Abstract
Nano-sized metal oxides with magnetic properties are a group of scientifically valuable materials whose application areas are increasingly diverse. This study investigates the structural, morphological, magnetic, photocatalytic, and hemolytic properties of CoCuFe2O4 nanoparticles (NPs) synthesized by co-precipitation method. Photocatalytic and biological properties of the samples were investigated by following crystal violet (CV) photocatalytic degradation reactions and hemolysis experiments, respectively. X-ray diffraction analysis (XRD) and scanning electron microscope (SEM) were used to analyze crystalline properties and morphological structure of nano-sized ferrites. Photocatalysis tests showed that CoCuFe2O4 NPs degraded 64.9% of the total organic dye after 420-min exposure to 254 nm irradiation. Coercivity (Hc) and saturation magnetization (Ms) of CoCuFe2O4 NPs were determined as 1000 Oe and 15.44 emu/g, respectively. Hemolysis tests showed an average hemolysis ratio (AHR) of 12.4% for human erythrocytes subjected to 5.0 mg mL−1 CoCuFe2O4 concentration, while it was only 2.2% for those of exposed to 1.0 mg mL−1 CoCuFe2O4. In addition, at both CoCuFe2O4 concentrations, spectrophotometric evidence was found indicating hemoglobin oxidation under the effect of reactive oxygen species (ROS) formed on the nanoparticle surface.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nano-metal oxides (NMOs) have received enormous attention due to their structural stability and high magnetic properties [1, 2] and regarded as valuable materials in medicinal area such as magnetic resonance imaging and magnetic hyperthermia [3, 4]. They have a talent to form biological interactions at the cellular level, enabling targeted drug delivery applications and node detections in tumors [5, 6]. They also have well-characterized catalytic performances towards the selective oxidation of carbon monoxide, decomposition of hydrogen peroxide, and degradation of synthetic colorants and have been extensively studied for both medicinal and environmental applications [7, 8].
Among NMOs, iron-containing derivatives having spinel configuration have magneto-crystalline anisotropy, high mechanical and chemical stability, and well-characterized magnetic properties [9,10,11]. Spinel ferrites have a general molecular formula of MFe2O4 and constituted by cubic structured unit cells. M represents a divalent metal cation such as Mn2+, Ba2+, Ni2+, Fe2+, Cu2+, Zn2+, or Co2+. Face-centered cubic lattice structure of spinel ferrites allows the formation of tetrahedral and octahedral coordination sites occupied by M2+ and/or Fe3+ ions. It is also possible to produce double ferrites having a general formula of M′1-xM″xFe2O4 by the isomorphic substitution of M. Spinel ferrites are very popular in scientific and technological area due to their adjustable physical, chemical, and physicochemical properties under the effect of external magnetic field and potential medical applications [12, 13]. Ferrites can exhibit high saturation magnetization depending on the particle size and morphological structure [14]. This feature allows to obtain ferrite-based materials that can respond quickly and efficiently to variable magnetic field applications. When evaluated in terms of photodegradation applications, the presence of a second metal doped into the ferrite structure allows to obtain photocatalysts with narrower band gaps, and therefore, highly efficient reactions can be proceeded even under ultraviolet/visible light [15]. Among spinel ferrites, especially cobalt ferrite (CoFe2O4) and copper ferrite (CuFe2O4) are of great interest in physical, chemical, and medical aspects due to the ability to control of their physicochemical properties as well as perfect mechanical properties and chemical stability [16, 17].
The mechanical properties and chemical stability of double ferrites and their superparamagnetic behavior that can vary depending on particle size [9] make these nano-sized materials tough competitors against traditional iron oxide structures such as magnetite (Fe3O4) and hematite (Fe2O3). Besides the ability of Fe3O4 and Fe2O3 to form reactive oxygen species, the biocompatibility of these species can also be improved by double ferrites obtained by isomorphic replacement of a second element atom with Fe.
Having inferior toxicity, high bio/cell compatibility, and showing high structural stability in physiological conditions are the main characteristics of materials planned to be used for biological and medicinal purposes. For example, superparamagnetic CoFe2O4 NPs proven as biocompatible were used in a controlled drug delivery system triggered by the application of an alternating magnetic field [18]. It was emphasized in another study that CoFe2O4 NPs display biocompatibility against both erythrocytes and breast cancer cells even without the presence of an exterior immune-specific coating [19]. Moreover, negligible hemolysis ratios offer significant biocompatibility which is essential for in vivo biomedical applications such as intravenous drug delivery, magnetic hyperthermia, and node tracer injections [20, 21].
Although being a heavy metal, Cu has a vital role in biological processes. In trace amounts, Cu is being required as co-factor of numerous enzymes involved in biological redox reactions [22]. Co is also vital as taking part in cyanocobalamin (C63H88CoN14O14P) and methylcobalamin (C63H91CoN13O14P) which are the most stable and biologically active forms of vitamin B12 [23]. On the other hand, elevated concentrations of both Co and Cu show toxic effects in biological systems. ROS produced by Co and Cu containing NPs may cause high levels of oxidative stress on tissues leading to failure of cell functions, inflammatory reactions, and cardiovascular diseases as well as cancer. However, some literature studies highlight the potential of nanostructures containing Co and Cu, especially in medical and biomedical applications. For example, in the study of Sattarahmady et al., dextrin-coated Co-Zn nano-ferrites (Zn0.5Co0.5Fe2O4) synthesized by co-precipitation technique were declared as having potential in MRI [24]. Properties of porphyrins attached on cobalt oxide surfaces were also investigated in terms of metalation which is one of the important interactions between porphyrins and metal oxide surfaces, due to the effect of metal ion incorporated in the porphyrin ring on chemical, electrical, and optical characteristics [25]. In another study, the applicability of cobalt protoporphyrin-loaded silica NPs in photoacoustic imaging by subcutaneous and myocardial injection approaches was proposed [26]. Another study reports the synthesis of ultrasmall CuO (Us-CuO) NPs showing enzyme-mimicking properties and broad-spectrum ROS scavenging ability. Also, researchers emphasize the cell-protective effects of Us-CuO NPs and their potential use in the treatment of ROS-related diseases [27].
Materials designed for medical purposes must be well-characterized in terms of their biocompatibility. Hemolysis tests are one of the well-accepted scientific ways to establish the biocompatibility of a synthetic material [28, 29]. Magnetic NPs, especially ferrites, are being studied for their usability in biomedical applications such as controlled drug release, hyperthermia applications, selective protein adsorption, and magnetic resonance imaging (MRI) [30,31,32,33]. Biological incompatibility between blood components (including RBCs) and iron-containing molecules arises from the oxidizing properties of hypervalent iron towards organic molecules [34]. A study conducted by Xiong et al. emphasized the enhanced reduction ability of Fe/Cu bimetallic particles and broadened reduction efficiency in the pH range of 3.0–9.0 in respect to pure Fe [35]. Reactivity of Fe3+ in biological systems can be adjusted by the incorporation of ferrite structures. Undesirable interactions between synthetic materials and blood components caused by the presence of Co and Cu ions in the structure of synthetic materials can be reduced or prevented by using ferrites. Ferrite structures show higher biocompatibility compared to conventional iron oxides. The synthesis of ferrites at nanoscale provides high photocatalytic, electrical, and magnetic properties in addition to biocompatibility. Thus, in this study, CoCuFe2O4 NPs were synthesized and investigated in terms of their material properties as well as biological potential. The aim of this study is to provide data about the advantages that CoCuFe2O4 NPs can provide in medical and biomedical fields.
Especially in the last few decades, design of new materials especially in the nanometer scale has become a very popular research area. Designing new materials with unique and/or improved properties for various application areas such as electronics, sensors, medicine, and biotechnology makes this area of research much more valuable. Up to date, many studies have been conducted on nano-sized metal oxides which were focused mainly on the material properties rather than the potential for use in medical area. Literature research shows that MFe2O4 and M′1-xM″xFe2O4 nanomaterials have a high potential especially in terms of biomedical applications [36,37,38]. However, there are only limited number of studies presented to the literature regarding the properties of CoCuFe2O4, and these studies have focused mainly on the synthesis conditions and catalytic properties of these NPs [39, 40]. Very few studies have been conducted on the biological properties of CoCuFe2O4 NPs. Thus, in order to prove the accuracy of the predictions on the potential of CoCuFe2O4 NPs, the interactions between the ferrite structures synthesized in this study and blood components were investigated by hemolysis tests. This study also reports the synthesis and material properties of CoCuFe2O4 NPs as well as the photocatalytic properties of CoCuFe2O4 NPs which have not been reported adequately.
Experimental part
Nanoparticle synthesis
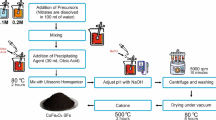
Synthesis procedure of CoCuFe2O4 nano-ferrites by co-precipitation method was summarized in Fig. 1. Iron(III) nitrate nonahydrate (Fe(NO3)3.9H2O, ≥ 99.95%, Sigma Aldrich), cobalt(II) nitrate hexahydrate (Co(NO3)2.6H2O, ≥ 99.0%, Sigma Aldrich), and copper nitrate trihydrate (Cu(NO3)2.3H2O, ≥ 99.0%, Sigma Aldrich) were used in the synthesis. In a typical experimental procedure to synthesize CoCuFe2O4 nano-ferrites, a 100-mL aqueous solution of Fe(NO3)3.9H2O, Co(NO3)2.6H2O, and Cu(NO3)2.3H2O was prepared with 0.2 M, 0.05 M, and 0.05 M concentrations, respectively. All reagents used in the nanoparticle synthesis were of analytical grade (Alfa Aesar). In nanoparticle syntheses, 30 µL of oleic acid was added to the reaction medium as the surfactant to prevent aggregation of NPs. Reaction mixture was continuously sonicated using an ultrasonic bath (Bandelin RK103H) for 2 h at 80 °C. Next, the pH of the reaction mixture was adjusted to pH 9 with NaOH added dropwise to form fine particles of solid hydroxides of metals (Eq. 1). Simultaneously, the mixture was maintained in ultrasonic bath for an additional 1 h to allow the transformation of metal hydroxides to metal oxides (Eq. 2). Next, distilled water was added to the precipitate and centrifuged (NUVE NF400) at 5000 rpm for 10 min. This purification process was repeated five times to remove the unreacted hydrates, unwanted impurities, and excess surfactant from the obtained product. CoCuFe2O4 NPs were than dried at 80 °C under vacuum for 8 h. CoCuFe2O4 NPs were calcined at 500 °C for 2 h at atmospheric conditions. As confirmed by XRD analysis, the final product obtained was NPs of cobalt–copper ferrite (CoCuFe2O4) with spinel structure:
Structural analysis
The crystal properties of the CoCuFe2O4 NPs were characterized by XRD measurements using fully computerized X-ray diffractometer (PANalytical X’pert Powder 3) with CuKα (λ = 1.5418 Å) radiation. Characterizations were performed at room temperature and patterns were recorded with a scanning speed of 3 (o)/min in the range of 2θ = 10° − 90° and a step size of 0.02°. The morphological characteristics of the CoCuFe2O4 NPs were investigated by using SEM (Zeiss EVO MA). Before SEM analysis, CoCuFe2O4 NPs were coated with gold–palladium under argon atmosphere to make the surface electrically conductive. Shape, surface morphology, length, and diameter of NPs were examined by SEM micrographs with different magnifications. Optical properties of the CoCuFe2O4 NPs were characterized by using a UV-Spectrometer (Shimadzu 2600) between 300 and 1000 nm. Magnetic characteristics were investigated through a vibrating sample magnetometer (Lake Shore 7304), operating in the temperature range of 15 K to 300 K.
Photocatalytic performance of CoCuFe 2 O 4 NPs
Photocatalytic degradation experiments of crystal violet (CV) in the presence of CoCuFe2O4 NPs were evaluated using an aqueous dispersion of NPs (1.0 mg mL−1) depending on the varying photodegradation time. CV solution with a concentration of 2.5 × 10−6 M was used in photocatalysis tests. In order to ensure that the CV reaches the equilibrium adsorption/desorption condition on the nanoparticle surfaces, magnetic stirring was applied continuously in the dark for 1 h before photocatalysis. Thereafter, aqueous dispersions of NPs in CV solution were exposed to 254 nm irradiation in cylindrical open top Pyrex reactors. The irradiation was carried out using a couple of UV lamps (45 W each) which was placed vertically on the reaction vessels at a distance of 40 cm. During the photodegradation process, 1.5 ml samples of CV dispersions were withdrawn at appropriate time intervals and subsequently centrifuged at 4000 rpm for 3 min to settle the NPs down. Supernatants were analyzed for undegraded CV content using Shimadzu UV mini 1240 UV–Vis spectrophotometer at λmax = 591 nm. Distilled water was used as a reference.
Blood compatibility tests
Susceptibility of erythrocytes (red blood cells, RBCs) to hemolysis in the presence of CoCuFe2O4 NPs was investigated. Venous blood samples were drawn from healthy adult volunteers (35–38 age female) and anticoagulated with the aqueous solution of 3.2% trisodium citrate. Blood:trisodium citrate ratio was 9:1. Blood:anticoagulant mixture was diluted with phosphate buffer solution (pH 7.35) and centrifuged at 5000 rpm for 3 min to precipitate the red blood cells (RBCs). After discharging the supernatant, RBCs were diluted with PBS. Solutions of RBC’s were prepared freshly and kept at room temperature during the tests. 1.0 ml of RBC stock solution was added to 5.0 ml of CoCuFe2O4 nanoparticle suspensions prepared at concentrations of 1.0 mg mL−1 and 5.0 mg mL−1. The prepared samples were incubated at 37 °C, which is the physiological temperature, for 3 h. NP erythrocyte suspensions were kept in the dark during the incubation period in order to avoid the effect of ROS that can be formed by the nanoparticles under the influence of ambient light. Each test was repeated twice, and the hemolysis percentages were averaged. After the incubation period, dispersions were centrifuged at 4000 rpm for 5 min, and absorbance of the supernatant was measured between 400 and 700 nm using a UV–Vis spectrophotometer. Absorption spectra were interpreted to detect the possible presence of hemoglobin (Hb) released into the environment following the lysis of RBCs.
The hemolysis ratio of each sample was calculated using the absorbance values at 540 nm corresponding to the absorption maxima of oxyhemoglobin (OxHb) in accordance with the following equation [41]:
Test results of RBCs in PBS alone were used as negative control (NC), whereas the results of RBCs in pure water was used as positive control (PC). AT refers for the absorbance value of test sample at 540 nm. Erythrocyte-CoCuFe2O4 interactions, CoCuFe2O4-induced erythrocyte deformations, and overall erythrocyte morphology before and after incubation with CoCuFe2O4 NPs were examined using a Nikon Eclipse E100 optical microscope.
Results and discussions
In this study, CoCuFe2O4 NPs were synthesized by co-precipitation which is a convenient method for the synthesis of NMOs. In this method, simple precursor solutions are used to synthesize NMOs with desired properties. Moreover, the co-precipitation method allows a rapid, simple, low-cost synthesis route to easily control the properties of the final product. In the following section, the structural, morphological, magnetic, photocatalytic, and biological properties of CoFe2O4 NPs synthesized by the co-precipitation method in this study are reported.
XRD analysis
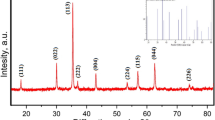
Characteristic powder XRD pattern confirmed the crystal structure of CoCuFe2O4 NPs (Fig. 2). Moreover, the formation of cubic structured spinel lattice was approved by all diffraction peaks for the CoCuFe2O4. No additional reflections were detected for the secondary phases in the XRD diffraction patterns corresponding to the oxides. In addition, it was determined that CoCuFe2O4 NPs belong to the Fd-3 m space group (PDF Card No.: 98–019-1044). The lattice parameter, a, was calculated using Eq. 4:
The crystallite size (D) of the CoCuFe2O4 NPs was calculated by Debye–Scherrer expression (Eq. 5) considering the full width at half maximum (FWHM) of the most intense peak (113):
In Eq. 5, λ, β, and θB are the X-ray wavelength of CuKα, the FWHM of the diffraction peak (113), and the Bragg diffraction angle, respectively. The calculated values of D and a, for CoCuFe2O4 NPs, were given in Table 1.
SEM analysis
The surface morphology of CoCuFe2O4 NPs synthesized by the co-precipitation method was investigated using scanning electron microscopy–energy dispersive spectroscopy (SEM–EDS) at an accelerating voltage of 30 kV. SEM images showed that CoCuFe2O4 NPs were not monodispersed in terms of size and shape and highly agglomerated (Fig. 3). SEM images showed that CoCuFe2O4 NPs were agglomerated as reported in previous studies [42, 43].
The chemical composition of CoCuFe2O4 NPs was given by the EDS spectrum reported in Fig. 4. The information obtained from the EDS spectrum was used in the identification of the presence of Co, Cu, and Fe in ferrite structure as well as for the confirmation of the successful result of Co and Cu co-doping into the Fe2O4 structure (Fig. 4).
Band gap calculation
The reflectance spectra of the CoCuFe2O4 NPs were obtained by UV–Vis diffuse reflectance measurements in 300–1000 nm wavelength range. Figure 5 inset graph shows the absorption edge which is close to 378 nm. The reflectance F(R) is proportional to the absorption coefficient (α). It was calculated by using the Kubelka–Munk function [44]:
The optical band gap \({E}_{g}\) for the photon energy (\(h\upupsilon\)) and the absorption coefficient (α) was calculated by using the following equation:
In Eq. (7), k and \({E}_{g}\) are the energy-independent constant and the optical band gap, respectively. \(F\left({R}_{\alpha }\right)\) is proportional to α and, n was taken 1/2 and 2 for direct and indirect band gaps, respectively. Since n is taken to be 1/2 for directly allowed transitions, Eq. (7) becomes
In other words, \({\left(F\left({R}_{\alpha }\right) h\upupsilon \right)}^{2}={k}^{2}\left(\mathrm{h\upsilon }-{E}_{g}\right)\). The slope of the graphs of \({\left(F\left({R}_{\alpha }\right) h\upupsilon \right)}^{2}\) was approximated by using a linear fit.
The direct band gap energy Eg was calculated by the linear approximation of the slope of the graph of (F(Rα) hυ)2 to the photon energy where F(Rα) = 0, namely, Eg = hυ. The extrapolation of linear fit onto photon energy axis gives the Eg value. Direct and indirect band gap energies of CoCu-ferrite NPs were found as 1.93 eV and 0.6 eV, respectively. Band gap energy depends on carrier concentration, crystallite size, lattice strain, grain size, and the size effect of the dopant metal atoms in CoCu-ferrite lattice. In the study of Chavan and Naik [45], Ni1−xMgxFe2O4 (x = 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5) NPs were synthesized by auto-combustion method, and band gap values of samples were found between 2.84 and 2.94 eV. In another study conducted by Khan et al. [46], the band gap value of bismuth ferrite (BiFeO3) NPs was calculated as 2.84 eV. Siva et al. [47] studied the material properties of CoCuFe2O4 NPs synthesized by hydrothermal method. In that study where the optical band gap was determined by Tauc equation, direct and indirect band gap values were calculated as 2.74 eV and 1.83 eV for CoCuFe2O4 NPs. Hammad et al. [48] explored the decreasing trend in band gap values of Cu1-xCoxFe2O4 (x = 0.2, 0.4, 0.6, 0.8, and 1.0) NPs (from 3.65 to 3.20 eV) with increasing content of Co2+. Thus, in the current study, the calculated band gap values for CoCuFe2O4 NPs were found to have improved compared to literature studies.
Magnetic behavior
The magnetic nature of the CoCuFe2O4 NPs was characterized by using a vibrating magnetometer. Magnetic measurements were conducted in the range of ± 1 T at room temperature. From the obtained data, the graph of magnetization dependent on magnetic field is shown in Fig. 6. The magnetic properties (coercivity (Hc) and saturation magnetization (Ms)) of CoCuFe2O4 spinel ferrites can be seen in Table 2 and Fig. 6. Moreover, it can be seen in Fig. 6 that the magnetization curve exhibits a narrow hysteresis.
The field dependence of the magnetization (M) close to the saturation value was calculated by using the formula below [49]:
where H is the applied magnetic field, β is a parameter related to the magneto-crystalline anisotropy, and Ms is the saturation magnetization. Calculated values (Ms, Hc, and β) for the CoCuF2O4 NPs are depicted in Table 2.
The plots of magnetization as a function of 1/H2 and linear fit for the CoCuFe2O4 NPs was depicted in Fig. 7. β and Ms values was found by the linear fitting of the slope of the graph as shown in Fig. 7 and tabulated in Table 2. In this study, Ms, Hc, and β values obtained for CoCuFe2O4 NPs were found to be compatible with the studies in the literature [44, 49].
Photocatalytic properties
Although oxidative stress has adverse effects on various biological systems, ROS produced by metal oxide particles can also be used to remove various pollutants such as paint, antibiotics, and biological wastes from the environment by photocatalytic degradation.
The photocatalytic properties of CoCuFe2O4 NPs were investigated by UV − Vis spectroscopy in the wavelength range of 400–700 nm. Absorbance values at 591 nm corresponding to the absorption maxima of CV were recorded at special time intervals and used to calculate the concentration of undegraded (remaining) CV in the solution. Time-dependent variation of the UV–Vis spectra lines of the non-photodegraded CV in an aqueous suspension of CoCuFe2O4 NPs shows the decrease of the characteristic band intensity at 591 nm over the range of 0–7 h (Fig. 8). This change under the effect of 254 nm irradiation indicates that the CV has been successfully photodegraded by CoCuFe2O4 NPs.
The decolorization percentages of CV solutions were calculated using the equation given as follows [50]:
where \({C}_{o}\) and \({C}_{t}\) define for the initial CV concentration and the concentration of CV at time t, respectively.
It was observed that CoCuFe2O4 NPs were able to degrade 64.9% of total CV at the end of 420 min. The mechanism for the degradation of CV in the presence of CoCuFe2O4 NPs follows the below mentioned route. Irradiation of CoCuFe2O4 NPs causes excitation and shift of electrons from valence band (VB) to the conduction band (CB) and electron–hole pairs \({(e}^{-}- {h}^{+})\) form (Eq. 11). Holes (\({h}^{+}\)) react with water molecules and form \({HO}^{*}\), while electrons in conduction band (\({e}^{-}\)) combines with O2 to produce \({O}_{2}^{-*}\) (Eq. 12–14). Formation of other reactive intermediates such as hydroperoxyl radical (\(H{O}_{2}^{*})\) and \({H}_{2}{O}_{2}\) is then followed (Eq. 15–16) [51]. \({HO}^{*}\), \(H{O}_{2}^{*}\), and \({O}_{2}^{-*}\) radicals are strong oxidizing agents towards CV and other organic molecules and lead to their degradation into non-toxic molecules such as \(C{O}_{2}\) and \({H}_{2}O\) (Eq. 17). Schematic illustration of the photocatalytic effect of CoCuFe2O4 and photodegradation mechanism of CV were given in Fig. 9:
To investigate the kinetics of photodegradation reactions, three different kinetic models were applied; those were zero order, first order, and second order [52,53,54]. Equations describing these kinetic models, rate constants (k, k1, and k2) and correlation coefficients (R2) were listed in Table 3. In each equation, C and Co represent the concentration of CV after a certain irradiation time (t) and initial concentration of CV at t = 0, respectively.
Among the three kinetic models tested, the kinetic expression that most successfully described the photodegradation reaction of CV by CoCuFe2O4 NPs was the zero-order kinetic model having the highest correlation coefficient (R2) 0.9932. CoCuFe2O4 NPs exhibited UV-irradiated photocatalytic activity which strongly depend on the formation of \({HO}^{*}\) radicals which are generated through the transition of photo-excited electrons from valence band to conduction band.
Blood compatibility tests
NPs can affect physical appearance and chemical parameters of various cells including the erythrocytes. Alterations in the morphology of erythrocytes change the elastic properties of cells and damage the rheological properties and respiratory functions [55, 56]. Cellular response in the presence of a synthetic material is an important subject of interest. Since the erythrocyte membrane is based on a similar principle of molecular organization with the cell membranes of other tissues which make up the biological system, possible responses of other membrane systems can be predicted by examining nanoparticle-induced erythrocyte cell disruptions and making reasonable corrections for different cell types. In this study, hemolytic activity tests were performed to examine the effects of CoCuFe2O4 NPs on RBCs. In Fig. 10a and b, aggregation of cell membranes of completely lysed erythrocytes incubated in pure water (due to high osmolarity) and healthy normal erythrocyte cells having a highly specific biconcave shape and a pallor area in the center is shown, respectively. Oxidative stress-induced erythrocyte deformations generated by CoCuFe2O4 NPs with 1.0 mg mL−1 and 5.0 mg mL−1 are shown in Fig. 10c and d, respectively. While lots of healthy normal RBCs are counted in 1.0 mg mL−1 treatment, RBCs treated with 5.0 mg mL−1 CoCuFe2O4 have different types of deformations (schistocytes, spherocytes, Heinz bodies). Heinz bodies and an increased degree of variation in cell morphology (poikilocytosis) [57] was clearly visible in Fig. 10d. When cells are exposed to oxidative stress, ROS start attacking the bonds between heme unit and globin moieties in the hemoglobin molecule. Globin units leave from the hemoglobin complex, form little aggregates, and stick to the inside of the RBC membrane. These inclusions composed of oxidized denatured hemoglobin are called Heinz bodies [58] (Fig. 10d). Heinz bodies form mainly during intense oxidative stress and hemolysis occurs after appearing inclusions in the erythrocytes [59]. In 1.0 mg mL−1 CoCuFe2O4 application, a fragmented RBC (schistocyte) was observed as evidence for erythrocyte lysis; nevertheless, the degree of hemolysis was below the acceptable level of 5%. On the contrary, at 1.0 mg mL−1 CoCuFe2O4 application (Fig. 10d), lots of deformed cells (poikilocytosis) including Heinz bodies and spherocytes were detected. Spherocytes which are observed in all hemolytic anemias are considered as evidence of extravascular factor-mediated hemolysis that forces the blood cell to have spherical shape [28, 60, 61].
As shown in Fig. 11, the supernatant of the 1.0 mg mL−1 CoCuFe2O4 application was colorless and transparent, demonstrating the negligible hemolysis ratio. However, at 5.0 mg mL−1 CoCuFe2O4 application, supernatant phase showed a bright red color, indicating the presence of an obvious hemolysis phenomena. While the AHR for human erythrocytes subjected to 5.0 mg mL−1 CoCuFe2O4 concentration was 12.4%, it was only 2.2% for those of exposed to 1.0 mg mL−1 CoCuFe2O4 which was lower than the allowed limit that is 5% [51, 62].
UV–Vis spectra of erythrocyte suspensions treated with PBS alone (negative control) and CoCuFe2O4 nanoparticles with 1.0 mg mL−1 and 5.0 mg mL−1 concentrations (after incubation and centrifugation). Insets: UV–Vis spectra of erythrocyte suspension in positive control test and after centrifugation digital photos of CoCuFe2O4 samples in 1.0 mg mL−1 (left) and 5.0 mg mL−1 (right) concentrations
The porphyrin ring system consists of four highly conjugated pyrrole rings linked by methane bridges. The heme moiety in Hb is the Fe(II) complex of protoporphyrin (\(Hb\left(F{e}^{2+}\right)\)), which carries a methyl group and a vinyl or propionic acid unit in each pyrrole ring. ROS produced on the surface of CoCuFe2O4 NPs would be responsible from the oxidation of hem iron into Fe(III) and the formation of methemoglobin (MHb). In MHb having the ferric state iron, the oxygen binding capacity of the molecule is significantly reduced. MHb ((\(Hb\left(F{e}^{3+}\right)OH)\) in Eq. 18 can also be formed in a small amount under the influence of various substances such as heavy metal ions or phenolics that are exposed in daily life [63, 64]. In a healthy metabolic process, it is reduced by intracellular enzymatic reactions and converted back to Hb having a Fe2+ carrying ferroprotoporphyrin system [65]. MHb reduction systems, in which NADH cytochrome b5 reductase and glutathione are primarily effective (Eq. 19–20), prevent MHb from exceeding 1% of the total Hb concentration [66]. Glutathione is an antioxidant which protects cells from the toxic effects of oxidants such as free radicals, peroxides, and heavy metals. Glutathione is found in erythrocytes and protects the cell from oxidative stress with the effect of enzymes such as superoxide dismutase and glutathione peroxidase [67]:
Another possible CoCuFe2O4–blood interaction may be the interaction of Co–Cu and Fe2+ in protoporphyrin ring system. Selective separation of some specific molecules is difficult due to interferences created by protein-based molecules which are abundant in blood. Chelating species such as Cu and Co in the structure of nano-sized materials make them suitable for being used in selective separation. Cu2+, Co2+, Ni2+, or Fe2+ containing NPs form covalent interactions and chelates especially with histidine-rich proteins. In a related literature study conducted by Thomas et al. [68], it was emphasized that ferrite-based NPs can be used in the chemical recognition of some molecules and in the selective separation and fractionation of blood proteins. In the study of Guo et al. [69], sol–gel-synthesized CuFe2O4 NPs were investigated for their chelating properties and photoactivities towards methylene blue. In another study, solvothermally synthesized CuFe2O4 particles were investigated for their performances to be used in the selective separation of hemoglobin- and histidine-bearing proteins [70]. Superparamagnetic CoFe2O4 NPs synthesized by seed-mediated growth method were also investigated for selective separation, enrichment, and characterization of phosphoproteins [71].
In this study, although the CoCuFe2O4 NPs seem to have good biocompatibility especially at 1.0 mg mL−1 concentration just by considering the hemolysis rates, when the full spectrum is examined, changes indicative of the potential interactions of the samples with blood components are striking. One of the most probable interactions of CoCuFe2O4 NPs and blood components is the oxidation of hem units as a direct result of ROS production by NPs. The decrease in intensity in the Q band at 1.0 mg mL−1 nanoparticle concentration is an indicator showing the presence of Fe3+ in the ferroprotoporphyrin system and proving the formation of MHb. At the same time, the characteristic change in the oxyhemoglobin Q bands is found as another evidence confirming MHb formation [72]. The UV spectrum of 1.0 mg mL−1 CoCuFe2O4 nanoparticle concentration also shows a significant blue shift and decrease in intensity in the Soret band, which are indicative of the oxidation of Hb to MHb because of oxidative stress created by ROS in erythrocytes (Fig. 11).
At 5.0 mg mL−1 CoCuFe2O4 NP concentration, the main and dominant interaction is between NPs and the erythrocyte cell wall. Erythrocyte membrane is a composite structure, primarily composed of a phospholipid bilayer bearing almost the same concentrations of both lipid and protein moieties [73, 74]. NPs inactivate proteins in the erythrocyte membrane, causing cell death. NPs form R–S–(Cu)2+–S–R complexes by interacting with proteins in the membrane structure, especially those carrying sulfhydryl (-SH) groups such as cysteine. These interactions reduce erythrocyte membrane permeability and ultimately result in lysis of the erythrocyte cell wall [75, 76]. In Fig. 11, along with the characteristic red color of hemoglobin leaking out of the cell as a result of lysis of the erythrocyte cell wall, a small amount of blue shift in the Soret band was also observed as an indicator of MHb formation. One of the main reasons for the deterioration of erythrocyte cell integrity is the destruction of the erythrocyte membrane by ROS such as superoxide and hydroxyl radicals. Thus, hemolysis at 5.0 mg mL−1 CoCuFe2O4 nanoparticle concentration can be attributed to the comparatively high amount of ROS formed by CoCuFe2O4 NPs, which is also consistent with the results of photodegradation experiments.
Conclusion
CoCuFe2O4 spinel ferrites were synthesized by the co-precipitation method using Fe(NO3)3.9H2O, Co(NO3)2.6H2O, and Cu(NO3)2.3H2O. NPs have a high crystalline quality; no secondary crystalline phases in addition to the cubic structured spinel lattice were observed and exhibited an agglomerated structure. SEM images showed the agglomerated morphology of CoCuFe2O4 NPs. UV–Vis diffuse reflectance spectra was interpreted, and direct and indirect band gap energies were determined as 1.93 eV and 0.6 eV, respectively. Photocatalytic properties of CoCuFe2O4 NPs were attributed to the formation of ROS such as HO* and O2–* triggered by the photo-excitation of electrons from valence band to conduction band. The decomposition of CV under the influence of ROS formed on the nanoparticle surface was determined as 64.9%. Three different kinetic models were tested to explain the CV photodegradation kinetics. Among them, it was concluded that the zero-order kinetics (R2 = 0.9932) was the best fitted model for the CV photodegradation reaction. Blood compatibility tests showed that CoCuFe2O4 did not cause hemolysis at 1 mg mL−1 concentration; however, a blue shift in the Soret band with a decrease in intensity and the disappearance of the Q bands demonstrated the formation of MHb. It was concluded that erythrocyte lysis at 5.0 mg mL−1 concentration was caused by high amount of ROS produced by NPs, which also caused oxidation of heme units on the porphyrin ring. Microscopic images of erythrocytes showed spherocytes, schistocytes, and Heinz bodies at a concentration of 5.0 mg mL−1 NPs, while large number of healthy cells was detected at 1.0 mg mL−1. Photocatalytic activity test results and RBC deformations were evaluated together, and it was concluded that NPs showed cytotoxic effects, especially at high concentrations, associated with ROS. It is hoped that the findings from this study will be developed with subsequent research and modifications, thus leading to the development of nanomaterials with better biocompatibility and adapted structural properties.
Data availability
The author confirms that the data supporting the findings of this study are available within the article.
References
Rajan A, Sharma M, Sahu NK (2020) Assessing magnetic and inductive thermal properties of various surfactants functionalised Fe3O4 nanoparticles for hyperthermia. Sci Report 10:1–15. https://doi.org/10.1038/s41598-020-71703-6
Yang X, Zheng G, Wang Q, Chen X, Han Y, Zhang D, Zhang Y (2022) Functional application of multi-element metal composite materials. J Alloys Compd 895:162622
Joshi R, Singh BP, Ningthoujam RS (2020) Confirmation of highly stable 10 nm sized Fe3O4 nanoparticle formation at room temperature and understanding of heat-generation under AC magnetic fields for potential application in hyperthermia. AIP Adv 10:105033. https://doi.org/10.1063/5.0022446
Mousavi SM, Hashemi SA, Zarei M, Bahrani S, Savardashtaki A, Esmaeili H, Ramavandi B (2020) Data on cytotoxic and antibacterial activity of synthesized Fe3O4 nanoparticles using Malva sylvestris. Data Br 28:104929. https://doi.org/10.1016/j.dib.2019.104929
Pachaiappan R, Manavalan K (2021) Role of metals, metal oxides, and metal sulfides in the diagnosis and treatment of cancer. In: Rajendran S, Naushad M, Durgalakshmi D, Lichtfouse E (eds) Metal, Metal Oxides and Metal Sulphides for Biomedical Applications. Environmental Chemistry for a Sustainable World, vol 58. Springer, Cham
Gholami A, Mousavi SM, Hashemi SA, Ghasemi Y, Chiang WH, Parvin N (2020) Current trends in chemical modifications of magnetic nanoparticles for targeted drug delivery in cancer chemotherapy. Drug Metab Rev 52:205–224. https://doi.org/10.1080/03602532.2020.1726943
Gaikwad P, Sabale S, Kurane R, Kakade B, Parase H, Dhabbe R, Kamble P (2021) Magneto-structural properties and reliability of (Mn/Ni/Zn) substituted cobalt-copper ferrite heterogeneous catalyst for selective and efficient oxidation of aryl alcohols. Inorg Nano-Met 1–14. https://doi.org/10.1080/24701556.2021.1980036
Yalcin B, Ozcelik S, Icin K, Senturk K, Ozcelik B, Arda L (2021) Structural, optical, magnetic, photocatalytic activity and related biological effects of CoFe2O4 ferrite nanoparticles. J Mater Sci Mater 32:13068–13080. https://doi.org/10.1007/s10854-021-05752-6
Ozcelik S, Yalçın B, Arda L, Santos H, Sáez-Puche R, Angurel LA, Ozcelik B (2021) Structure, magnetic, photocatalytic and blood compatibility studies of nickel nanoferrites prepared by laser ablation technique in distilled water. J Alloys Compd 854:157279. https://doi.org/10.1016/j.jallcom.2020.157279
Senol SD, Yalcin B, Ozugurlu E, Arda L (2020) Structure, microstructure, optical and photocatalytic properties of Mn-doped ZnO nanoparticles. Mater Res Express 7:015079. https://doi.org/10.1088/2053-1591/ab5eea
Heiba ZK, Mohamed MB, Arda L, Dogan N (2015) Cation distribution correlated with magnetic properties of nanocrystalline gadolinium substituted nickel ferrite. J Magn Magn Mater 391:195–202. https://doi.org/10.1016/j.jmmm.2015.05.003
Nikazar S, Barani M, Rahdar A, Zoghi M, Kyzas GZ (2020) Photo-and magnetothermally responsive nanomaterials for therapy, controlled drug delivery and imaging applications. Chem Select 5:12590–12609. https://doi.org/10.1002/slct.202002978
Kossatz S, Grandke J, Couleaud P, Latorre A, Aires A, Crosbie-Staunton K, Calero M (2015) Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Breast Cancer Res 17:66. https://doi.org/10.1186/s13058-015-0576-1
Junaid M, Qazafi IA, Khan MA, Gulbadan S, Ilyas SZ, Somaily HH, Amin MA (2022) The influence of Zr and Ni co-substitution on structural, dielectric and magnetic traits of lithium spinel ferrites. Ceram Int 48(10):14307–14314. https://doi.org/10.1016/j.ceramint.2022.01.320
Jacinto MJ, Ferreira LF, Silva VC (2020) Magnetic materials for photocatalytic applications - a review. J Solgel Sci Technol 96:1–14. https://doi.org/10.1007/s10971-020-05333-9
Bahrami M, Derikvand Z (2022) Fabrication of a new magnetic CoFe2O4/ZrMCM-41 nanocomposite: simple construction and application for fast reduction of Cr (IV) and nitroaromatic compounds. J Molec Str 1254:132367. https://doi.org/10.1016/j.molstruc.2022.132367
Zhang J, Liu P, Ren Y, Du Y, Geng C, Ma J, Zhao F (2022) Treatment of shale gas produced water by magnetic CuFe2O4/TNTs hybrid heterogeneous catalyzed ozone: efficiency and mechanisms. J Hazard Mater 423:127124
Amiri M, Salvati-Niasari M, Akbari A (2019) Magnetic nanocarriers: evolution of spinel ferrites for medical applications. Adv Colloid Interface Sci 265:29–44. https://doi.org/10.1016/j.cis.2019.01.003
Oh Y, Moorthy MS, Manivasagan P, Bharathiraja S, Oh J (2017) Magnetic hyperthermia and pH-responsive effective drug delivery to the sub-cellular level of human breast cancer cells by modified CoFe2O4 nanoparticles. Biochimie 133:7–19. https://doi.org/10.1016/j.biochi.2016.11.012
Chapman MC, Lee AY, Hayward JH, Joe BN, Price ER (2020) Superparamagnetic iron oxide sentinel node tracer injection: effects on breast MRI quality. J Breast Imaging 2:577–582. https://doi.org/10.1093/jbi/wbaa083
Ansari SM, Bhor RD, Pai KR, Mazumder S, Sen D, Kolekar YD, Ramana CV (2016) Size and chemistry controlled cobalt-ferrite nanoparticles and their anti-proliferative effect against the MCF-7 breast cancer cells. ACS Biomater Sci Eng 2:2139–2152. https://doi.org/10.1021/acsbiomaterials.6b00333
Zarrabi M, Afzal E, Ebrahimi M (2018) Manipulation of hematopoietic stem cell fate by small molecule compounds. Stem Cells Dev 27:1175–1190. https://doi.org/10.1089/scd.2018.0091
Sunitha Y, Kumar S (2021) An assessment of vitamin B12 through determination of cobalt by X-ray fluorescence spectrometry. Radiat Phys Chem 188:109583. https://doi.org/10.1016/j.radphyschem.2021.10958
Sattarahmady N, Zare T, Mehdizadeh AR, Azarpira N, Heidari M, Lotfi M, Heli H (2015) Dextrin-coated zinc substituted cobalt-ferrite nanoparticles as an MRI contrast agent: in vitro and in vivo imaging studies. Colloids Surf B 129:15–20. https://doi.org/10.1016/j.colsurfb.2015.03.021
Wahler T, Schuster R, Libuda J (2020) Self-metalation of anchored porphyrins on atomically defined cobalt oxide surfaces: in situ studies by surface vibrational spectroscopy. Chemistry 26:12445. https://doi.org/10.1002/chem.202001331
Yao M, Ma M, Zhang H, Zhang Y, Wan G, Shen J, Wu R (2018) Mesopore-induced aggregation of cobalt protoporphyrin for photoacoustic imaging and antioxidant protection of stem cells. Adv Funct Mater 28:1804497. https://doi.org/10.1002/adfm.201804497
Liu T, Xiao B, Xiang F, Tan J, Chen Z, Zhang X, Deng J (2020) Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat Commun 11:1–16. https://doi.org/10.1038/s41467-020-16544-7
Agarwal V, Gupta V, Bhardwaj VK, Singh K, Khullar P, Bakshi M (2022) Hemolytic response of iron oxide magnetic nanoparticles at the interface and in bulk: extraction of blood cells by magnetic nanoparticles. ACS Appl Mater Interfaces 14:6428–6441
Yadav S, Maurya PK (2022) Recent advances in the protective role of metallic nanoparticles in red blood cells. Biotech 12:1–13
Bai C, Hu P, Liu N, Feng G, Liu D, Chen Y, Zhang Y (2020) Synthesis of ultrasmall Fe3O4 nanoparticles as T 1–T 2 dual-modal magnetic resonance imaging contrast agents in rabbit hepatic tumors. ACS Appl Nano Mater 3:3585–3595. https://doi.org/10.1021/acsanm.0c00306
Vangijzegem T, Stanicki D, Panepinto A, Socoliuc V, Vekas L, Muller RN, Laurent S (2020) Influence of experimental parameters of a continuous flow process on the properties of very small iron oxide nanoparticles (VSION) designed for T1-weighted magnetic resonance imaging (MRI). Nanomaterials 10:757. https://doi.org/10.3390/nano10040757
Du H, Akakuru OU, Yao C, Yang F, Wu A (2022) Transition metal ion-doped ferrites nanoparticles for bioimaging and cancer therapy. Transl Oncol 15:101264
Kumari S, Manglam MK, Kumar L, Seal P, Borah JP, Kumar Zope M, Kar M (2022) Magnetic properties and hyperthermia action of cobalt zinc ferrite fibers. J Sol-Gel Sci Technol 101:546–561. https://doi.org/10.1007/s10971-022-05737-9
Sharma VK, Bielski BH (1991) Reactivity of ferrate (VI) and ferrate (V) with amino acids. Inorg Chem 30:4306–4310. https://doi.org/10.1021/ic00023a005
Xiong Z, Lai B, Yang P, Zhou Y, Wang J, Fang S (2015) Comparative study on the reactivity of Fe/Cu bimetallic particles and zero valent iron (ZVI) under different conditions of N2, air or without aeration. J Hazard Mater 297:261–268. https://doi.org/10.1016/j.jhazmat.2015.05.006
Jiang K, Zhang L, Bao G (2021) Magnetic iron oxide nanoparticles for biomedical applications. Curr Opin Biomed Eng 20:100330. https://doi.org/10.1016/j.cobme.2021.100330
Kumar G, Katoch G (2021) Recent advances in processing, characterizations and biomedical applications of spinel ferrite nanoparticles. Materials Research Foundations 112:62–120. https://doi.org/10.21741/9781644901595-2
Nosheen S, Irfan M, Abidi SH, Syed Q, Habib F, Asghar A, Waseem B, Soomro B, Butt H, Akram M (2021) A review: development of magnetic nan o vectors for biomedical applications. GSC Adv Res Rev 8:85–110. https://doi.org/10.30574/gscarr.2021.8.2.0169
Matloubi Moghaddam F, Pourkaveh R, Ahangarpour M (2018) Cobalt-copper ferrite nanoparticles catalyzed click reaction at room-temperature: green access to 1, 2, 3-triazole derivatives. Chem Select 3:2586–2593. https://doi.org/10.1002/slct.201800134
Dave PN, Sirach R, Deshpande MP (2022) Evaluating the effect of nanosized CoCuFe2O4 for thermal decomposition of nitrotriazolone high energetic material. Chem Select 7:e202202071. https://doi.org/10.1002/slct.202202071
Yalcin B, Erbil C (2018) Effect of sodium hydroxide solution as polymerization solvent and activator on structural, mechanical and antibacterial properties of PNIPAAm and P (NIPAAm–clay) hydrogels. Polym Compos 39:E386–E406. https://doi.org/10.1002/pc.24490
Dadaei M, Naeimi H (2020) An environment-friendly method for green synthesis of pyranopyrazole derivatives catalyzed by CoCuFe2O4 magnetic nanocrystals under solvent-free conditions. Polycycl Aromat Compd 42:204–217. https://doi.org/10.1080/10406638.2020.1725897
Moghaddam FM, Pourkaveh R, Ahangarpour M (2017) Nano CoCuFe2O4 catalyzed coupling reaction of acid chlorides with terminal alkynes: a powerful toolbox for palladium-free ynone synthesis. Catal Commun 102:71–75. https://doi.org/10.1016/j.catcom.2017.08.029
Jamil H, Dildar IM, Ilyas U, Hashmi JZ, Shaukat S, Sarwar MN, Khaleeq-ur-Rahman M (2021) Microstructural and optical study of polycrystalline manganese oxide films using Kubelka-Munk function. Thin Solid Films 732:138796. https://doi.org/10.1016/j.tsf.2021.138796
Chavan P, Naik LR (2017) Investigation of energy band gap and conduction mechanism of magnesium substituted nickel ferrite nanoparticles. Phys Status Solidi 214:1700077. https://doi.org/10.1002/pssa.201700077
Khan HAA, Ullah S, Rehman G, Khan S, Ahmad I (2021) First principle study of band gap nature, spontaneous polarization, hyperfine field and electric field gradient of desirable multiferroic bismuth ferrite (BiFeO3). J Phys Chem Solids 148:109737. https://doi.org/10.1016/j.jpcs.2020.109737
Siva KV, Kumar A, Chelvane JA, Arockiarajan A (2022) Structural, magnetic, magnetostrictive and optical properties of Mn and Cu codoped cobalt ferrite. Mater Sci Eng, B 284:115885. https://doi.org/10.1016/j.mseb.2022.115885
Hammad TM, Kuhn S, Amsha AA, Hempelmann R (2021) Investigation of structural, optical, and magnetic properties of Co2+ ions substituted CuFe2O4 spinel ferrite nanoparticles prepared via precipitation approach. J Aust Ceram Soc 57:543–553. https://doi.org/10.1007/s41779-020-00556-z
Mirzaee S, Mahdavifar S, Shayesteh SF (2018) Experimental and theoretical investigations of magnetic properties of Co ferrite/polyvinyl alcohol nanocomposites. J Supercond Nov Magn 31:217–223. https://doi.org/10.1007/s10948-017-4166-6
Yalcin B, Akcan D, Yalcin IE, Alphan MC, Senturk., Ozyigit II, Arda L, (2020) Effect of Mg doping on morphology, photocatalytic activity and related biological properties of Zn1-xMgxO nanoparticles. Turk J Chem 44:1177–1199. https://doi.org/10.3906/kim-2004-9
Bhaskar N, Sulyaeva V, Gatapova E, Kaichev V, Khomyakov M, Kolodin A, Basu B (2022) On the origin of better hemocompatibility of the BCxNyOz coatings. Appl Surf Sci 576:151760. https://doi.org/10.1016/j.apsusc.2021.151760
Frolova LA, Khmelenko OV (2020) The study of Co–Ni-Mn ferrites for the catalytic decomposition of 4-nitrophenol. Catal Lett 151:1522–1533. https://doi.org/10.1007/s10562-020-03419-1
Rani M, Shanker U, Chaurasia AK (2017) Catalytic potential of laccase immobilized on transition metal oxides nanomaterials: degradation of alizarin red S dye. J Environ Chem Eng 5:2730–2739. https://doi.org/10.1016/j.jece.2017.05.026
Oladoja NA, Anthony ET, Ololade IA, Saliu TD, Bello GA (2018) Self-propagation combustion method for the synthesis of solar active Nano Ferrite for Cr (VI) reduction in aqua system. J Photochem Photobiol 353:229–239. https://doi.org/10.1016/j.jphotochem.2017.11.026
Pleskova SN, Gornostaeva EE, Kryukov RN, Boryakov AV, Zubkov SY (2018) Changes in the architectonics and morphometric characteristics of erythocytes under the influence of magnetite nanoparticles. Cell Tissue Biol 12:127–134. https://doi.org/10.1134/S1990519X18020086
Zemlyanova MA, Zaitseva NV, Ignatova AM, Stepankov MS, Toropov LI, Kol’dibekova YV, (2021) Study of hematological parameters and morphometric indices of erythrocytes in rats exposed to calcium oxide nanoparticles. Bull Exp Biol Med 170:665–668. https://doi.org/10.1007/s10517-021-05128-0
Corrons JLV, Bain BJ (2022) Haemoglobin Bristol-Alesha in a child with non-spherocytic severe haemolytic anaemia and marked anisochromic poikilocytosis with basophilic stippling and amorphous intracellular content. Blood Cells Mol Dis 94:102652. https://doi.org/10.1016/j.bcmd.2022.102652
Thiagarajan P, Parker CJ, Prchal JT (2021) How do red blood cells die? Front Physiol 12:318
Mameri A, Bournine L, Mouni L, Bensalem S, Iguer-Ouada M (2021) Oxidative stress as an underlying mechanism of anticancer drugs cytotoxicity on human red blood cells’ membrane. Toxicol in Vitro 72:105106. https://doi.org/10.1016/j.tiv.2021.105106
Johnson S (2021) Review on spherocytosis. J Hematol Thrombo Dis 9:445
Agarwal V, Gupta V, Bhardwaj VK, Singh K, Khullar P, Bakshi MS (2021) Avoiding hemolytic anemia by understanding the effect of the molecular architecture of Gemini surfactants on hemolysis. Langmuir 37:3709–3720. https://doi.org/10.1021/acs.langmuir.1c00154
Singh S, Singh G, Bala N (2021) Synthesis and characterization of iron oxide-hydroxyapatite-chitosan composite coating and its biological assessment for biomedical applications. Prog Org Coat 150:106011. https://doi.org/10.1016/j.porgcoat.2020.106011
Iolascon A, Bianchi P, Andolfo I, Russo R, Barcellini W, Fermo E (2021) SWG of red cell and iron of EHA and EuroBloodNet, Recommendations for diagnosis and treatment of methemoglobinemia. Am J Hematol 96:1666–1678. https://doi.org/10.1002/ajh.26340
Arnhold J (2019) Cell and tissue destruction: mechanisms, protection, disorders. Academic Press
Cortazzo JA, Lichtman AD (2014) Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth 28:1043–1047. https://doi.org/10.1053/j.jvca.2013.02.005
Keohane E, Otto CN, Walenga J (2019) Rodak's Hematology-E-Book: Clinical Principles and Applications. Elsevier Health Sciences
Chu L, Wu Y, Xu X, Phillips L, Kolodrubetz D (2020) Glutathione catabolism by Treponema denticola impacts its pathogenic potential. Anaerobe 62:102170. https://doi.org/10.1016/j.anaerobe.2020.102170
Thomas SL, Thacker JB, Schug KA, Maráková K (2021) Sample preparation and fractionation techniques for intact proteins for mass spectrometric analysis. J Sep Sci 44:211–246. https://doi.org/10.1002/jssc.202000936
Guo X, Wang K, Xu Y (2019) Tartaric acid enhanced CuFe2O4-catalyzed heterogeneous photo-Fenton-like degradation of methylene blue. Mater Sci Eng B 245:75–84. https://doi.org/10.1016/j.mseb.2019.05.015
Zheng J, Lin Z, Liu W, Wang L, Zhao S, Yang H, Zhang L (2014) One-pot synthesis of CuFe2O4 magnetic nanocrystal clusters for highly specific separation of histidine-rich proteins. J Mater Chem B 2:6207–6214. https://doi.org/10.1039/C4TB00986J
Chen B, Hwang L, Ochowicz W, Lin Z, Guardado-Alvarez TM, Cai W, Ge Y (2017) Coupling functionalized cobalt ferrite nanoparticle enrichment with online LC/MS/MS for top-down phosphoproteomics. Chem Sci 8:4306–4311. https://doi.org/10.1039/C6SC05435H
Denzer ML, Mowery C, Comstock HA, Maheswarappa NB, Mafi G, VanOverebeke DL, Ramanathan R (2020) Characterization of the cofactors involved in non-enzymatic metmyoglobin/methemoglobin reduction. In Vitro Meat Muscle Biol 4:1–10. https://doi.org/10.22175/mmb.9507
Cho Y, Woo JH, Kwon OS, Yoon SS, Son J (2019) Alterations in phospholipid profiles of erythrocytes deep-frozen without cryoprotectants. Drug Test Anal 11:1231–1237. https://doi.org/10.1002/dta.2600
Sathi A, Viswanad V, Aneesh TP, Kumar BA (2014) Pros and cons of phospholipid asymmetry in erythrocytes. J Pharm Bioallied Sci 6:81. https://doi.org/10.4103/0975-7406.129171
Tkachenko A, Onishchenko A, Klochkov V, Kavok N, Nakonechna O, Yefimova S, Posokhov Y (2020) The impact of orally administered gadolinium orthovanadate GdVO4: Eu3+ nanoparticles on the state of phospholipid bilayer of erythrocytes. Turkish J Biochem 45:389–395. https://doi.org/10.1515/tjb-2019-0427
Azzam EMS, Zaki MF (2016) Surface and antibacterial activity of synthesized nonionic surfactant assembled on metal nanoparticles. Egypt J Pet 25:153–159. https://doi.org/10.1016/j.ejpe.2015.04.005
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yalcin, B. Exploration of the potential of Co/Cu co-doped Fe2O4 for medical applications: nanostructure, catalytic properties, and blood compatibility. J Nanopart Res 24, 271 (2022). https://doi.org/10.1007/s11051-022-05645-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05645-7