Abstract
Key message
Mimosa luisana seeds germinate after 7 years of storage, suggesting that they have a long span life; an interesting characteristic for environmental restoration of semi-arid zones.
Abstract
Mimosa luisana is endemic to Mexico, provides ecosystem services and is economically and culturally important. This species exhibits morphological, anatomical and physiological qualities that make it potentially valuable in ecological restoration. This study evaluated the effects of seed age on seed germination, survival seedlings and growth of M. luisana, on the assumption that seed age positively influences the parameters related to germination. Mature fruits were collected at the semiarid Tehuacán–Cuicatlán Valley and the seeds were extracted. Healthy seeds were measured and weighed to obtain a uniform sample. Seed moisture content, imbibition rate, germination percentage, survival seedlings and growth were quantified. Seeds that were 84 months old showed the lowest moisture content (4.65%) and imbibition rate of unscarified seeds increased as seeds were older. Scarification considerably promoted germination, which was epigeal and phanerocotylar. Regardless of seed age, seedling growth was slow, with the presence of foliar cotyledons which persisted after the appearance of the protophylls, and the characteristics of an adult plant were observed until the day 22 after sowing. Mimosa luisana seeds are long-lived and the germination percentage depends on the age of the seed and whether or not it was scarified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Mexico, there are ca. 105 species of Mimosa L. (Leguminosae), 54% of which are endemic; thus, it is considered the most diverse genus of the mimosoids in the country (Grether et al. 2015). Mimosa luisana Brandegee is a species endemic to the semiarid Tehuacán–Cuicatlán Valley, which is located in the states of Puebla and Oaxaca, Mexico (Martínez-Bernal and Grether 2006). Mimosa luisana is considered a multipurpose species (Camargo-Ricalde et al. 2001) that forms resource islands (Camargo-Ricalde et al. 2002), which are reservoirs of arbuscular mycorrhizal fungal spores (Camargo-Ricalde and Dhillion 2003; Chimal-Sánchez 2015) and nitrogen-fixing bacteria (Camargo-Ricalde et al. 2010a) and also as a nurse plant for the columnar cactus Neobuxbaumia tetetzo (F.A.C.Weber ex K.Schum.) Backeb., a species endemic to this area (Valiente-Banuet and Ezcurra 1991).
Due to the fact that M. luisana provides ecosystem services, to its economic and cultural importance to local inhabitants (Camargo-Ricalde et al. 2001) and to its morphological, anatomical and physiological characteristics, this species has the potential to restore degraded environments within the Tehuacán–Cuicatlán Valley (Dhillion et al. 2004; Montaño-Arias et al. 2015, 2017). Through wood anatomical analysis, Montaño-Arias et al. (2017) determined that this species is resistant to drought events in adulthood, but its drought tolerance in other life cycle stages is unknown. There are studies that support the use of M. luisana in ecological restoration; however, the longevity of its seeds is unknown. These data would be particularly important to take in consideration, especially because the changes in precipitation regimens, predicted by the Intergovernmental Panel on Climate Change (IPCC).
The botanical characteristics of M. luisana are well known; however, the effect of seed longevity on germination is unknown. We consider that this knowledge is important because it could contribute to the understanding of the dynamic of plant establishment in an adverse environment, where Mimosa makes important association with micro- and macro-biota, as part of the ecosystem.
The seed is a very important stage of the life cycle of angiosperms since the establishment and growth of the species depend on it. Anatomical studies on the seed coat of M. luisana, described an external layer of macrosclereids and an internal layer of osteosclereids, whose make the seed coat hard and impermeable (Montaño-Arias 2016). According to some studies, seeds need scarification to germinate in a wide range of temperatures (Camargo-Ricalde et al. 2004; Montaño-Arias et al. 2015). Within the genus Mimosa, seed longevity has been poorly explored, although some studies on Leguminosae describe seeds with long life span, for 10 years (Moreno-Casasola 1973; Parra 1984), 50 years (Ewart 1908) and 81 years.
Research on germination of Mimosa seeds is increasing but it is still a research challenge. These studies investigate the optimal temperatures for germination (Camargo-Ricalde and Grether 1998; Camargo-Ricalde et al. 2004; Pavón et al. 2011; Montaño-Arias et al. 2015), and very few are focused on the effect of seed longevity on germination (Silveira et al. 2014).
In other mimosoid legumes, such as Leucaena leucocephala cv. Cunningham (González et al. 2012) and Albizia lebbeck (L.) Benth. (González et al. 2009), a negative correlation between seed age and germination percentage was found. According to González et al. (2009), seed age can affect the time of seedling emergence; for example, Gliricidia sepium (Jacq.) Kunth ex Walp. showed lower emergence percentage compared to Albizia lebbeck; however, at beginning of emergence, there were no differences between newly collected and older seeds. Seedling vigour is another variable associated with seed longevity; it was found that the seedlings size decreased as seeds get older González et al. (2009).
In the particular case of Mimosa, Gómez-Pompa et al. (1976) reported that naturally aged seeds of M. pudica L. showed a low germination percentage, while Silveira et al. (2014) found that the seeds of M. foliolosa Benth. subsp. pachycarpa (Benth.) Barneby, artificially stored for 12, 24 or 36 months, showed an accelerated germination process and an increased germination percentage.
Considering the biological and ecological relevance of M. luisana and the lack of studies referring the effect of seed longevity on germination, the present study explores the physiological implications of the seed longevity for plant establishment analysing: seed moisture content, seed imbibition rate, germination percentage and seedlings survival, under the assumption that seed age critically influences these parameters.
Methods
Mimosa luisana naturally occurs and is endemic to the scrublands of the semiarid area of Tehuacán-Cuicatlán Valley, in the Mexican states of Puebla and Oaxaca. A population located at the state of Puebla (18°15′ 23.7ʺ N, 97°09′03.3ʺ W, at 2,232 masl), was selected based on the predominance of Mimosa luisana. Mature fruits were collected in November of 2010, 2011, 2016 and 2017, the season of maximum productivity. Each year, samples were obtained from eight individuals (trees) with the same height and coverage (2.0 and 2.0 m, respectively). Voucher specimens were deposited at the official herbarium of the Universidad Autónoma Metropolitana, Iztapalapa campus (Herbario Metropolitano, UAMIZ) (Table 1).
All seeds were extracted from mature fruits manually and those that showed no evidence of infestation by bruchids (healthy seeds) were selected under a stereomicroscope (Nikon, SMZ800, Japan) for the experiments. According to the year of collection, seeds were organised into four age groups: 84, 72, 12 and 0 months old (recently collected) as control.
Considering that seed size and weigh can influence the germination rate (Matilla 2004; Skogen et al. 2010), we measured the length, width and thickness of the seeds with a digital calliper (Absolute Digimatic, CD-6" CS) in all seeds of each age group. Individual seed were weighted (g) individually with an analytical balance (Denver Instrument, APX-100, US). Seeds in the range of 2.0–2.5 mm length, 2.0–2.4 mm width and 1.8–2.2 mm thickness, and 0.08–0.10 g weight were used for the experiments. Seeds were stored in airtight glass containers, in the dark at 20 °C, and 50–60% relative humidity (RH) until germination experiments.
Moisture content (MC)
Seed moisture content (%) was measured as follows: The “initial weight” was obtained with an analytical balance (Denver Instrument, APX-100), from three replicates of 30 seeds per age group (90 seeds per treatment); subsequently seeds were placed in an incubator (Rios Rocha, S.A., Model EC-33) at 65 °C until constant weight was achieved, to obtain the “Final weight”. The MC (%) was calculated according to ISTA (2013):
Treatments and experimental design
The experimental design was factorial 4 × 2: four seed age groups 84, 72, 12 and 0 months old (recently collected, control) and two scarification conditions: unscarified (UNS, control) and mechanically scarified (S) giving a total of eight treatments. Seeds were mechanically scarified, with a nail clipper, cutting the seed coat at the opposite side of the micropyle, to prevent the embryonic axis damage (Fig. 1a, b). All treatments were arranged completely randomised and replicates will be indicated for each experiment described below.
Seed and seedlings of Mimosa luisana. a unscarified seed; b scarified seed; c Mimosa seedling with cotyledons and protophyll, and d Mimosa plant with cotyledons persistent and metaphylls with two pairs of pinnae each with four pairs of leaflets. C cotyledon, P protophyll, M micropyle, ME metaphyll, LF leaflets, SS scarified seed. Scale: a, b = 0.9 mm; c, d = 1 cm
Imbibition rate (IR)
There were three replicates with 30 seeds per treatment (90 per treatment). Each replicate was placed in 20 mL of water at the optimal germination temperature (25 °C) (Montaño-Arias et al. 2015). The imbibition process was monitored every 3, 6, 12, 24 and 48 h, until the seed stop imbibition. At each interval, the seeds were extracted, the excess water was removed using Whatman filter paper No. 2 and their initial weight was recorded with an analytical balance (Denver Instrument, APX-100). The seeds were then dried in an incubator (Rios Rocha, S.A., Model EC-33) at 65 °C until constant weight was achieved and this was considered as final weight.
The IR (%) was calculated according to Jacobo-Pereira et al. (2016) as follows:
Germination experiment
A total of 100 seeds per age group were tested for germination. Twenty scarified seeds were sown in each of five sterile Petri dishes (9 cm diameter), with filter paper (Whatman no. 2), and seeds were moistened with deionised water and kept at 25 °C in a controlled environment chamber (Conviron T 38/Lb/AP); 25 °C, with 14 h light and 10 h darkness. Seeds were considered germinated when they showed emerged radicle of 1 mm (Bewley and Black 1994). All experiments lasted 8 days, when the scarified seeds reached 100% germination.
The following variables were evaluated according to Piedrahita (1997, 1998), Enríquez-Peña et al. (2004), Weng and Hsu (2006) and Montaño-Arias et al. (2015):
-
I.
Germination percentage (GP) was calculated as GP = Ng × 100/Ns,
where Ng total germinated seeds and Ns Total sowed seeds.
-
II.
Half-maximal germination (G50) was the number of days after sowing needed to reach 50% of the total germination, estimated in this study through the interpolation of one day before and 1 day after 50% of total germination was achieved.
-
III.
Saturation rate (SR) was the germination time related to the total germination percentage: SR = Σ(niti)/N,
where ni number of seeds germinated in 1 day (i).
ti number of days after sowing;
N total number of seeds sown.
-
IV.
Germination rate (GR) was calculated as follows: GR = Σ (Ni)/t,
where Ni number of seeds germinated in 1 day (i); t time from sowing until germination of the last seed.
Survival and growth
Seedlings (15 days after sowing, from the scarified seeds (treatments 84S, 72S, 12S, 0S)) were transplanted into 0.5 kg polypropylene pots (10 cm diameter × 50 cm hight) containing a mixture 3:1:1 of native soil:agrolita:volcanic rock (0.2 mm diameter). Native soil was obtained from Tehuacán–Cuicatlán Valley, the place of seed harvest. Pots were placed in the controlled environment chamber, at 25 °C, with 14 h light and 10 h darkness. The seedlings were watered every other day with 300 mL of deionised water. The seedlings were monitored daily, assessing (1) percentage of survival (green erect seedling and turgid), (2) seedling height (mm), measured from the base to the apex of its distal leaves, (3) appearance of the protophylls (Fig. 1c), and (4) appearance of the metaphylls (Fig. 1d). The terminology used is according to Duke and Polhill (1981).
Statistical analysis
Data were examined by a two-way ANOVA test (P < 0.05), followed by a comparison of means test (Tukey’s HSD, P < 0.05) (Sokal and Rohlf 1995). All analyses were performed with NCSS software (Hintze 2001).
Results
Moisture content (MC) and imbibition rate (IR)
The oldest seed groups (84 months and 7 years) exhibited the lowest MC, while there were no significant differences among the seeds of the other three ages (Table 2).
Both, S and UNS seeds, imbibe up to 80% of their total absorption capacity after 3 h. It should be noted that the S seeds imbibed up to 0.5 mL (six times its size), while the UNS seeds (control) were only able to absorb 0.2 mL (three times its size). The calculation of the IR revealed a significant interaction between the treatments (F = 10.57, P < 0.001), which indicates that the IR depended on the age of the seed and the scarification treatment (Table 3).
Germination experiment
Seed germination was epigeal and phanerocotylar. Our results showed that scarification promoted germination as expected (Table 3). The control-UNS treatment began its germination between the second and the seventh day, and its germination percentage (GP) was low (< 10%); while the germination of all scarified (S) seeds, regardless their age, began between the first and second day after sowing and their GP was significantly high (> 85%, F = 4.47, p < 0.05, Table 4).
Factor analysis of the GP showed a significant interaction between treatments (Table 4); however, there was a difference between the S and UNS seeds, as scarification accelerated the germination process (Table 3).
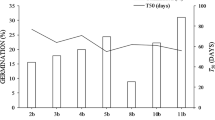
In the S seed treatment, it was observed that the lowest GP was achieved by the 84- and 72-month-old seed groups, while the recently collected and the 12-month-old seeds achieved the highest GP (Table 3). It should be noted that the G50 showed no differences among treatments, indicating that 2 days are required to reach 50% germination (Table 4).
The same behaviour was observed in the SR; analysis also revealed a significant interaction in this variable (Table 4 and Fig. 2a), but there was a clear separation between the unscarified and scarified group. Intra-group analysis produced a similar pattern; the 0-UNS seeds required on average 0.5 days to reach only 5% of final germination; this group showed no significant differences compared to the other age’s categories. As for the S seeds, those that were recently collected required 3 days to reach a final GP of 99%, while seeds of 84, 72 and 12 months needed only 2 days to reach final GP of 86%, 86% and 100%, respectively.
Statistical analysis related to the GR revealed a significant interaction between the treatments (Table 4), regardless of seed age; it was observed that when the seeds were scarified, a higher number of seeds germinate every day. The 12-month-old seeds (12S) were the treatment that showed the highest germination rate (nine seeds per day) (Fig. 2b).
Survival and growth
The seedlings exhibited a low percentage of survival. Seedlings from the oldest seeds tended to live less; seedlings from the 84-month-old group showed a survival of 45% at 10 days but only 10% of seedlings survive 15 days. On the same number of days (10 days), seedlings from 72- and 12-month-old showed a 90% of survival, and both manage to reach 20 days (5% and 10%, respectively) (Table 5). Interestingly, the recently collected seeds (0 month old) showed a survival of 70% at 10 days, and they lived more (22 days) but with a very low percentage of survival (5%) (Table 5).
The surviving seedlings, regardless of treatments grew between 0.5 and 1.0 mm per day. The cotyledons were foliar and they persisted after the appearance of the protophyll (Fig. 1c, d). The seedlings developed the protophyll, with four pairs of leaflets (Fig. 1c), 13 days after sowing. Eighteen days after sowing, the seedlings developed the first bipinnate leaf, with two pairs of pinnae, each with three or four pairs of leaflets. Between 20 and 22 days after sowing, the metaphylls emerged with two pairs of pinnae each with four pairs of leaflets (Fig. 1d).
Discussion
Moisture content (MC) and imbibition rate (IR)
The MC is an important parameter for the preservation of seeds viability, so it is the first aspect to consider in their storage (Giamminola et al. 2012). Seeds loose their MC with time. Our seeds from the 84-month-old group showed a low moisture content (4.65% ± 0.34%), while the recently harvest seeds (0 months) had a MC of 6.48% ± 0.06%, which suggests that they are orthodox seeds (Roberts 1973; Giamminola et al. 2012). The MC data together with the germination results indicate that M. luisana seeds remain viable 7 years after collection indicating a large life span as other mimosoids like M. glomerata Forssk.[= Dichrostachys cinerea (L.) Wight & Arn.] (Crocker 1938), M. pudica (Ewart 1908; Moreno-Casasola 1973) and M. foliolosa subsp. pachycarpa (Silveira et al. 2014) and others legumes like Astragalus massiliensis Lain., Cassia multljuga Rich., Cytisus austriacus Linn., Leucaena leucocephala Linn. and Melilotus lutea Gueld (Crocker 1938).
Seed longevity has been attributed to the presence of a hard and impermeable testa. According to Montaño-Arias (2016), M. luisana exhibits an impermeable testa with two layers of sclereid, characteristic that supports the notion of their long life span. Silveira et al. (2014) reported that the seeds of M. foliolosa subsp. pachycarpa have a long life span; however, they mentioned that older and unscarified seeds showed higher germination percentage than younger unscarified seeds. In contrast, in the present study, there were no significant differences in GP between seed age groups of unscarified seeds; which indicate that intact seeds of M. luisana do not deteriorate with time but for scarified seeds GP showed statistical difference between groups 72, 84 and 0, 12.
Nevertheless, MC is inversely correlated with IR since lower moisture gives the seed a greater ability to imbibition, the first step in the initiation of the germination process (Bewley and Black 1994). According to Moreno et al. (2006), imbibition is determined by the permeability of the testa. But in this study, IR was evaluated in S and UNS seeds of different ages groups and it was observed that the IR depended on seed age: the older the seed, the lower the MC and consequently the greater the imbibition. Furthermore, IR depends on the scarification; if the seed is scarified, water enters easily, while without scarification, the testa function as a barrier and it inhibits water entrance.
Germination experiment
Epigeal and phanerocotylar germination has been reported in other Mimosa species such as M. adenantheroides (M. Martens & Galeotti) Benth., M. calcicola B. L. Rob., M. lacerata Rose, M. polyantha Benth., M. purpusii Brandegee and M. texana (A. Gray) Small var. filipes (Britton & Rose) Barneby (Camargo-Ricalde et al. 2004) and also M. luisana (Montaño-Arias et al. 2015). Our study confirms that M. luisana seed germination required scarification, as was observed in other species of the genus by Camargo-Ricalde and Grether (1998), Leal and Biondi (2007), Biondi and Leal (2008), Chauhan and Johnson (2008) and Jayasuriya et al. (2013). When our seeds were scarified, germination began at the first days after sowing, and showed the highest GP, similar to the results reported in M. bimucronata (DC.) O. Kuntze (Ribas et al. 1996), M. aculeaticarpa Ortega var. biuncifera (Benth.) Barneby (Pavón et al. 2011), M. setosa Benth. (Sperandio et al. 2013), M. quitensis Benth. (Achipiz-Fajardo et al. 2014) and M. aculeaticarpa var. aculeaticarpa (Montaño-Arias et al. 2015). As a general conclusion, we found that there is a significant interaction between the age of the seed and the treatment received (scarification), so the GP depends on these two factors. Our results showed that the scarified seeds always germinated quicker than unscarified seeds. Recently collected and scarified seeds took more 3 days to reach their final GP, while the older (12, 72 and 84 months old) scarified seeds, took only 2 days. Similar results were reported for M. adenantheroides, M. calcicola, M. lacerata, M polyantha, M. purpusii and M. texana var. filipes (Camargo-Ricalde et al. 2004), M. luisana (Camargo-Ricalde et al. 2004; Montaño-Arias et al. 2015) and M. foliolosa subsp. pachycarpa (Silveira et al. 2014). In our study, seeds of 84 and 72 months showed decreased in the final-GP, which is consistent with the report for M. pudica (Gómez-Pompa et al. 1976).
Survival and growth
In Mimosa seedlings, these aspects are practically unexplored (Parra 1984; Camargo-Ricalde and Grether 1998; Santiago et al. 2001; Niroula et al. 2009; Silveira et al. 2014). When the seeds of M. luisana were scarified they exhibited a rapid GR; however, the growth of the seedling, under the same germination conditions (25 °C, 14 h light and 10 h darkness) was slow. The seedling survival is very important and it has several ecological implications. According to Sánchez et al. (2005), a rapid GR implies an early emergence of seedlings in the field that could ensure the establishment of the plants. But in the case of M. luisana, their seedlings and adults are consumed by goats, which are considered one of the dispersers of this species (Giordani et al. 2015). If M. luisana seedlings are slow-growing and are eaten by goats, this may explain why very few seedlings or young plants have been observed in the field (pers. obs.). Additionally, it is necessary to consider the low water availability in its natural ambient, characterised by a semiarid climate with low precipitation; which is another limiting factor for the emergence and establishment in natural conditions. It should be borne in mind that in this study, 25 °C was established as the optimal temperature for germination (Montaño-Arias et al. 2015) and plants were maintained at this temperature the whole experiment; however, the seedlings mortality rate was high, suggesting that this temperature may not be optimal for their growth. Soil is known to be an important factor for seedling establishment; according to Pavón et al. (2011), when native soil is used, there is a greater seedling survival. However, this was not the case for M. luisana in our study, although we use a mixture of native soil. Other important factor that could be affecting seedling survival is the failure to form mycorrhizae, this mutualistic association improves uptake of nutrients and water (Camargo-Ricalde et al. 2010b; Peña-Becerril et al. 2016). It has been found that arbuscular mycorrhizal fungi play a key role in the establishment, growth and survival of some Mimosa species in semi-arid environments, providing protection against drought, pathogenic fungi and nematode (de Souza et al. 2016). No information was found about the optimal growing temperature for mimosas, and in our knowledge, this is the first study to evaluate the effect of seed longevity on germination, and growth of M. luisana. The information generated in the present study is an important variable that needs to be taken into consideration, given the predictions of the IPCC, where fluctuations in climatic parameters are expected (Christensen et al. 2007).
Mimosa luisana seedling displayed the characteristics of an adult plant after 22 days. For future research, it is recommended to study the variables affecting seedling establishment (temperature, water, soil and light). An important conclusion is that M. luisana seeds have a long life span, desirable characteristic for restoration of semi-arid zones in Mexico in the context of Global warming.
References
Achipiz-Fajardo J, Gálvez-Campo GM, Morales-Velasco S, Vivas-Quila NJ (2014) Guarango (Mimosa quitensis) opción forrajera para sistemas ganaderos de clima frio. Biotecnología en el Sector Agropecuario y Agroindustrial 12:71–80
Bewley JD, Black M (1994) Seeds: germination, structure and composition. In: JD Bewley, M Black (eds) Seeds: Physiology of Development and Germination. Plenum Press, New York
Biondi D, Leal L (2008) Tratamentos pré-germinativos em sementes de Mimosa strobiliflora Burkart. Sci. Agraria 9:245–248
Camargo-Ricalde SL, Dhillion SS (2003) Endemic Mimosa species can serve as mycorrhizal “resource islands” within semiarid communities of the Tehuacán-Cuicatlán Valley, Mexico. Mycorrhiza 13:129–136
Camargo-Ricalde SL, Grether R (1998) Germinación, dispersión y establecimiento de plántulas de Mimosa tenuiflora (Leguminosae) en México. Rev Biol Trop 46:1–12
Camargo-Ricalde SL, Grether R, Martínez-Bernal A, García-García V, Barrios-del-Rosal S (2001) Especies útiles del género Mimosa (Fabaceae-Mimosoideae) en México. Bol Soc Bot Méx 68:33–44
Camargo-Ricalde SL, Dhillion SS, Grether R (2002) Community structure of endemic Mimosa species and environmental heterogeneity in a semi-arid Mexican valley. J Veg Sci 13:697–704
Camargo-Ricalde SL, Dhillion SS, García-García V (2004) Phenology, and seed production and germination of seven endemic Mimosa species (Fabaceae-Mimosoideae) of the Tehuacán-Cuicatlán Valley. Mexico J Arid Environ 58:423–437
Camargo-Ricalde SL, Reyes-Jaramillo I, Montaño NM (2010a) Forestry insularity effect of four Mimosa L. species (Leguminosae-Mimosoideae) on soil nutrients of a Mexican semiarid ecosystem. Agroforest Syst 80:385–397
Camargo-Ricalde SL, Montaño NM, Reyes-Jaramillo I, Jiménez-González C, Dhillion SS (2010b) Effect of mycorrhizae on seedlings of six endemic Mimosa L. species (Leguminosae-Mimosoideae) from the semi-arid Tehuacán-Cuicatlán Valley, Mexico. Trees Struct Funct 24:67–78
Chauhan BS, Johnson DE (2008) Seed germination and seedling emergence of giant sensitive plant (Mimosa invisa). Weed Sci 56:244–248
Chimal-Sánchez E (2015) Influencia de cuatro especies del género Mimosa L. (Leguminosae) en la diversidad y potencial de inóculo de hongos micorrizógenos arbusculares en un ecosistema semiárido de México. Master’s Thesis, Universidad Autónoma Metropolitana, Unidad Iztapalapa. 98 pp.
Christensen JH, Hewitson B, Busuioc A, Chen A, Gao X, Held I, Jones R, Kolli RK, Kwon W-T, Laprise R, Magaña-Rueda V, Mearns L, Menéndez CG, Räisänen J, Rinke A, Sarr A, Whetton P (2007) Regional climate projections. In: Solomon S, Qin D, Manning M, Marquis M, Averyt KB, Tignor M, LeRoy-Miller H, Chen Z (eds) Climate change: the physical science basis. Summary for Policymarkers. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 847–940
Crocker W (1938) Life-span of seeds. Bot Rev 4:235–274
de Souza TA, Rodriguez-Echeverría S, de Andrade LA, Freitas H (2016) Arbuscular mycorrhizal fungi in Mimosa tenuiflora (Willd.) Poir from Brazilian semi-arid. Braz J Microbiol 47:359–366. https://doi.org/10.1016/j.bjm.2016.01.023
Dhillion SS, Aguilar-Støen M, Camargo-Ricalde SL (2004) Integrative ecological restoration and local involvement in the Tehuacán-Cuicatlán Valley, Mexico. Environ Conserv 13:1–3
Duke JA, Polhill RM (1981) Seedlings of Legumes. In: Polhill RM, Raven PH (eds) Advances in legume systematics. Part 2: 941.949. The Royal Botanic Gardens, Kew
Enríquez-Peña E, Suzán-Azpiri H, Malda-Barrera G (2004) Viabilidad y germinación de semillas de Taxodium mucronatum (Ten.) en el estado de Querétaro. México México Agrociencia 38:357–381
Ewart AJ (1908) On the longevity of seeds. Proc R Soc Vic 21:1–210
Giamminola EM, Morandini MN, De Viana ML (2012) Respuesta a la desecación y a la temperatura de almacenamiento del germoplasma de Prosopis nigra (Grisebach) Hieron. y Ziziphus mistol Griseb. Gestión y Ambiente 15:19–25
Giordani L, Baraza E, Camargo-Ricalde SL, Moe SR (2015) The domestic goat as a potential seed disperser of Mimosa luisana (Leguminosae, Mimosoideae) in the Tehuacán-Cuicatlán Valley. Mexico J Trop Ecol 31:91–94
Gómez-Pompa A, Vázquez-Yanes C, Del Amo S, Butanda A (eds). (1976) Investigaciones sobre la regeneración de selvas altas en Veracruz, México. Cia. Editorial Continental, México. 676 pp.
González Y, Sánchez JA, Reino J, Montejo L (2009) Efecto de los tratamientos de hidratación-deshidratación en la germinación, la emergencia y el vigor de las plántulas de Albizia lebbeck y Gliricidia sepium. Pastos y Forrajes 32:255–262
González Y, Reino J, Sánchez JA, Machado R (2012) Efecto del almacenamiento al ambiente en semillas de Leucaena leucocephala cv. Cunningham sometidas a hidratación parcial. Pastos y Forrajes 35:393–399
Grether R, Camargo-Ricalde SL, Martínez-Bernal A, Montaño-Arias S, Fraile ME (2015) Diversity and geographical distribution patterns of the genus Mimosa (Mimosoideae) in the United States, Mexico, and Central America. In: Fortunato EH (ed) V Conferencia Internacional de Leguminosas (VILC). Fundación Centro de Integración Comunicación, Cultura y Salud (CICCUS). Buenos Aires, Argentina, p 224
Hintze J (2001) NCSS 2001. NCSS, LLC. Kaysville, Utah, USA. www.ncss.com
ISTA (2013) International rules for seed testing: edition 2013. Bassersdorf, Zurich, p 433
Jacobo-Pereira C, Romo-Campos R, Flores J (2016) Seed germination of Magnolia pugana (Magnoliaceae), an endemic and endangered species from Western Mexico. Bot Sci 94:575–584. https://doi.org/10.17129/botsci.512
Jayasuriya GK, Wijetunga AS, Baskin JM, Baskin CC (2013) Seed dormancy and storage behaviour in tropical Fabaceae: a study of 100 species from Sri Lanka. Seed Sci Res 23:257–269
Leal L, Biondi D (2007) Comportamento germinativo de sementes de Mimosa dolens Vell. Pub. UEPG Ci Exatas Terra Ci. Agr Eng 13:37–43
Martínez-Bernal A, Grether R (2006) Mimosa. En: Novelo A. y Medina-Lemus R. (eds.). Flora del Valle de Tehuacán-Cuicatlán. Fascículo 44. Instituto de Biología, Universidad Nacional Autónoma de México, México. 1–108 p.
Matilla A (2004) Ecofisiología de la germinación de semillas. En: Sánchez A, Reigosa-Roger MJ, Pedroi-Bonjoch N. La ecofisiología Vegetal: Una ciencia de síntesis. pp. 901–922. Thomson-Paraninfo, España.
Montaño-Arias SA (2016) Respuestas ecoanatómicas y ecofisiológicas de dos taxa de Mimosa (Leguminosae) ante el cambio climático. Doctoral thesis, Universidad Autónoma Metropolitana, Unidad Iztapalapa, Ciudad de México. 253 pp.
Montaño-Arias SA, Camargo-Ricalde SL, Grether R, Díaz-Pontones D (2015) Effect of scarification and temperature on seed germination of two Mexican species of Mimosa (Leguminosae-Mimosoideae). Bot Sci 93:649–659
Montaño-Arias SA, Camargo-Ricalde SL, Grether R, Díaz-Pontones D (2017) Ecoanatomía de la madera de dos taxa mexicanos del género Mimosa (Leguminosae-Mimosoideae). Acta Bot Mex 118:105–120. https://doi.org/10.21829/abm118.2017.1203
Moreno F, Plaza GA, Magnitskiy SV (2006) Efecto de la testa sobre la germinación de semillas de caucho (Hevea brasiliensis Muell.). Agron Colomb 24:290–295
Moreno-Casasola P (1973) Estudio sobre viabilidad y latencia de semillas tropicales. Undergraduate thesis, Universidad Nacional Autónoma de México, UNAM, México, pp 78
Niroula B, Parajuli D, Jha S (2009) Ecophysiology of Mimosa pudica L. at Biratnagar. Eastern Nepal Our Nature 7:177–181. https://doi.org/10.3126/on.v7i1.2568
Parra GP (1984) Estudio de la morfología externa de plántulas de Calliandra gracilis, Mimosa albida, Mimosa arenosa, Mimosa camporum y Mimosa tenuiflora. Rev Fac Agron (Maracay) 13:311–350
Pavón NP, Ballato-Santos J, Pérez-Pérez C (2011) Germinación y establecimiento de Mimosa aculeaticarpa var. biuncifera (Fabaceae-Mimosoideae). Rev Mex Biodivers 82:653–661
Peña-Becerril JC, Monroy-Ata A, Orozco-Almanza MS, García-Amador EM (2016) Establishment of Mimosa biuncifera (Fabaceae) inoculated with arbuscular mycorrhizal fungi in greenhouse and field drought conditions. Rev Biol Trop 64:791–803
Piedrahita CE (1997) Germinación de semillas de Jacaranda copaia bajo condiciones contrastantes de luz, Colombia. Cron For Medio Ambient 12:1–4
Piedrahita CE (1998) Aumento del vigor en semillas de Pinus patula (Schlecht. & Cham.) por el efecto de osmoacondicionamiento. Cron For Medio Ambient 13:1–21
Ribas FLL, Fossati LC, Nogueira AC (1996) Superação da dormência de sementes de Mimosa bimucronata (DC.) O. Kuntze (maricá). Rev Bras Sementes 18:98–101
Roberts EH (1973) Predicting the storage life of seeds. Seed Sci Technol 1:499–514
Sánchez JA, Reino J, Muñoz BC, González Y, Montejo L, Machado R (2005) Germinación y vigor de plántulas de Leucaena leucocephala cv. Cunningham en respuesta a tratamientos de hidratación-deshidratación. Pastos y Forrajes 28:209–230
Santiago AMP, Nogueira RJMC, Lopes EC (2001) Crescimento em plantas jovens de Mimosa caesalpiniifolia Benth., cultivadas sob estresse hídrico. Ecossistema 26:23–30
Silveira FAO, Negreiros D, Ranieri BD, Silva CA, Araújo LM, Fernandes W (2014) Effect of seed storage on germination, seedling growth and survival of Mimosa foliolosa (Fabaceae): implications for seed banks and restoration ecology. Trop Ecol 55:385–392
Skogen KA, Senack L, Holsinger KE (2010) Dormancy, small seed size and low germination rates contribute to low recruitment in Desmodium cuspidatum (Fabaceae). J Torrey Bot Soc 137:355–365
Sokal RR, Rohlf FJ (1995) Biometry. Freeman and Company, San Francisco, p 887
Sperandio HV, Lopes JC, Matheus MT (2013) Superação de dormência em sementes de Mimosa setosa Benth. Com Sci 4:385–390
Valiente-Banuet A, Ezcurra E (1991) Shade as a cause of the association between the cactus Neobuxbaumia tetetzo and the nurse shrub Mimosa luisana. J Ecol 79:961–971
Weng J-H, Hsu F-H (2006) Variation of germination response to temperature in Formosan lily (Lilium formosanum Wall.) collected from different latitudes and elevations in Taiwan. Plant Prod Sci 9:281–286
Acknowledgements
The first author wishes to thank the “Consejo Nacional de Ciencia y Tecnología of México” (CONACyT) for the financial support Granted (228993/211528) for the postdoctoral stay of the first author, under the advice of Zavaleta-Mancera. The authors also wish to thank Rosalva García Sánchez, of the Facultad de Estudios Superiores Zaragoza, UNAM, for the technical facilities. We also thank to David Manuel Díaz Pontones for the revision of the manuscript.
Author information
Authors and Affiliations
Contributions
Author contributions
SAM-A and HAZ-M conceived and study designed. SAM-A performed the field work and experimental development. RG and SLC-R performed the analysis and interpretation of the data. SAM-A wrote the manuscript with the help of HAZ-M, SLC-R and RG. All the authors contributed to the discussion, review and approval of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Buckeridge.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Montaño-Arias, S.A., Zavaleta-Mancera, H.A., Camargo-Ricalde, S.L. et al. Effect of seed age on germination, seedling survival and growth of Mimosa luisana (Leguminosae). Trees 35, 231–239 (2021). https://doi.org/10.1007/s00468-020-02031-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-020-02031-5