 237:111–119
Sharifi RS (2012) Study of nitrogen rates effects and seed biopriming with PGPR on quantitative and qualitative yield of safflower (Carthamus tinctorius L.). Tech J Engin Appl Sci 2(7):162–166
Silva LR, Pereira MJ, Azevedo J, Mulas R, Velasquez E, González-Andrés F, Valentão P, Andrade PB (2013) Inoculation with Bradyrhizobium japonicum enhances the organic and fatty acids content of soybean (Glycine max L. Merrill) seeds. Food Chem 141:3636–3648
Soriano D, Huante P, Gamboa-debuen A, Orozco-segovi A (2014) Effects of burial and storage on germination and seed reserves of 18 tree species in a tropical deciduous forest in Mexico. Oecologia 174:33–44
Srimathi RA, Kulkarni HD, Venkatesan KR (1995) Recent advances in research and management of Sandal (Santalum album L) in India. Associated Publishing Co, New Delhi, p 416
Srinivasan VV, Sivaramakrishnan VR, Rangaswamy CR, Ananthapadmanabha HS (1992) Sandal. Santalum album Linn Indian Council of Forestry Research and Education, ICFRE, Bangalore, India, p 186p
Sutheesh K, Jijeesh CM, Divya TP (2016) Evaluation of organic and inorganic pre-treatment for better seed germination and seedling vigour in Santalum album L. Plant Arch 16(1):143–150
Yadav J, Verma JP, Tiwari KN (2010) Effect of plant growth promoting Rhizobacteria on seed germination and plant growth chickpea (Cicer arietinum L.) under in vitro conditions. Biol Forum Int J 2:15–18
Yemm EM, Willis AJ (1954) The estimation of carbohydrates in plant extracts by Anthrone. Biochem J 57:508–514
Acknowledgement
The authors acknowledge Kerala Agricultural University for providing the financial requirement of the work. The support and facilities provided by the Department of Silviculture and Agroforestry, College of Forestry, Kerala Agricultural University is remembered with immense gratitude. We also acknowledge Dr. T. Girija and Dr. Hrideek T. K. for their sincere support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chitra, P., Jijeesh, C.M. Biopriming of seeds with plant growth promoting bacteria Pseudomonas fluorescens for better germination and seedling vigour of the East Indian sandalwood. New Forests 52, 829–841 (2021). https://doi.org/10.1007/s11056-020-09823-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-020-09823-0