Abstract
For the effective use of native plants for mineland revegetation, an understanding of seed dormancy break and germination requirements, and seed storage tolerance is indispensable. In the present study, eight native species (Bauhinia pulchella, Bauhinia longipedicellata, Dioclea apurensis, Mimosa camporum, Mimosa acutistipula var. ferrea, Mimosa pudica, Parkia platycephala, and Stryphnodendron pulcherrimum) from the metalliferous savannas (cangas) and forests of Carajás Mineral Province, eastern Amazon-Brazil, were studied to determine seed size, seed quality (X-ray and tetrazolium tests), germination, and dormancy break requirements (boiling water, acid and mechanical scarification), and seed storage behavior. Our results showed considerable variation in seed size and percentage germination among the species. There was a strong relationship between seed size and germination, and the germination was greater for larger seeds from forests than smaller seeds from canga. All three scarification methods increased germination of M. camporum and M. acutistipula var. ferrea. Seeds of D. apurensis, M. acutistipula var. ferrea, M. pudica, and P. platycephala did not show a significant decline in germination after storage, indicating possible orthodox behavior. In contrast, B. pulchella, B. longipedicellata, M. camporum, and S. pulcherrimum showed behavior typical of recalcitrant or intermediate seeds since the germination of these species was reduced after storage. Further studies addressing seed dormancy break and seed storage in other native species are encouraged for a better use of native species in mineland revegetation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of native species for revegetation purposes, which is mandatory in Brazilian conservation units (IBAMA 2011; ICMBio 2014), is increasingly recognized as an effective means to restore ecosystem functions and biodiversity on impacted minelands around the world (Macdonald et al. 2015; Lu et al. 2017; Gastauer et al. 2018a). However, successful revegetation with native species adapted to local conditions requires extensive knowledge about propagation, growth, nutrient requirements, and functional adaptations of these species (e.g., Oliveira et al. 2015; Carvalho et al. 2018a). Species producing large numbers of viable seeds remain the most adequate and economic way to revegetate minelands (Dürr et al. 2015). In addition, the use of native species can also contribute to reduced loss of biodiversity and ecosystem services (Skirycz et al. 2014; Souza Filho et al. 2016; Gastauer et al. 2018b), especially in areas that require large-scale revegetation. Thus, knowledge of seed quality and how to optimizing germination is indispensable for the mineland revegetation (Salazar et al. 2015). The Carajás Mineral Province (CMP) in the eastern Amazon harbors the world’s largest mineral reserves, and open-cast mining for iron, copper, manganese and nickel ores requires large-scale revegetation of degraded lands with native species (Gastauer et al. 2018b).
Seeds of wild species differ in size and physiological adaptation to specific environments. Species with larger seeds tend to have more energy and nutrient reserves than smaller seeds, while smaller seeds show a higher probability of dispersal, and possess the capacity to form persistent soil seed banks (Szentesi and Jermy 1995; Khurana and Singh 2004; Kumar et al. 2015). While larger seeds with high water content are generally associated with late successional species, early successional species have small seeds with low water content. The latter are often considered to be produced in large quantities all year round and to have specialized dormancy mechanisms that detect environmental changes indicating the arrival of favorable conditions for seedling establishment (Casas et al. 2017). Thus, seed dormancy can be important to synchronize germination during favorable growth periods and increasing competitive abilities in seasonal habitats such as savannas, but they may also require dormancy breaking treatments for nursery propagation.
Dormancy can be either exogenous or endogenous (Baskin and Baskin 2014). Exogenous dormancy is caused by impermeable or hard seed coats developed during maturation and seed drying and usually obstructs water uptake. In natural ecosystems, this kind of dormancy is broken by alternating temperatures or passage through the animal gut (Fuzessy et al. 2015). Artificially, exogenous dormancy can be overcome by mechanical scarification, elevated temperatures or acid treatments (Baskin and Baskin 2014). Endogenous dormancy, in contrast, comprises physiological or morphological dormancy, such as underdeveloped or undifferentiated embryos (Bradbeer 2013) and chemical inhibitors (Shu et al. 2016).
Storage tolerance constitutes one of most important seed traits in large-scale revegetation programs (Hay and Probert 2013). As suggested by Roberts (1973), seeds can be classified as orthodox, intermediate or recalcitrant (unorthodox). Orthodox seeds can tolerate long-term storage at room or subzero temperatures; whereby, recalcitrant or unorthodox seeds usually show high moisture contents and do not withstand long-term storage, desiccation or low temperature. Pioneer species that characterize the initial stages of forest succession generally possess orthodox seeds, while climax species are predominantly recalcitrant (Ribeiro et al. 2013). Some species exhibit intermediate behaviors, tolerating reductions in water content to levels below those of recalcitrant seeds but not to the same extent as orthodox seeds (Rodrigues-Junior et al. 2015; Walters 2015).
The differences in seed quality among and within species (especially in wild, nondomestic species) may be a result of incomplete pollination (e.g., Bommarco and Vaissiere 2012; Metsare et al. 2015), incomplete seed filling or nonsynchronous seed maturation (Miller-Rushing and Primack 2008). Thus, more information about seed germination, dormancy break, and storage tolerance is still needed to provide efficient techniques for successful mineland revegetation with native species (Hay and Probert 2013; Macdonald et al. 2015). To provide this information, the aim of this study was to outline how scarification and storage influence the germination of eight native species from the metalliferous savannas (cangas) and forests of CMP, eastern Amazon-Brazil. For that, our study also addresses the seed size and seed quality.
Materials and methods
Seed material
Seed measurements and germination tests were carried out on eight Fabaceae species native to the CMP (Fig. 1). These include early successional species (B. pulchella, D. apurensis, M. camporum, M. acutistipula var. ferrea, and M. pudica) occurring on metalliferous savannas over iron duricrusts, locally known as cangas, and late successional species (B. longipedicellata, P. platycephala, and S. pulcherrimum) from evergreen and seasonal forest species. Seeds were collected from different locations around the CMP by the Cooperativa dos Extrativistas da Flona de Carajás (CoEx), a cooperative that provides native seeds for revegetation purposes. The species used in this study present a large occurrence in the CMP, and the phenology of these species is well known by local population working at CoEx. Seeds of these species were collected at maturity from different areas and grouped as seed lots, and then transferred to paper bags and stored in seed chamber at low temperature and air humidity.
Seed morphology
Measurements were carried out on 100 seeds each of the eight Fabaceae species. Length, width and thickness were measured using digital calipers. Weight was measured using a precision analytical balance (0.0001 g). Seed moisture was determined by weighing four replicates of twenty-five seeds before and after drying in an oven at 105 °C until constant weight. The results were expressed as the percentage of water on a fresh weight basis according to the International Seed Testing Association (Ista 2004).
Seed viability
X-ray images were captured from four lots of 25 seeds of each species. Seeds were arranged in clear acrylic plates on double-sided adhesive tape and subjected to radiation using a Faxitron HP X-ray device, Model 43855A X at 45 kV for 25 s. The X-ray plates were evaluated based on the presence and morphology of an embryo and endosperm in each seed. Percentages of full seeds with an entire embryo, damaged seeds, and empty seeds were determined.
Seed viability was assessed by a tetrazolium test. Seeds were initially scarified with sandpaper and premoistened in a paper towel for 18 h. The teguments were removed with a scalpel from the seeds, which were then placed in 50 mL plastic cups with a 0.5% solution of 2,3,5-triphenyl tetrazolium chloride for 18 h under 25 °C in a germination chamber in the absence of light. For each species, four subsamples of 25 seeds were tested. After each staining period, the tetrazolium solution was drained, and the seeds were washed in running water and placed in water. They were then placed in a refrigerator until the evaluation time. Viable seeds were those that presented a bright, light-pink color, tissues with a normal and firm appearance, and an embryonic axis with an intense red color. The nonviable seeds were those showing more than 50% discolored cotyledons, an intense red coloration, an embryonic axis with discolored areas, and an intense red color reaching the central cylinder. The results were expressed as percentages of viable seeds.
Seed germination and dormancy break requirements
Seed germination tests were performed in a germination chambers at 25 °C at a constant irradiance of 26 μmol m−2 s−1 with a 12-h photoperiod. The number of germinated seeds were recorded daily for 30 days, with germination defined as radicle emergence of 2 mm.
The scarification methods applied were (1) mechanical: external tegument of the seed scratched with sandpaper; (2) boiling water: immersion in water at a temperature of 100 °C for 5 min, followed by cooling to room temperature; (3) immersion in 90% sulfuric acid (H2SO4) for 7 min, for softening the waxy seed coat, followed by washing with abundant running water.
After the scarification treatments, seeds were immersed in a 1% sodium hypochlorite solution for 3 min to ensure surface sterility and then washed in sterile water for 1 min.
Seed storage
Four samples containing 25 seeds of each species were subjected to mechanical scarification and then dried in silica gel for 4 days. Non-stored seeds with mechanical scarification were considered the control treatment. The seeds were placed in sealed plastic bags and then stored in the dark at − 18.5 °C for 30, 60, or 90 days. After each storage time, the seeds were prehumidified with sterile water and placed in the germination conditions, as previously described above.
Statistical analysis
We used one-way analysis of variance (ANOVA) to outline the influence of the treatments on percentage of germination. Comparisons of mean values of length, width, thickness, 100-seed weight, water content, and seed quality were performed using the post hoc Tukey HSD test after checking for normality of the distribution of each variable using a Shapiro–Wilk test. We have used the Pearson’s correlation analysis to evaluate the interaction between germination and seed morphological traits. All analyses were performed using R Environment (R Development Core Team 2018).
Results
Seed morphology
Seed dimensions differed significantly between natural habitat, successional status, life form, and plant species (Table 1). The length, width, thickness, and 100-seed weight were higher in forest than canga species. Among life forms, shrubs showed smaller and more lightweight seeds than liana and tree species. Germination percentages were greater for larger seeds than smaller seeds. The seed size × germination interaction was significant for both canga (length r = 0.597 p value = 0.005; width r = 0.699 p value = 0.001; thickness r = 0.738 p value = 0.004) and forest habitats (length r = 0.613 p value = 0.034; width r = 0.553 p value = 0.062; thickness r = 0.481 p.value = 0.114) (Fig S1—Supplementary material). In general, the length, width, and thickness of seeds ranged from 2.82 to 10.15, 2.30 to 6.98, and 0.82 to 3.84 mm, respectively. The highest values of length and width were observed in the seeds of B. longipedicellata. The highest values of seed thickness were found in D. apurensis. In contrast, the lowest seed length and width were detected in Mimosa pudica, and the lowest thickness was observed for M. acutistipula var. ferrea.
Among life form, liana showed higher 100-seed weight than shrub and tree species. The 100-seed weight varied from 0.45 g (M. pudica) to 16.55 g (D. apurensis). The 100-seed weight for two species was higher than 10.0 g (B. longipedicellata and D. apurensis), while that for M. camporum and M. pudica was close to 0.5 g.
The initial water content did not differ statistically between habitats, life forms, and successional strategies (Table 1). The initial water content ranged from 8 to 13%, and the highest values were observed for B. pulchella, while the lowest water contents were found for P. platycephala and M. pudica.
Seed viability
The evaluation of the internal morphology of seeds using X-ray showed that in all species more than 80% of all the examined seeds presented an internal cavity completely filled by embryo and endosperm tissue, although significant differences among species were detected (Table 2, Fig. S2). The percentage of physically perfect seeds (full seeds: embryo + endosperm) did not differ between canga species (early successional status) and forest species (late successional). The percentage of damaged seeds (seeds with insect predation or empty) was higher in shrub and tree than liana species.
In general, tetrazolium tests indicate seed viability higher than 80% for all species (Table 2, Fig. S3). Viability percentages of seeds did not differ between habitats, life forms, and successional strategies. The highest value of viable seeds was observed for P. platycephala (90% viable seeds). Fig. S3 shows the color ranges observed during the tetrazolium evaluation. Light pink or bright red coloration indicates living, firm and vigorous tissues from viable seeds. In contrast, unviable seeds assumed a partial milky white color, suggesting dead tissue, or an intense red color throughout, indicating an accelerated process of tissue deterioration.
Seed germination and dormancy break requirements
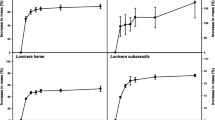
Considerable variation in germination was observed without seed scarification (Fig. 2). Seeds of forest species germinated to higher percentages than canga species, while liana and tree species were similar, but higher than shrub species. Very low germination without scarification was found for all tested species from the Mimosa genus and from S. pulcherrimum.
Germination percentage of Fabaceae seeds in response to the scarification methods. Different lowercase letters indicate differences among scarification methods within the same natural habitat, life form, successional status, and species. Different uppercase letters indicate differences among scarification methods among each natural habitat, life form, and successional status (p value < 0.05, Tukey’s test). Error bar indicates + SE (n = 4)
In general, mechanical scarification was the best scarification method for both canga and forests species (Fig. 2). Among life form, the use of boiling water and mechanical scarification promoted higher germination for liana species, while for shrub species sulfuric acid was similar to mechanical scarification. Among species, B. longipedicellata reduced the germination in response to both boiling water and the acid treatment, while D. apurensis and P. platycephala responded significantly to boiling water and the mechanical treatment, with germination increasing over 90% for both species.
After storage at − 18.5 °C for up to 90 days, we observed that shrub and tree species from cangas and forests exhibited significant decreases in percentage germination (Fig. 3). In such circumstances, seeds of D. apurensis, M. pudica, and P. platycephala had average germination close to 90%. However, we observed that 90 days of storage significantly reduced the seed germination B. longipedicellata, S. pulcherrimum, B. pulchella, and M. camporum (Fig. 3)
Discussion
Seed traits and germination requirements have not been widely reported for native species of the eastern Amazon, especially for plants used to revegetate mining areas such as the CPM. We have shown substantial variation in length, width, thickness, and 100-seed weight of eight Fabaceae species that differ regarding life form and natural habitat. We found that late successional species from forests have larger seeds than those of earlier stages of canga. The 100-seed weight showed over two-fold difference between seeds from forest and canga. Overall, large seed species can produce seedlings with a greater survival rate than those from smaller seeds under intense stressed conditions (Deb and Sundriyal 2017; Barak et al. 2018). However, seedlings from small seeded, particularly of fast-growing species, may be able to cope with mild drought events by morphogenetic and physiological plastic response in a better way than large seeded species (Khurana and Singh 2004).
In the present study, classification of the internal seed cavity by X-ray showed a high percentage of seeds with their internal cavity occupied by the embryo and endosperm. Also, we found that liana species showed a higher percentage of species with full internal structures than shrub or tree species. However, low seed germination was found in natural conditions for most species studied, while a high germination percentage was observed after applying scarification methods, especially when mechanical scarification was used. These results suggest that despite the good internal condition found by the X-ray analysis, the native Fabaceae species used in this study have physical dormancy and thus require scarification to allow water to permeate the seed coat and break dormancy. This confirms previous results from Baskin and Baskin (2014) who reported that non-domesticated Fabaceae species typically present physical dormancy and require scarification to allow water uptake to increase germination.
The tetrazolium test has been used for over 60 years to give a rapid assessment of seed viability (Kuhn and Jerchel 1941; Carvalho et al. 2018b). However, few studies have investigated the use of the tetrazolium test for seed viability of native Amazon species (e.g., Souza et al. 2015; Grzybowski et al. 2017). In the present study, the tetrazolium tests for seed viability indicated that, for most species, full seeds contained a high proportion of viable tissues, and no difference among seeds from different natural habitats, life forms, and successional strategies was observed. The variation in color intensity from staining observed among species may be indicative of tissue aging and differences in seed vigor. Our results for the tetrazolium analysis were significantly higher than those for the germination test in the control treatment, especially for D. apurensis, M. camporum, M. acutistipula var. ferrea, M. pudica, and S. pulcherrimum, which were found to have low germination without the use of scarification methods. This result reinforces the presence of dormancy in the seeds of these species. Thus, given correct dormancy breaking treatment, the seed germination of the species tested should be successful.
Dormancy break was promoted by mechanical scarification, boiling water or sulfuric acid treatments in many of the species, resulting in a germination reaching a percentage equivalent to that of viable seeds. Nevertheless, the maximal percentage germination after all three dormancy breaking treatments in B. pulchella, B. longipedicellata and M. acutistipula var. ferrea were found to be lower than the percentage of viable seeds indicated by the tetrazolium tests or by X-ray analysis. This indicates that some seeds of these species may present physiological seed dormancy as well. In M. pudica and P. platycephala, the maximum germination rate exceeded the percentage of detected viable seeds. In the present study, we applied standardized tetrazolium tests, following recommendations for a wide range of seeds (e.g., Moore 1962; Jurado and Westoby 1992; Camargo-Ricalde et al. 2004), requiring modifications and adjustments of the test to analyze the germinability of seeds from these species.
Understanding the germination characteristics of native plant species from the CMP provides an important basis for mineland revegetation. In the present study, the germination of nonscarified seeds from forest species was higher than in canga species. Among life form, seeds of shrub showed lower germination than liana and tree for nonscarified seeds. Within species, M. camporum, M. acutistipula, M. pudica, and S. pulcherrimum displayed low values of germinability. The greater seeds germination of canga and forest was promoted mainly by mechanical scarification. The overall increment in seed germination after scarification described here was also observed in several other Mimosa species (Rosa et al. 2012; Pereira et al. 2013; Dayrrel et al. 2015), which may be linked to the impermeable seed coat observed in these species. However, some scarification methods, such as immersion in boiling water for M. pudica or immersion in acid for seeds of D. apurensis and P. platycephala, did not increase the seed germination of these species, and the methods made most of the seeds rigid (hard) at the end of the evaluation period of the germination tests, indicating that these scarification methods were not sufficient to overcome the barrier for water absorption or that the treatments may have negatively affected the seed embryo and/or endosperm tissues. In some cases, treatments devised to overcome seed dormancy can be highly specific because seed populations show large degrees of impermeability of the seed coat (Kak et al. 2009). Varela et al. (1991) found that boiling water did not overcome the seed dormancy of S. pulcherrimum, and these authors suggested the use of mechanical scarification to surpass seed coat dormancy. As reported above, mechanical scarification was effective to enhance the seed germination of S. pulcherrimum.
Seed storage is indispensable for most tree nurseries, and maintaining a high viability of collected native seeds represents one of the challenges ensuring adequate seedling production. The results found here showed that not all species were able to maintain seed viability along 90 days of storage, i.e., some species of shrub and tree showed decrease in seed germination, while that of liana species was unaffected. Within species, M. camporum and S. pulcherrimum were found to have germination reduced by 50 and 30%, respectively. In contrast, we provide strong evidence that seed storage (90 days) does not significantly reduce the germination ability of D. apurensis, M. acutistipula var. ferrea, M. pudica, and P. platycephala, and these species should be classified as orthodox seeds. Based on the present study, B. pulchella, S. pulcherrimum, and M. camporum are either in the recalcitrant or intermediate category of seed storage behavior. Our data have important implications for the use of native species in revegetation programs. First, they help us understand seed germination and how to overcome seed dormancy. We found 35% germination without scarification methods, while 85% germination was found after mechanical scarification; the data reported here suggest that seed dormancy can be effectively overcome by scarification methods. Second, our results also provide key and practical information for seed storage behavior. Only three species were found to reduce their seed germination after 90 days of storage, while five species showed a high percentage of germination after storage. Therefore, the combination of scarification methods and storage behavior of native Fabaceae species can be an important and fundamental tool to guarantee the most efficient way to use native species in revegetation activities in several areas, such as that enclosed by the CMP.
Conclusion
Our findings indicate considerable variation in terms of seed size, quality, and germination among eight native species of canga and forest of CMP. The scarification methods led to a significant germination gain, mainly mechanical scarification in all species. This illustrates the need to considerer seed scarification in Fabaceae species from Amazon prior to application for rehabilitation proposes.
Additionally, the species D. apurensis, M. acutistipula var. ferrea, M. pudica, and P. platycephala show higher storage tolerance, so their physiological behavior corresponds to the orthodox type. Seeds of B. pulchella, B. longipedicellata, M. camporum, and S. pulcherrimum seeds shown the typical recalcitrant or intermediate behavior since the germination of these species was reduced after storage. Further studies addressing overcoming seed dormancy break and seed storage in other native species are encouraged to enhance the use of native species in mineland revegetation.
References
Barak R, Lichtenberger TM, Wellman-Houde A, Kramer AT, Larkin DJ (2018) Cracking the case: seed traits and phylogeny predict time to germination in prairie restoration species. Ecol Evol 8:5551–5562
Baskin CC, Baskin JM (2014) Seeds: ecology, biogeography, and evolution of dormancy and germination, 2nd edn. Elsevier, Amsterdam
Bommarco R, Vaissiere LMB (2012) Insect pollination enhances seed yield, quality, and market value in oilseed rape. Oecologia 169:1025–1032
Bradbeer JW (2013) Seed dormancy and germination. Springer, Berlin
Camargo-Ricalde SL, Dhillion SS, García-García V (2004) Phenology, and seed production and germination of seven endemic Mimosa species (Fabaceae-Mimosoideae) of the Tehuacán-Cuicatlán Valley, Mexico. J Arid Environ 58:423–437
Carvalho JM, Ramos SJ, Furtini Neto AE, Gastauer M, Caldeira CF, Siqueira JO, Silva MLS (2018a) Influence of nutrient management on growth and nutrient use efficiency of two plant species for mineland revegetation. Restor Ecol 26:303–310
Carvalho SMC, Torres SB, Souza EC, Souza DMM, Pereira KTO, Paiva EP, Matias JR, Santos BRV (2018b) Viability of Carica papaya L. seeds by the tetrazolium test. J Agric Sci 10:335–340
Casas RR, Willis CG, Pearse WD, Baskin CC, Baskin JM, Cavender-Bares J (2017) Global biogeography of seed dormancy is determined by seasonality and seed size: a case study in the legumes. New Phytol 214:1527–1536
Dayrrel RLC, Gonçalves-Alvim SJ, Negreiros D, Fernandes GW, Silveira FAO (2015) Environmental control of seed dormancy and germination of Mimosa calodendron (Fabaceae): implications for ecological restoration of a highly threatened environment. Braz J Bot 38:395–399
Deb P, Sundriyal RC (2017) Effect of seed size on germination and seedling fitness in four tropical rainforest tree species. Indian J For 40:313–322
Dürr C, Dickie JB, Yang XY, Pritchard HW (2015) Ranges of critical temperature and water potential values of the germination of species worldwide: contribution to a seed trait database. Agric For Meteorol 200:222–232
Fuzessy LF, Cornelissen TG, Janson C, Silveira FAO (2015) How do primates affect seed germination? A meta-analysis of gut passage effects on neotropical plants. Oikos 125:1069–1080
Gastaruer M, Silva JRS, Caldeira CF, Ramos SJ, Souza Filho PWM, Furtini Neto AF, Siqueira JO (2018a) Mine land rehabilitation: modern ecological approches for more sustainable mining. J Clean Prod 172:1409–1422
Gastauer M, Souza Filho PWM, Ramos SJ, Caldeira CF, Silva JR, Siquiera JO, Furtini Neto AE (2018b) Mine land rehabilitation in Brazil: goals and techniques in the context of legal requirements. Ambio. https://doi.org/10.1007/s13280-018-1053-8
Grzybowski CRS, Nascimento WMO, Silva RC, Vieira ESN, Panobianco M (2017) Physiological potencial and conservation of murici (Brysonima crassifólia) seeds. Rev Brasil Frutic 39:e-475
Hay FR, Probert RJ (2013) Advances in seed conservation of wild plant species: a review of recent research. Conserv Physiol 1(1):cot030
IBAMA (Instituto Brasileira do Meio Ambiente) (2011) Instrução normativa N 03/2011, de 1 de abril de. http://www.ibama.gov.br/phocadownload/fauna/fauna_exotica/2011_ibama_in_03_2011_e_alteracoes_criacao_de_fauna_exotica_amadora.pdf. Accessed 10 Apr 2018
ICMBio (Instituto Chico Mendes de Conservação da Biodiversidade) (2014) Instrução normativa ICMBio N 11, de 11 de Dezembro de 2014. http://www.icmbio.gov.br/cepsul/images/stories/legislacao/Instrucao_normativa/2014/in_icmbio_11_2014_estabelece_procedimentos_prad.pdf. Accessed 10 Apr 2018
ISTA (2004) Handbook on seed sampling. International Seed Testing Association, Bassersdorf
Jurado E, Westoby M (1992) Germination biology of selected central Australian plants. Aust J Ecol 17:341–348
Kak A, Pandey C, Gupta V, Bhardwaj M, Dashora K (2009) Effect of sulphuric acid pretreatment on breaking hard seed dormancy in diverse accessions of four wild Corchorus species. Seed Sci Technol 37:568–572
Khurana E, Singh JS (2004) Germination and seedling growth of five tree species from tropical dry forest in relation to water stress: impact of seed size. J Trop Ecol 20:385–396
Kuhn R, Jerchel D (1941) Reduktion von Tetrazoliumsalzen durch Bakterien, garende Hefe und keimende Samen. Ber der Deutsh Bot Ges 60:299–305
Kumar SPJ, Prasad SR, Banerjee R, Thammineni C (2015) Seed birth to death: dual functions of reactive oxygen species in seed physiology. Ann Bot 116:663–668
Lu Y, Ranjitkar S, Harrison RD, Xu J, Ou X, Ma X, He J (2017) Selection of native tree species for subtropical forest restoration in Southwest China. PLoS One. https://doi.org/10.1371/journal.pone.0170418
Macdonald SE, Landhausser SM, Skousen J, Franklin J, Frouz J, Hall S, Jacobs DF, Quideau S (2015) Forest restoration following surface mining disturbance: challenges and solutions. New For 46:703–732
Metsare MM, Ilves A, Haldna M, Kull T, Tali K (2015) Four seed-quality measures in orchids with different pollination systems. Acta Bot Gallica 162:263–269
Miller-Rushing A, Primack RB (2008) Global warming and flowering times in Thoreau’s Concord: a community perspective. Ecology 89:332–342
Moore RP (1962) Tetrazolium as a universally acceptable quality test for viable seed. Proc Int Seed Test Assoc 27:795–805
Oliveira RS, Galvão HC, Campos MCR, Eller CB, Pearse S, Lambers H (2015) Mineral nutrition of campos rupestres plant species on contrasting nutrient-impoverished soil types. New Phytol 205:1183–1194
Pereira SR, Laura VA, Souza ALT (2013) Superação de dormência de sementes como estratégia para restauração florestal de pastagem tropical. Pesq Agropec Bras 48:148–156
R Core Team (2018) R: a language and environment for statistical computing. R Core Team, Vienna
Ribeiro LC, Pedrosa M, Borghetti F (2013) Heat shock effects on seed germination of five Brazilian savanna species. Plant Biol 15:152–157
Roberts EH (1973) Predicting the storage life of seeds. Seed Sci Technol 1:499–514
Rodrigues-Junior AG, Faria JMR, Vaz TAA, José AC (2015) Loss of desiccation tolerance and storage behavior in germinating seeds of Senna multijuga: implications for seed germination and conservation. New For 46:283–291
Rosa FC, Reiniger LRS, Golle DP, Muniz MFB, Curti AR (2012) Overcome dormancy and in vitro germination of seeds of Mimosa scabrella Bentham. Semin Cienc Agrar 33:1021–1026
Salazar A, Hodges SR, Maschinski J (2015) Chemical scarification improves seed germination of Trema lamarckiana (Cannabaceae), a potential tree species to restore South Florida endangered ecosystems. Seed Sci Technol 43:291–296
Shu K, Liu X, Xei Q, He Z (2016) Two faces of one seed: hormonal regulation of dormancy and germination. Mol Plant 9:34–35
Skirycz A, Castilho A, Chapparo C, Carvalho N, Tzotzos G, Siqueira JO (2014) Canga biodiversity, a matter of mining. Front Plant Sci 5:1–9
Souza Filho PWM, Souza EB, Silva Junior RO, Nascimento WR, Mendonça BRV, Guimarães JTF, Dall`Agnol R, Siqueira JO (2016) Four decades of land-cover, land-use and hydroclimatology changes in the Itacaiúnas River watershed, southeastern Amazon. J Environ Manag 167:175–184
Souza MP, Palhares D, Pereira LAR, Silveira CES (2015) Micropopagation of Apeiba tibourbou Aubl (Tilaciaceae), a multipurpose species with wide distribution in forest of Brasil. Am Intern J Bot 3:31–40
Szentesi A, Jermy T (1995) Predispersal seed predation in leguminous species: seed morphology and bruchid distribution. Oikos 73:23–32
Varela VP, Broski E, Sá STV (1991) Tratamentos pré-germinativos de sementes de espécies florestais da Amazônia: IV. Faveira camuzê—Stryphnodentron pulcherrimum (Willd.) Hochr Leguminosae. Rev Bras Sem 13:87–90
Walters C (2015) Orthodoxy, recalcitrance and in-between: describing variation in seed storage characteristics using threshold responses to water loss. Planta 242:397–406
Acknowledgements
Funding was provided by Instituto Tecnológico Vale and CNPq Grant 305831/2016-0 (SJR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramos, S.J., Caldeira, C.F., Gastauer, M. et al. Native leguminous plants for mineland revegetation in the eastern Amazon: seed characteristics and germination. New Forests 50, 859–872 (2019). https://doi.org/10.1007/s11056-019-09704-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-019-09704-1