Abstract
Spontaneous combustion of dried water-immersed coal (DWIC) wastes many coal resources, and so the effect of water immersion on the characteristic temperature during coal low-temperature oxidation and its possible reasons were investigated. The characteristic temperatures of coal oxidation were obtained by thermogravimetry. The possible causes for the changes in characteristic temperatures were analyzed from the pore structure and microscopic function groups by means of liquid nitrogen adsorption/desorption experiment and in-situ Fourier transform infrared spectroscopy. The results show that, in the early stages of coal low-temperature oxidation, the characteristic temperatures of DWIC samples were lower compared to those of raw coal samples. The differences between the characteristic temperatures of the DWIC and raw coal first increased but then decreased as the temperature rose. When the coal’s temperature rose to the maximum weight gain temperature, the differences had almost disappeared. Water-immersion reduced the coal’s specific surface area but increased the average diameter of its pores and the total pore volume. These changes in the coal pores were beneficial for oxygen migration and transport. During the whole low-temperature oxidation stage, the DWIC hydroxyl concentration is higher compared to that of raw coal, and this made the initial reaction of coal and oxygen more likely to occur. In the late low-temperature oxidation stages, the methyl concentration had a considerable influence on the coal–oxygen reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite continuing adjustments in the world's energy industries, coal is still one of its most important basic energy sources (Wang et al., 2018a; Zhang et al., 2020). In 2019, coal still accounted for 27% of the world’s primary energy consumption (BP, 2020). However, in underground mines, coal spontaneous combustion impedes safe and efficient coal mining and it is a common problem and concern for the coal industry in many countries (Elick et al., 2013; Karaoulis et al., 2014; Rúa et al., 2018). In the coal mines in western China that produce from multiple seams, water commonly accumulates in the upper goaf zones and this causes the residual coal in that goaf to be immersed in water for long periods of time (Zhao et al., 2016). Air leakage from mining-induced fissures and from drill holes will dry out the soaked coal after a period of time (Shao et al., 2018; Ti et al., 2018; Wang et al., 2019), and dried water-immersed coal (DWIC) is formed (Song et al., 2018). During subsequent coal mining, the DWIC is easily oxidized and self-heated or even spontaneous combustion can occur (Tang and Xue, 2017). This phenomenon causes a huge waste of coal resources.
Researchers have carried out numerous studies on DWIC and some believe that long-term water immersion dissolves some of organic and inorganic matter on the surface of the coal (Yang et al., 2017) resulting in DWIC and raw coal having considerably different physical and chemical properties. For example, Zhai et al. (2020b) used a laser thermal conductivity analyzer to investigate the changes in thermal properties of bituminous coal after water immersion. They found that the thermal diffusivity, specific heat capacity, and thermal conductivity of the water-immersed coal were significantly reduced and this would make the coal more prone to oxidation and self-heating. This would significantly increase the risk of spontaneous combustion. Tang and Xue (2017) studied the microstructure of coal samples after long-term water immersion and reported that long-term water immersion dissolved some of the refractory compounds and made the water-immersed coal more prone to spontaneous combustion. Zhong et al. (2019) showed that the initial heat release temperature of water-immersed coal was lower than that of raw coal and the amount of heat released during the low-temperature oxidation was higher. Yang et al. (2017) conducted experiments on coal samples from three different mines. Their results showed that during oxidation the oxygen consumption and production of oxidation products such as CO, CO2, and C2H4 were greater for water-immersed coal than they were for raw coal. They also believe that the influence of water-immersion on coal was significantly correlated with coalification. Most of the studies mentioned above considered changes in the coal’s structure and properties or the coal–oxygen reaction.
The spontaneous combustion of coal is a very complex physical and chemical process and one of the most important stages is low-temperature oxidation (Green et al., 2012; Qi et al., 2015). During the thermogravimetric analysis of coal spontaneous combustion, the characteristic temperatures can reflect qualitative changes in coal–oxygen reaction, and these temperatures are commonly used to divide the spontaneous combustion stages (Qi et al., 2017; Zhai et al., 2020a). By comparing the different characteristic temperatures of different coal samples, one can judge the progress of the coal–oxygen reaction (Deng et al., 2017; Wang et al., 2018; Zhang et al., 2021). In coal, the pores act as channels for gas storage, diffusion, and migration. These properties determine the coal’s capacity for physical and chemical oxygen adsorption (Wang et al., 1999; Parsa et al., 2017). The water is not only adsorbed by the coal’s surface pores but it also infiltrates the capillary pores. This will increase the water vapor pressure, increase the pores’ volume, and may even generate new pores (Wang et al., 2018b; Zhai et al., 2019). Long-term water immersion inevitably leads to changes in the coal’s pore structure and this will affect the coal’s oxygen adsorption and the coal–oxygen reaction (Yu et al., 2020). On the microscopic level, coal spontaneous combustion is caused by the reaction of oxygen and functional groups on the coal’s surface. Differences in the number and types of functional groups lead to the different tendency of coal spontaneous combustion (Xiao et al., 2018; Xie et al., 2018).
For this study, thermogravimetry was used to determine characteristic temperatures to judge the effects of water immersion on the coal low-temperature oxidation. The reason by which this occurs was analyzed by comparing the differences of the microstructural and the functional group concentrations between raw coal and DWIC coal. This study provides a theoretical basis for understanding the mechanisms that cause spontaneous combustion of DWIC in goaf.
Materials and Methods
Coal Samples
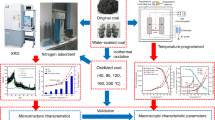
Two kinds of coal with different degrees of coalification were used for this research. The samples were collected from Cilinshan (CLS meager coal) Coal Mine in China’s Shanxi Province and Xiayukou (XYK lean coal) Coal Mine in China’s Shaanxi Province. The coal samples’ surface oxide layers were removed and then the samples were crushed and ground to sub-200 mesh powders; these were so-called raw coal samples. Portions of the above two coal samples were put into conical bottles with the same amount of water and immersed at room temperature for 30 d after which the samples were filtered, drained, and then transferred to a vacuum-drying oven and dried for 72 h at 30 °C to obtain DWIC samples. The “raw coal” samples were not immersed in water but they underwent the same drying process. Both the DWIC and the raw coal were stored in glass bottles to prevent oxidation before they were used in the experiments. Proximate analyses of the coal samples are listed in Table 1.
As Table 1 shows, the DWIC samples have less ash than the raw coal samples but higher proportions of volatiles and fixed carbon. These differences are due to the dissolution of organic matter, inorganic matter, and incombustible substances from the DWIC samples during the 30 days of water immersion (Tang and Xue, 2017).
Experimental Procedures
Thermogravimetric Experiments
The coal sample’s weight loss during heating was analyzed by the thermogravimetric method. TG209C (Netzsch-Gerätebau GmbH, Selb Germany), a Thermogravimetric Analyzer, was used for the experiments. In these experiments, a 1 g portion of the sub-200 mesh coal sample was used for each test. The initial temperature for each heating experiments was 30 °C, the final temperature was 800 °C, and the samples were heated at a rate of 10 °C/min. The sample’s weight was recorded at 10 °C intervals; the air flow was 200 ml/min.
Liquid Nitrogen Adsorption/Desorption Experiments
The liquid nitrogen adsorption experiments were carried out at 77 K using an ASAP2020 (Micromeritics, Norcross GA, USA) specific surface area and pore analyzer. The nitrogen adsorption capacity of each coal sample was measured at different pressures. The samples’ specific surface areas and mean pore sizes were calculated using the Brunauer–Emmett–Teller (BET) method and their total pore volumes were calculated by the Barrett–Joyner–Halenda (BJH) method.
The Frankel–Halsey–Hill (FHH) fractal method, a method based on the liquid nitrogen adsorption/desorption curve was used to characterize the coal’s structural properties. The equation used was Eq. 1 (Pfeifer et al., 1989; Sun et al., 2015):
where P is gas adsorption equilibrium pressure (MPa), V is gas adsorption volume (cm3 g−1) of the equilibrium pressure, P0 is saturated vapor pressure (MPa) of the gas, C is a constant, and A is a power exponent; A represents the pre-exponential factor (which depends on fractal dimension) and its value is the slope of \({\text{ln}}V - {\text{ln}}\left( {{\text{ln}}\frac{{P_{0} }}{P}} \right)\) curve.
Researchers have put forward two different methods for calculating the fractal dimension (D) (Qi et al., 2002; Pyun and Rhee, 2004), thus:
There is no consensus as to which method is most suitable for the pore structure of coal; therefore, this paper uses both methods.
In-situ Fourier Transform Infrared Spectroscopy
The heating and oxidation process of coal samples were scanned with a VENTEX80 in-situ Fourier transform infrared (FTIR) spectrometer (Bruker, Germany). The air flow through the sample reaction tank for the experiments was 200 ml/min, the initial temperatures were 30 °C, and the final temperatures were 400 °C. The sample heating rate was 10 °C/min. The FTIR scanning range was 400–4000 cm−1, the resolution of the infrared spectrometer was 4 cm−1, and 32 scans were used to obtain each spectrum. Samples were scanned, and data were recorded every 10 °C. A flowchart illustrating the sample preparation, experiments, and data analysis and interpretation is shown in Figure 1.
Results and Discussion
Thermogravimetric Analysis
Previous studies have shown that characteristic temperatures of the coal oxidation process can be identified (Qi et al., 2017; Zhai et al., 2020a). Figure 2 shows the thermogravimetry and differential thermogravimetry (TG/DTG) curves for the coal samples. Although the curves of raw coal and DWIC are not identical, their general shapes and trends are similar.
As shown in Figure 2, the DTG curve per sample and the DTG = 0 line intersect at two points. The first intersection is the point at which the weight gain from oxygen inhalation is balanced by the mass loss due to heating and vaporization. The second intersection is the temperature of maximum weight after which the coal and oxygen react rapidly and the sample enters the volatile combustion stage. The low-temperature oxidation stage refers to the temperature range before the maximum weight temperature is reached (Zhong et al., 2019). There were five characteristic temperatures indicated in the low-temperature oxidation stage of each sample shown in Figure 2. These are the critical temperature (T1), the dry temperature (T2), the active temperature (T3), the acceleration temperature (T4), and the maximum weight gain temperature (T5).
The critical temperature, T1, is the temperature at which the coal–oxygen reaction rate begins to accelerate significantly. Table 2 lists the characteristic temperatures and it can be seen from the table that the T1 of the two DWIC samples were lower than the T1 for the two raw coal samples. The ΔT1 of the CLS and the XYK samples were 13 °C and 5 °C, respectively. The dry temperature, T2, is the temperature at which the weight of a coal sample is at its lowest during low-temperature pyrolysis. The T2 of the two DWIC samples were lower than those of the raw coals, the ΔT2 being 24 °C for the CLS samples and 15 °C for the XYK pair. The T2 differences were greater than the T1 differences. The active temperature, T3, is the temperature at which the coal begins to gain weight. The T3 for the two DWIC samples were lower than the raw coal sample’s T3 with differences of 21 °C (for the CLS samples) and 24 °C (for the XYK samples). The acceleration temperature, T4, is the temperature at which the weight gain rate is at its maximum. The T4 of the two DWIC samples were lower than the raw coal’s T4. The ΔT4 of CLS and the XYK samples were 15 °C and 1 °C, respectively. The maximum weight gain temperature, T5, is the temperature at which the coal reaches its maximum weight through oxygen inhalation. The CLS DWIC T5 was 2 °C higher than that of the CLS raw coal and the XYK DWIC T5 was essentially the same as that of the XYK raw coal.
The data from the experiments showed that during the low-temperature oxidation stage, the first three characteristic temperatures of the CLS and XYK DWIC coal samples were significantly lower than the characteristic temperatures of the raw coal samples. The characteristic temperature differences decreased noticeably after T3. Over the course of the low-temperature oxidation stage, the characteristic temperature differences first increased and then decreased. This temperature increase followed by a decrease suggests that the DWIC samples were prone to react with oxygen, but that the changes caused by water immersion became very small when the temperature rose to the volatile combustion stage. The influence of water immersion on the coal–oxygen reaction was mainly in the early portion of the low-temperature reaction.
Pore Structure Analysis
Specific Surface Area, Total Pore Volume, and Average Pore Size
The results of the liquid nitrogen adsorption/desorption tests (the specific surface areas, the average pore sizes, and the total pore volumes) for the coals’ pores calculated by the BET and BJH methods are listed in Table 3. It can be seen from this table that, compared to the raw coal samples, the DWIC sample had smaller specific surface areas but larger total pore volumes and average pore diameters. This is because the coal samples’ specific surface areas are dominated by the surface area of micro-pores (Zhai et al., 2019), but the process of water immersion leads to larger pores and thus reduced proportion of micro-pores.
Fractal Dimension
Fractal dimensions for the four coal samples were calculated from the liquid nitrogen adsorption/desorption curves. Those curves are shown in Figure 3.
As shown in Figure 3, when the relative pressure, P0/P, is less than 0.5, the adsorption and desorption curves overlap. At this pressure ratio, adsorption mainly occurred in the micro-pores and the main forces between the coal and the nitrogen molecules were Van der Waals forces. When P0/P was greater than 0.5, adsorption hysteresis began to take place. At this higher-pressure ratio, adsorption occurred mainly in the meso-pore and macro-pore, and nitrogen adsorption was driven predominantly by capillary condensation (Zhang et al., 2014). The coal–nitrogen adsorption mechanism in different pressure stages is different; therefore, calculations of fractal dimension for different pressure stages must also be carried out differently (Lv et al., 2020); when P0/P is < 0.5, the fractal dimension is D1, and when P0/P is > 0.5, the fractal dimension is D2. The D1 fractal dimension is the dimension of pore surfaces reflecting the roughness of those surfaces whereas D2 is the fractal dimension of the pore structure and reflects the complexity of the pore structure. D1 and D2 have a low correlation. Because the desorption curve is more stable, desorption curves were used for the calculations (Lv et al., 2020). Logarithmic curves for nitrogen adsorption volume (V) and relative pressure (P0/P) are shown in Figure 4. D1 and D2 were calculated using both Eqs. 2 and 3; the results of the calculations are listed in Table 4. According to the FHH theory, generally, Eq. 2 should be used to calculate D1, Eq. 3 should be used to calculate D2, and the D value is between 2 and 3 (Pfeifer et al., 1989; Zhang et al., 2014). If the value of D less than 2, it will make the D meaningless in practice. In this work, we calculated D using both Eqs. 2 and 3. It can be seen from Table 4 that D2 calculated by Eq. 2 is less than 2 for all four samples. Therefore, Eq. 2 was used to determine D1, and Eq. 3 was used to determine D2.
It can be seen from Table 4 that D1 values for the two DWIC samples were lower compared to those of the raw coal. This indicates that the surfaces of the DWIC samples’ pores were smoother than the surfaces of pores in the raw coals. The difference between the CLS DWIC D2 and CLS raw coal D2 is insignificant but the XYK DWIC D2 is larger than the XYK raw coal D2. This means that the XYK DWIC pore structure is more complex. It has been stated that D1 in coal mainly reflects the fractal characteristics of micro-pores (plus some of the meso-pores) whereas D2 reflects the fractal characteristics of meso-pores and macro-pores (Lv et al., 2020). Macro-pores contribute the largest proportion of total pore volume in these coal samples but the specific surface area is dominated by micro-pores. Therefore, it can be concluded that, after water-immersion, the proportion of micro-pores decreased and the proportion of meso-pores and macro-pores increased. This is consistent with the results of the pore structure parameter calculations and it means that the porosity and permeability of the coal increased after water immersion. This is conducive to oxygen transport and allows the coal–oxygen reaction to proceed more quickly.
Fourier Transform Infrared Spectroscopy Analysis
According to previous studies, infrared spectra can be resolved by the position of the absorption peak of each group (Ma et al., 2019). This investigation mainly studied the hydroxyl and aliphatic hydrocarbon groups including the free hydroxyl group with the 3697–3684 cm−1 peak position, the intra-molecular hydrogen bond at the 3624–3613 cm−1 peak position, the methylene shear vibration at the 1379–1373 cm−1 peak position, and the methyl shear vibration at the 1449–1439 cm−1 peak position. The three-dimensional in-situ infrared figures of coal samples are shown in Figure 5. The strengths of the infrared spectral peaks changed as the temperature increased as shown by the peak intensity versus temperature fitting curves in Figures 6, 7, 8, and 9. According to the thermogravimetric results, the low-temperature oxidation stage in these coal samples did not exceed 400 °C; therefore, the FTIR measurement was only recorded from 30 °C to 400 °C.
It can be seen from Figures 6 and 7 that, during the entire heating process, the free hydroxyl group and intra-molecular hydrogen bond absorption peak strengths for the DWIC samples were significantly greater than the strengths for such peaks for the raw coal samples. According to the Beer–Lambert Law (Xin et al., 2014), these peak strengths show that the free hydroxyl group and intra-molecular hydrogen bonds were more abundant in the DWIC than in the raw coal and that they increased as the temperature rose. The rising curves indicate that the rate at which this group and these bonds were being produced was greater than the rate at which they were being destroyed. In addition, it can be seen from Figure 8 that, in the initial heating stage, the methylene concentration of the raw coal was higher compared to those of the DWIC samples. In the T1–T2 range, the methylene concentration in the DWIC was higher. As the temperature increased, the sample with higher methylene concentration alternated but the difference was not significant. As for the methyl concentration, Figure 9 shows that the methyl concentration of the CLS raw coal was greater than that of the CLS DWIC at 30 °C but when the temperature rose to 47 °C, the methyl concentration of the DWIC began to exceed that of the raw coal. When the temperature rose to around 334 °C, the raw coal’s methyl concentration again surpassed that of the DWIC. The methyl concentration of the XYK raw coal exceeded that of the XYK DWIC for the second time at 230 °C. Although the temperature was different from that of the CLS coal sample, the trends of changes of methyl shear vibration absorption peak intensities with temperature were similar. Comparing these data with the data in Table 2 shows that, when the temperature rose to cause the methyl concentration of the raw coal to be greater than that of the DWIC, the characteristic temperature difference between raw coal and DWIC began to decrease. When the temperature reached T5, the characteristic temperature differences between raw coal and DWIC were very small. Hydroxyl and aliphatic hydrocarbon groups are key to coal spontaneous combustion cyclic chain reaction (Wang et al., 2016) and the differences in their abundances will influence the course of the reactions. From the changes in methyl concentration with characteristic temperature, it can be concluded that in the later low-temperature oxidation periods, methyl concentration had a considerable influence on the coal–oxygen reaction.
Progression of Oxidation Reaction
When coal oxidation was beginning and the coal–oxygen reaction was initiated, physical oxygen adsorption was dominant. The adsorption force was weak but adsorption progressed rapidly and adsorption equilibrium was reached easily (Qi et al., 2017). At this stage, the coal samples absorbed more gas than they desorbed and their weight increased. As the temperature increased, the quantity absorbed decreased and the kinetic energy of the gas molecules increased. The energy required for coal to adsorb gas increased and adsorption equilibrium could no longer be maintained; gas desorption accelerated and the coal lost weight. As the temperature continued to rise, gas in the coal pores began to desorb, chemical adsorption and chemical reactions sped up, gas was released, and the weight loss rate increased to maximum and reached the critical temperature. When the coal was immersed in water, water condensed in the deep pores, and this increased the pore volume, some of the primary coal gas was expelled. In addition, during immersion, some of the active chemical groups on the coal’s surface were chemically adsorbed spontaneously and they underwent chemical reactions at room temperature. This consumed hydroxyls and oxygen, and gave off heat (Wang et al., 2016). According to the results presented in sections Pore Structure Analysis and Fourier Transform Infrared Spectroscopy Analysis above, the hydroxyl and oxygen concentrations in the DWIC were higher than they were in the raw coal. Therefore, at the early low-temperature stage, the coal–oxygen reaction was completed earlier in DWIC than it was in the raw coal; that is, the DWIC’s T1 values were lower.
When the coal temperature rose to T2, the reaction rate accelerated and the gas production increased, some of the bridge bonds and side chains in the coal molecules began to crack, more active structures were produced, and oxygen absorption increased (Qi et al., 2017). The weight gains from oxygen inhalation and the weight losses from water evaporation and gas desorption reached a dynamic equilibrium. According to the results presented in the section Fourier Transform Infrared Spectroscopy Analysis above, the methyl and methylene molecule concentrations in DWIC at temperatures near T2 were higher than they were in the raw coal. This made the DWIC react more readily. When the temperature rose to T3, the ring macromolecules in the coal broke their bonds more quickly and active structures were produced at a higher rate. Oxygen adsorption increased dramatically and the coal’s weight gain from oxygen absorption began to increase (Qi et al., 2017). At this stage, the methylene concentration in the DWIC and the raw coal samples was essentially the same. The methyl concentrations in the DWIC samples were higher compared to those in the raw coal and the T3 values of the DWIC samples reached 21 °C and 24 °C earlier than the raw coal samples. When the temperature rose to T4, the coal’s weight gain rate reached its maximum after which the oxygen adsorption capacity of the active structures gradually reached their maxima and the coal temperature reached T5. The methylene concentrations of the coal samples at this stage were virtually the same and after the methyl concentration of the raw coal exceeded that of the DWIC, the different of the characteristic temperatures decreased rapidly. It can be inferred that, at this stage, the methyl concentrations had considerable influence on the progress of the coal–oxygen reaction. During these reactions, the differences between the CLS and the XYK coal samples might be related to their different degrees of coalification.
Conclusions
For this study, thermogravimetry, liquid nitrogen adsorption/desorption, and in-situ Fourier transform infrared spectrometry were used to explore the influence of water immersion on the characteristic temperatures of low-temperature coal oxidation and its mechanism. The study’s main conclusions are as follows.
-
1.
The most important stage in coal oxidation is the low-temperature oxidation stage. This stage has five characteristic temperatures, namely critical temperature, dry temperature, active temperature, acceleration temperature, and maximum weight temperature. The differences between the characteristic temperatures of DWIC and raw coal first increase but then decrease as the temperature rise. When the reaction reaches the volatile combustion stage, the effect of water immersion on the macroscopic characteristic temperature of coal oxidation is small.
-
2.
Compared with raw coal, the specific surface area of DWIC decreases but the average pore size and total pore volume increase. The fractal results show that the pore surfaces of the DWIC samples are smoother compared to those of raw coal, and the DWIC pore structure is more complex. The porosity of the DWIC increases, which is a beneficial property for oxygen migration and transport.
-
3.
Hydroxyl and aliphatic hydrocarbon groups are key to the coal–oxygen reaction, and their concentration will affect the progress of the reaction. During the whole low-temperature oxidation stage, the DWIC hydroxyl concentration is higher than the hydroxyl concentration in the raw coal. In the late low-temperature oxidation stages, the methyl concentration has a considerable influence on the coal–oxygen reaction.
References
Deng, J., Zhao, J. Y., Huang, A. C., Zhang, Y. N., Wang, C. P., & Shu, C. M. (2017). Thermal behavior and microcharacterization analysis of second-oxidized coal. Journal of Thermal Analysis and Calorimetry, 127(1), 439–448.
Elick, J. M. (2013). The effect of abundant precipitation on coal fire subsidence and its implications in Centralia, PA. International journal of coal geology, 105, 110–119.
Green, U., Aizenshtat, Z., Ruthstein, S., & Cohen, H. (2012). Stable radicals formation in coals undergoing weathering: effect of coal rank. Physical Chemistry Chemical Physics, 14(37), 13046–13052.
Karaoulis, M., Revil, A., & Mao, D. (2014). Localization of a coal seam fire using combined self-potential and resistivity data. International Journal of Coal Geology, 128, 109–118.
Looney, B. (2020). BP statistical review of world energy 2020.
Lv, X., Liang, X., Xu, P., & Chen, L. (2020). A numerical study on oxygen adsorption in porous media of coal rock based on fractal geometry. Royal Society Open Science, 7(2), 191337.
Ma, T., Chen, X., & Zhai, X. (2019). Thermogravimetric and infrared spectroscopic studies of the spontaneous combustion characteristics of different pre-oxidized lignites. RSC Advances, 9(56), 32476–32489.
Parsa, M. R., Tsukasaki, Y., Perkins, E. L., & Chaffee, A. L. (2017). The effect of densification on brown coal physical properties and its spontaneous combustion propensity. Fuel, 193, 54–64.
Pfeifer, P. Y. J. M. W. J., Wu, Y. J., Cole, M. W., & Krim, J. (1989). Multilayer adsorption on a fractally rough surface. Physical Review Letters, 62(17), 1997.
Pyun, S. I., & Rhee, C. K. (2004). An investigation of fractal characteristics of mesoporous carbon electrodes with various pore structures. Electrochimica Acta, 49(24), 4171–4180.
Qi, G., Wang, D., Zheng, K., Xu, J., Qi, X., & Zhong, X. (2015). Kinetics characteristics of coal low-temperature oxidation in oxygen-depleted air. Journal of Loss Prevention in the Process Industries, 35, 224–231.
Qi, H., Ma, J., & Wong, P. Z. (2002). Adsorption isotherms of fractal surfaces. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 206(1–3), 401–407.
Qi, X., Li, Q., Zhang, H., & Xin, H. (2017). Thermodynamic characteristics of coal reaction under low oxygen concentration conditions. Journal of the Energy Institute, 90(4), 544–555.
Rúa, M. O. B., Baena, P. B., & Aragón, A. (2018). Optimization of techniques for the extinction and prevention of coal fires produced in final walls as a result of spontaneous combustion in the Cerrejón mine–Colombia. Environmental Science and Pollution Research, 25(32), 32515–32523.
Shao, Z., Jia, X., Zhong, X., Wang, D., Wei, J., Wang, Y., & Chen, L. (2018). Detection, extinguishing, and monitoring of a coal fire in Xinjiang China. Environmental Science and Pollution Research, 25(26), 26603–26616.
Song, S., Qin, B., Xin, H., Qin, X., & Chen, K. (2018). Exploring effect of water immersion on the structure and low-temperature oxidation of coal: A case study of Shendong long flame coal, China. Fuel, 234, 732–737.
Sun, W., Feng, Y., Jiang, C., & Chu, W. (2015). Fractal characterization and methane adsorption features of coal particles taken from shallow and deep coalmine layers. Fuel, 155, 7–13.
Tang, Y., & Xue, S. (2017). Influence of long-term water immersion on spontaneous combustion characteristics of Bulianta bituminous coal. International Journal of Oil, Gas and Coal Technology, 14(4), 398–411.
Ti, Z., Zhang, F., Pan, J., Ma, X., & Shang, Z. (2018). Permeability enhancement of deep hole pre-splitting blasting in the low permeability coal seam of the Nanting coal mine. PLoS ONE, 13(6), e0199835.
Wang, K., Deng, J., Zhang, Y. N., & Wang, C. P. (2018). Kinetics and mechanisms of coal oxidation mass gain phenomenon by TG–FTIR and in situ IR analysis. Journal of Thermal Analysis and Calorimetry, 132(1), 591–598.
Wang, H., Dlugogorski, B. Z., & Kennedy, E. M. (1999). Experimental study on low-temperature oxidation of an Australian coal. Energy and fuels, 13(6), 1173–1179.
Wang, S., Li, C., & Yang, L. (2018a). Decoupling effect and forecasting of economic growth and energy structure under the peak constraint of carbon emissions in China. Environmental Science and Pollution Research, 25(25), 25255–25268.
Wang, K., Tang, H., Wang, F., Miao, Y., & Liu, D. (2019). Research on complex air leakage method to prevent coal spontaneous combustion in longwall goaf. PLoS ONE, 14(3), e0213101.
Wang, Z., Su, W., Tang, X., & Wu, J. (2018b). Influence of water invasion on methane adsorption behavior in coal. International Journal of Coal Geology, 197, 74–83.
Wang, D. M., Xin, H. H., Qi, X. Y., Dou, G. L., Qi, G. S., & Ma, L. Y. (2016). Reaction pathway of coal oxidation at low temperatures: a model of cyclic chain reactions and kinetic characteristics. Combustion and Flame, 163, 447–460.
Xiao, Y., Ren, S. J., Deng, J., & Shu, C. M. (2018). Comparative analysis of thermokinetic behavior and gaseous products between first and second coal spontaneous combustion. Fuel, 227, 325–333.
Xie, X., Zhao, Y., Qiu, P., Lin, D., Qian, J., Hou, H., & Pei, J. (2018). Investigation of the relationship between infrared structure and pyrolysis reactivity of coals with different ranks. Fuel, 216, 521–530.
Xin, H. H., Wang, D. M., Qi, X. Y., Qi, G. S., & Dou, G. L. (2014). Structural characteristics of coal functional groups using quantum chemistry for quantification of infrared spectra. Fuel Processing Technology, 118, 287–295.
Yang, Y., Li, Z., Si, L., Gu, F., Zhou, Y., Qi, Q., & Sun, X. (2017). Study governing the impact of long-term water immersion on coal spontaneous ignition. Arabian Journal for Science and Engineering, 42(4), 1359–1369.
Yu, Z., Xueqing, Z., Wen, Y., Haihui, X., Sherong, H., & Yu, S. (2020). Pore structure and its impact on susceptibility to coal spontaneous combustion based on multiscale and multifractal analysis. Scientific Reports, 10(1), 1–15.
Zhai, X., Ge, H., Wang, T., Shu, C. M., & Li, J. (2020a). Effect of water immersion on active functional groups and characteristic temperatures of bituminous coal. Energy, 205, 118076.
Zhai, X. W., Pan, W. J., Wu, S. B., & Ge, H. (2020b). Laboratory experimental study on water-soaked–dried bituminous coal’s thermal properties. Journal of Thermal Analysis and Calorimetry, 139(6), 3691–3700.
Zhai, X. W., Wang, B., Wang, K., & Obracaj, D. (2019). Study on the influence of water immersion on the characteristic parameters of spontaneous combustion oxidation of low-rank bituminous coal. Combustion Science and Technology, 191(7), 1101–1122.
Zhang, D., Cen, X., Wang, W., Deng, J., Wen, H., Xiao, Y., & Shu, C. M. (2021). The graded warning method of coal spontaneous combustion in Tangjiahui Mine. Fuel, 288, 119635.
Zhang, S., Tang, S., Tang, D., Huang, W., & Pan, Z. (2014). Determining fractal dimensions of coal pores by FHH model: Problems and effects. Journal of Natural Gas Science and Engineering, 21, 929–939.
Zhang, Y., Zhang, Y., Li, Y., Li, Q., Zhang, J., Yang, C. (2020). Study on the characteristics of coal spontaneous combustion during the development and decaying processes. Process Safety and Environmental Protection, 138, 9–17.
Zhao, K., Xu, N., Mei, G., & Tian, H. (2016). Predicting the distribution of ground fissures and water-conducted fissures induced by coal mining: a case study. SpringerPlus, 5(1), 1–23.
Zhong, X., Kan, L., Xin, H., Qin, B., & Dou, G. (2019). Thermal effects and active group differentiation of low-rank coal during low-temperature oxidation under vacuum drying after water immersion. Fuel, 236, 1204–1212.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (No. 51974236). The authors also acknowledge Key Laboratory of Coal Fire and Hazard Prevention in Shaanxi Province, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhai, X., Song, B., Wang, B. et al. Study on the Effect and Mechanism of Water Immersion on the Characteristic Temperature during Coal Low-Temperature Oxidation. Nat Resour Res 30, 2333–2345 (2021). https://doi.org/10.1007/s11053-021-09854-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11053-021-09854-0