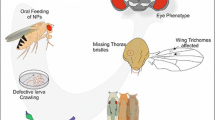
Abstract
Tungsten oxide nanoparticles (WO3 NPs) have now been employed by various products including electronics, smart screens, gas-biosensors, water purifiers, disinfectants, and biomedical applications. Despite this wide-ranging adoption, little research has investigated their potential endpoint biomarkers in different in vivo models. We therefore propose the use of Drosophila melanogaster as a testing model in assessing genotoxic risks of exposure to WO3 NPs. Our study examined toxicity, phenotypic alterations, locomotor behavior (climbing assay), intracellular oxidative stress (ROS), DNA damage (Comet assay), and somatic recombination (wing spot assay) in Drosophila melanogaster after exposure to WO3 NPs (43.71 ± 1.59 nm) and microparticulated (MPs) of WO3. Drosophila larvae were exposed to the test materials via ingestion at doses ranging between 1 and 10 mM, and two greatest doses of NPs (5 and 10 mM) were found to cause mutagenic/recombinogenic effects, while the MPs caused no effects. Wing-spot assay detected genotoxic activity of NPs mostly through somatic recombination, and Comet assay showed DNA damage after exposure to NPs at certain doses (1, 2.5, 5, and 10 mM). Other observations included ROS generation in hemocytes, phenotypic alterations in the mouths and wings of adult flies, and impaired locomotor behavior. This is the first research to report genotoxic evidence on the impact of WO3 exposure in Drosophila larvae, highlighting the significance of this model organism in exploring the potential biological impact of nanoparticles and MPs of WO3. The results of our in vivo testing should make a vital contribution to the existing database on the genotoxicity of WO3 NPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lately, huge strides in the sphere of nanotechnology have culminated in more than 20-fold increase in the amount of nanomaterial production. Nanoparticles (NPs), ranging in size between 1 and 100 nm, are key components of nano-based products [1]. Certain characteristics of NPs, including surface area, diameter, and shape, give them some properties considerably different from their microparticulated (MPs) (> 100 nm), which are prized across a wide range of fields [2]. Such broad-spectrum of NP utilization is predicted to cause a significant increase in their leakage or escape into diverse environments [3]. They can find their way into the organisms dwelling in these environments, raising concerns over their hazardous health impact on animal and plant life. Human populations could be exposed to NPs through natural routes like ingestion, inhalation, and dermal contact [4].
There is still limited research into the toxic and genotoxic impacts of NPs despite the potential risks associated with their acute and chronic exposure [5, 6]. Earlier research in the relevant literature employing various in vivo testing models to explore the toxic and genotoxic effects of several NPs showed that some could induce significant genotoxicity, along with oxidative stress [5, 7, 8]. Tungsten trioxide (or tungsten (VI) oxide (WO3) is a thermally stable and valuable semiconductor compound with a wide bandgap (2.5–2.8 eV). Industrial applications of WO3 include utilization as pigment for tint and ceramics on account of its yellowish color [9]. Its nanoparticle form, WO3 NPs, attracts considerable attention thanks to the large surface area of particles and their stability at extreme temperatures [10]. Different nano forms of WO3, such as nanocrystals and nanosheets, have been widely used in applications like memory devices, micro/optoelectronics [11], smart screens [12] gas-sensing devices [13], polluted water purifiers [14], disinfectants [15], and photoelectrocatalysis [16]. On top of that, current efforts are geared towards using WO3 in biomedical applications, since they offer several advantages in this field such as improved visibility of target tissues in CT scans. Besides, WO3 nanorods have been employed as both therapeutic and diagnostic agents in tumor CT imaging and photothermal therapy [8].
Although we still have no reliable figure as to the total global production of WO3, the US market is forecast at 9.9 thousand metric tons in 2021, while China is estimated to reach 67.5 thousand metric tons by 2026 (https://www.reportlinker.com/p04159772/?utm_source=GNW).
In spite of its heavy use across various industries and prevalence of its compounds in the nature, potential biological endpoints of WO3 NPs have yet to be fully illuminated by using reliable in vivo models. Limited number of in vitro studies, on the other hand, report that WO3 NPs may have some toxic potential. There is also evidence that WO3 NPs may cause mutagenicity in a bacteria species called Salmonella typhimurium [17]. Also, cytotoxic effects of WO3 NPs have been determined in different cell lines (Caco-2, 3T3, A549 cell, AGS cell line of human stomach cancer, and cultured primary rat hepatocytes) [18,19,20,21]. In addition, the genotoxic and mutagenic effects caused by WO3 NPs were explored by in vitro studies employing micronucleus (MN) tests and Comet assays in cultured human lymphocytes [22]. So far, very little in vivo research has been carried out to explore the toxicity of WO3 NPs to living organism, and yet they revealed that chronic or acute exposure to WO3 NPs could exert toxic effects on hamsters and rats [8, 23,24,25,26]. Exposure via oral route is the most common entrance way of the NPs into the human body, which is well reflected in in vivo model organisms that are often exposed to such materials via ingestion [20].
Over the recent decades, researchers have been striving to find a way to avoid the high costs and prevailing ethical issues related to the handling of mammalian testing models in experiments, trying alternative in vivo model organisms with a capacity to act as near-perfect counterparts of higher vertebrates [27,28,29,30,31]. To that end, a number of non-mammalian organisms including Daphnia magna [32, 33], Caenorhabditis elegans [29, 30], and Drosophila melanogaster [34,35,36] have been used in search of an alternative in vivo model organism.
Research previously established that certain engineered NPs might escape or leak into the nature during their fabrication, transfiguration, and utilization, causing significant pollution and endangering plant and animal life. Despite their common usage, there still exists rather little scientific evidence as to the cytotoxic and genotoxic capacity of WO3 NPs across different fauna and flora. A nanomaterial’s capacity to produce toxicity is principally regulated by the particle size, shape, surface area, form, and surface coating or modification [37]. More recently, toxicity research into NPs has reported that several types of NPs could bring about oxidative stress, ROS generation, and detrimental consequences to genetic makeup and essential components of cell membranes mainly including lipids and proteins [38, 39].
Around 75% of human genes linked to diseases have been discovered to exhibit matches or homologs in the genes of Drosophila fruit flies [40], and since then this species has been adopted as a popular genetic model by researchers to study various diseases including gastrointestinal infections [41], heart and circulatıry problems [42], oxidative stress [43], carcinoma [44], genetic disorders [45], and neurodegenerative disorders [46, 47]. Furthermore, several biological commonalities are conserved between humans and fruit flies, which include gene expression, cell proliferation, cell signaling, differentiation, homeostatic cellular balance, and cell death [48]. Best of all, Drosophila enables bypassing frustrating ethical rules in place to restrict testing on mammals or higher vertebrates [49, 50]. A new field commonly referred to as Drosophotoxicology [51, 52] involves the development of biological data from toxicological research using D. melanogaster [53]. Also, this organism is among those recommended by the researchers [54, 55] including the European Center for the Validation of Alternative Methods (ECVAM) [56] to minimize experiments on higher vertebrates like mice.
Our study aimed to characterize as many as endpoint biomarkers for MPs and nano forms of WO3 through a series of experiments on Drosophila. For an in-depth analysis and risk assessment, we adopted a combined approach, and as far as we are aware, our study is to become the first in vivo study to investigate this compound’s cytotoxicity, genotoxicity, and morphologic alterations in fruit flies. To that end, we conducted several tests and microscopy imaging techniques, which included quantification of egg-to-adult viability, climbing assays to measure overall locomotion in exposed adult flies (direct cytotoxicity), observation of morphological alterations, Comet assays to detect the genotoxic capacity of WO3 compounds (as revealed by DNA strand breaks), SMART assays to analyze activities like genetic mutations and recombinations, and finally, determination of ROS generation. We firmly believe that our study findings will contribute significantly to our present understanding of risks pertaining to WO3 exposure.
Experimental details
Chemicals
Tungsten oxide (VI) NPs (mean diameter: 30 nm, nanopowder with 99.99% purity, CAS No. 1314–35-8) and its MPs (tungsten oxide (VI)) (99% purity; CAS No. 1314–35-8, amorphous powder) were obtained from Acros Organics (Belgium). We procured hydrogen peroxide (H2O2, CAS No. 7722–84-1) and ethyl methanesulfonate (EMS, CAS No. 62–50-0) from Sigma Chemical (USA). WO3 NPs and MPs were dispersed in deionized water, a common solvent in previous research [57], and as for positive control substance in SMART and Comet testing, we used EMS at 1 mM [57, 58], EMS at 4 mM [57,58,59,60], respectively. In the tests conducted for the quantification of ROS levels, we employed 0.5 mM of hydrogen peroxide solution (H2O2) [57, 58, 60].
Characterization and dispersion of tungsten oxide (IV) nanoparticles
For the characterization of WO3 NPs, we utilized the following instruments: Environmental Scanning Electron Microscopy (ESEM, FEI QUANTA 260F) (Hillsboro, Oregon, USA), TEM, (Tecnai G2 F30) (Austin, Texas, USA) and Malvern Zetasizer Nano-ZS zen3600 laser Doppler velocimetry (LDV) (Worcestershire, UK), and Malvern Zetasizer Nano-ZS zen3600 dynamic light scattering (DLS) (Worcestershire, UK). In elemental analyses of test materials, we utilized energy-dispersive X-ray spectroscopy (EDX) (Malvern, UK), which is generally considered a well-established technique for the chemical evaluation of nanomaterials. We prewetted WO3 NPs in 0.5 vol% ethanol, and then dispersed the NPs in a stabilizing agent (BSA 0.05%) in Milli-Q water, purified by a Milli-Q lab water system. After that stage, once in the dispersion medium, we sonicated the NPs at 20 kHz for 30 min in a Branson digital sonifier (S-250D, Cambridge, MA, USA) to create a 2.56 mg/mL stock dispersion, as per the Nanogenotox protocol [61].
Toxicity (viability) of WO3 NPs and MPs
In our experiments, we used a wild-type strain of Drosophila commonly referred to as Oregon-R+ to determine the toxic potential of WO3 NPs and MPs. Toxic doses of these materials were previously specified in detail by earlier research (genotoxicity, ROS production in cells, locomotor behavior, and phenotypic changes). Following exposure to study compounds, egg-to-adult viability was measured in percentages. The steps in this procedure were as follows: 50 eggs for each dose of WO3 NPs and its MPs (1, 2.5, 5, and 10 mM) were placed into 5 plastic test vials containing food media (4 g). Agar-based food media was initially treated with different doses of WO3 NPs (10 mL) and its MPs (0, 1, 2.5, 5, and 10 mM), which accounted for nominal doses of 231.84, 579.6, 1159.2, and 2318.4 μg/mL, and following dispersion in the media they accounted for 0.5796, 1.449, 2.898, and 5.796 mg/g food. We decided on the ideal doses of study compounds in reference to findings of earlier studies using in vivo and in vitro models [8, 20, 21, 24, 26]. The upper threshold for WO3 NP and MPs doses was 10 mM, and for each test dose we performed 5 replications in total. After the eggs had been exposed to the study compounds throughout the stages of development, we noted the number of adult flies that successfully survived in order to quantify the survival rate. Throughout the paper, we presented the doses of WO3 NPs and MPs in mM to maintain coherence and unity.
Intracellular oxidative stress (ROS) detection in Drosophila hemocytes
Oxidative stress, often caused by disturbances in the balance between free radicals and antioxidants, is a crucial phenomenon governing the extent of the toxic and genotoxic capacity of fabricated NPs. In detecting any possible elevation in ROS production, we utilized 2′,7′ dichlorodihydrofluorescein diacetate (DCFH-DA), a useful cell-permeable fluorogenic probe commonly used to quantify ROS levels in the cells of living organisms. Accordingly, our tests detected increased ROS in the hemocytes of 96 h-old 3rd instar Drosophila larvae upon exposure to both WO3 NPs and MPs for 24 h. During these experiments, the steps we followed, in line with our previous studies [57, 58, 60], were as follows: First, we collected samples of Drosophila hemocytes, then incubated the samples in 5 μM DCFH-DA at 24 °C for 30 min. Second, the cells were examined under fluorescence microscopy equipped with a green filter (485-nm excitation and 528-nm emission). As a negative control, we used sterile distilled water, while 0.5 mM of hydrogen peroxide (H2O2) was employed as positive control. In the final step, the images created through microscopy studies were transferred to a software package called ImageJ for further analysis [62].
Phenotypic alterations
In an attempt to probe whether exposure to WO3 NPs and MPs would induce any alterations in the phenotype, we examined a total of 50 Oregon R+ adult flies through a stereomicroscope [34, 58], performing assessments in all body parts of flies (including eyes, legs, wings, and abdomen) and observed alterations and abnormalities mainly in the mouth and wings.
Climbing assay
Locomotor behavior of the flies was measured through climbing assays in accordance with the procedures proposed by previous studies [58, 63,64,65,66,67,68]. For this experiment, 10 flies randomly selected from control and study groups were transferred into separate vials, and before assessing the parameters of climbing behavior, they were kept there for 15 min at room temperature to allow acclimatization. The flies were moved to the bottom of the vial by a gentle tap on the vial, and the number of flies that managed to climb above the 7 cm mark in 10 s was recorded to assess their climbing ability. For each group, we repeated this climbing assay ten times after exposure to different doses of test materials.
Genotoxicity detection with Drosophila wing spot test and Comet assay
The wing-spot assay (somatic mutations and recombination test, SMART)
SMART assays, which yield results in a short period of time at significantly low costs, rapidly identify allelic imbalance and chromosome instability in larval somatic cells [69]. They have been used by many studies into genotoxicity and antigenotoxicity of environmental contaminants like plastics and nanomaterials [58, 70, 71]. In SMART assay, we employed two distinct strains of fruit flies: flare-3 (flr3/In (3LR) TM3, Bds) and multiple wing hairs (mwh/mwh). More detailed information on these strains was given in early research [70]. Prior to this research protocol, we first crossbred virgin female flare-3 and male mwh flies to obtain trans-heterozygous flies for two recessive mutations, and then we cultured such trans-heterozygous eggs in test vials that were added food media (containing agar, sugar, corn in powder form) for 8 h. Once the eggs had turned into 72 ± 4 h-old larvae, we placed them into plastic vials for exposure to the study compounds. Before this exposure, we carried out a viability test in the larvae, and the final test doses for WO3 NPs and MPs were determined as follows: 1, 2.5, 5, and 10 mM. At all tested doses, the viability rate was calculated to be above 70%. Exposure vials contained 4 g instant Drosophila food media including 10 mL of WO3 NPs and its MPs and also negative (sterile distilled water) as well as positive control solutions (EMS, 1 mM). Drosophila larvae (72 ± 4 h-old) were exposed to WO3 NPs and MPs via ingestion throughout the larval-pupal transition. In line with the standards elaborated in great length in earlier research, the experiments were carried out under normal conditions (25 ± 1 °C, humidity at around 65%). Once the pupation was completed, adult flies were immersed in ethanol (70%) at 4 °C until they were ready for preparation of wing slides. Next, their wings were dissected from the body and fixed on the microscopy slides to allow detection of any possible clone formations through a light microscope (400 ×). Presence of mwh single spots is considered to indicate recombination and substitution of certain genes, or deletion of wild-type segments, whereas presence of flr3 single spots could indicate deletion of wild-type alleles [69]. On the other hand, both mwh and flr3 twin spots observed on the fly’s wing blades usually result from gene recombination during mitosis [69]. In our study, we examined a total of 40 flies at each series by following step-by-step commonly adopted procedures for the wing-spot assay and scoring described by previous reports [6, 57, 58].
Comet assay
Third instar (72 ± 4 h-old) Drosophila larvae belonging to the Oregon R+ strain were exposed to WO3 NPs, its MPs, as well as positive control substance (4 mM EMS) and negative control (sterile distilled water). The exposure to such compounds was achieved as follows: instant Drosophila food media (4 g) was mixed with 10 mL of varying doses of study compounds (0.1, 1, 2.5, and 5 mM). The larvae were exposed (through ingestion) to the mixture for 1 day (24 ± 2 h). Then, hemocyte cells were collected following the steps in the research protocol proposed by Irving et al. [72]. Comet assays were carried out on isolated hemocytes by following the procedure specified by Singh et al. [73] along with some minor changes. Trypan blue exclusion assay was used to assess the viability of the hemocytes [74]. As well as low melting point agarose (75% LMA, 120 µL), slides coated with normal melting point (1% NMP) agarose were employed to spread the hemocytes (20 µL). Then, coverslips were placed on the slides to allow keeping them on ice (for 5 min) and to embed the isolated hemocytes into the slides. After the removal of these coverslips, we applied a second LMA (80 µL) spreading and kept them on ice for 5 min once again [73, 75]. In a dark chamber, the slides were saturated in lysing solution (10 mM Trizma base, 100 mM EDTA, 2.5 M NaCl, 1% Triton X-100, and 1% N lauroyl sarcosinate pH 10) at 4 °C for 2 h. We did not use dimethyl sulfoxide during the preparation of the lysing solution, as it could give rise to some extra damage in the fly tissues [76, 77]. Besides, all such procedures were carried out under dim light conditions to avoid any potential DNA breakage owing to strong light. The slides were transferred into a running buffer for electrophoresis (1 mM EDTA and 300 mM NaOH, pH ~ 12.8) and kept there for as long as 20 min (300 mA and 1 V/cm). Tris buffer solution (pH 7.5) was used to wash the slides, which was repeated 3 times for 5 min. After that, they were fixed with cold 70% ethanol for 5 min and dried prior to EtBr (20 mg/ml) staining. To acquire images with fluorescence microscopy (filter 515–560 nm), the slides were stained for 20 min, after which Comet assays were performed on CaspLab (version 1.2.3b2) [78]. Such experiment protocols are realized by strictly following procedures described in detail by earlier research [79]. For each exposure series (300 cells in total), 100 cells randomly chosen in triplicate were analyzed. Damage in DNA was measured as the percentage of DNA in the tail (% DNA tail), so mean and standard error values were calculated accordingly.
Statistical analysis
The conditional binomial test, tabulated in detail by Kastenbaum and Bowman [80], was used to assess the differences in the frequency of each type of wing spot in exposed flies and negative controls, and α = β = 0.05 was considered statistically significant. Mann–Whitney-Wilcoxon nonparametric U-test [81] and the multiple decision method [82] were employed to decide whether there were positive, weakly positive, negative, or inconclusive responses to the test materials (P ≤ 0.05). While Kolmogorov–Smirnov and Shapiro–Wilk test assessed the normality of variance, Levene’s test analyzed the homogeneity of variance. Data displaying normal distribution and equal variance were analyzed by the Student’s t-test (Comet assay and ROS production) and one-way ANOVA (climbing assay) on SigmaPlot 11.0 (SPSS). Non-parametric Mann–Whitney U-test analyzed data exhibiting unequal variance or skewed distribution (toxicity/viability). Unless specified otherwise, all research data were presented as means of two independent experiments, as well as duplicates of each test, and the figures represent arithmetic means ± standard error values.
Results
Physicochemical characterization of WO3 NPs
The physicochemical characterization of WO3 NPs (mean diameter 30 nm) was achieved by means of TEM, ESEM, EDX, LDV, and DLS, whose results are presented in Figs. 1, 2. Figure 1A shows the examples of ESEM images, while Figs. 1C, 2B present relevant TEM images. Spectroscopy images obtained with EDX are shown in Fig. 1B. Details of WO3 characterization are presented in Figs. 1, 2. The mean diameter of WO3 NPs was 43.71 ± 1.59 nm, measured through respective TEM images. The histogram graph in Fig. 2A illustrates particle size distribution. Average size of WO3 was 43.71 ± 1.59 nm, zeta potential − 22 ± 5.26 mV (Fig. 2C), and the mean diameter calculated by DLS was 756.8 ± 161.9 nm (Fig. 2D). This zeta potential value indicates the good dispersion of WO3 NPs. The Polydispersity index (PDI) determined by DLS was 0.483 for WO3 NPs. PDI gives information about the size distribution in a sample or agglomeration, corresponding to the measurement of the heterogeneity of a chemical based upon size [83]. In order to avoid any potential aggregation, all experimental applications were carried out with freshly prepared NPs solutions. On the other hand, energy-dispersive X-ray (EDX) spectroscopy via ESEM was performed to assess the chemical composition of WO3 and showed that oxygen (O) peak was 84.49% and tungsten (W) peak of 15.51% (atomic % by element) (Fig. 1B).
Toxicity of WO3 NPs and its MPs
Toxic capacity of study compounds was measured with viability assays by calculating the egg-to-adult survival rate. The results of these assays showed no significantly impaired survival at doses of 1, 2.5, 5, and 10 mM. On the other hand, Drosophila eggs exposed to our negative control substance (sterile distilled water) exhibited perfect viability at 100%. Following exposure to 1, 2.5, 5, and 10 mM (greatest dose) of WO3 NPs, the eggs showed the following viability rates: 96, 95, 96, and 94%, respectively, while exposure to the MPs resulted in slightly higher rates at 98, 99, 97, and 96% (Fig. 8). Drosophila larvae were also exposed to non-toxic doses (0.1, 1, 2.5, and 5 mM) of WO3 NPs and its MPs to conduct ROS assays, genotoxicity studies (SMART and Comet assay), climbing assays, and to assess alterations in phenotypes.
Oxidative stress in hemocytes after exposure to WO3 NPs and its MPs
Closely associated with the accumulation of ROS in cells, oxidative stress can be accepted as an indicator of hazardous consequences of exposure to WO3 NPs and MPs, as it induces the generation of ROS in several cells within the organism. Therefore, we explored ROS levels in the 3rd instar larvae hemocytes upon exposure to WO3 NPs and its MPs by means of fluorescent dye, 6-carboxy 2,7′-dichlorodihydro-fluorescein diacetate (DCFH-DA) under fluorescent microscopy. Exposure to WO3 NPs at doses of 1, 2.5, 5, and 10 mM brought about statistically significant increases in ROS accumulation (P ≤ 0.001), with a direct concentration–response relationship (Fig. 3A). This is especially observed for WO3 NPs (Fig. 3B). ROS generation at the highest dose (10 mM) was considerably greater following exposure to MPs (103%) as compared to WO3 NPs (153.1%), suggesting that WO3 NPs caused greater ROS induction than WO3 MPs (Fig. 3A). WO3 NPs exposure was found to induce some ROS build-up in hemocytes. However, WO3 MPs induced no ROS formation (Fig. 3A).
Intracellular ROS generation in hemocytes of unexposed 3rd instar larvae (distilled water only) and of those exposed to varying doses of WO3 NPs and MPs (A). Hemocytes were incubated in 5 μM DCFH-DA for 30 min at 24 °C and then observed under fluorescence microscopy. ImageJ analysis quantified the fluorescence intensity of hemocytes in larvae exposed to WO3 NPs and MPs (B). Positive control substance was 0.5 mM H2O2. ***P ≤ 0.001 as compared to the negative control by Student’s t-test
Phenotypic alterations
Experiments with non-toxic doses (1, 2.5, 5, and 10 mM) of WO3 NPs and its MPs were carried out in 3rd instar larvae of Drosophila to probe whether any alterations specific to fly phenotypes would be observed in the wings, head, thorax, abdomen, or legs of the flies. All doses of WO3 NPs caused such abnormalities in the mouth and wing parts (Figs. 4B, E and Figs. 5B, E). The mouth of the flies was enlarged, and the wings were defective in shape following exposure to WO3 NPs (1, 2.5, 5, and 10 mM). On the other hand, no such abnormalities were detected after exposure to WO3 MPs at doses of 1, 2.5, 5, and 10 mM.
Phenotypic alterations in D. melanogaster after exposure to WO3 NPs and MPs. Normal mouth phenotype after exposure to WO3 MPs at all doses (A) and abnormal mouth phenotypes after exposure to WO3 NPs at doses 1 mM (B), 2.5 mM (C), 5 mM (D), and 10 mM (E). The dark-colored circles in the image show the defective areas
Phenotypic alterations in D. melanogaster after exposure to WO3 NPs and MPs. Normal wing phenotype after exposure to WO3 MPs at all doses (A) and abnormal wing phenotypes after exposure to WO3 NPs at doses 1 mM (B), 2.5 mM (C), 5 mM (D), and 10 mM (E). The dark-colored circles in the image show the defective areas
Climbing behavior
Climbing test, conducted to quantify a Drosophila fly’s ability to climb upwards, is a useful assay to assess whether locomotor behavior is affected. Our climbing tests, after chronic exposure to the WO3 NPs and its MPs (1, 2.5, 5, and 10 mM) for 7 days, detected significant differences between the climbing behavior of the study and control groups. All three doses of WO3 NPs (1, 2.5, 5, and 10 mM) caused impairment in climbing performances (86 ± 2.3, 80 ± 3.1, 62 ± 2.6, and 57 ± 3.3%, respectively) as compared to the control group, while exposure to WO3 MPs at doses of 1, 2.5, 5, and 10 mM produced milder effects (91 ± 2.6, 86 ± 3.4, 82 ± 3.7, and 77 ± 3.2%, respectively) (Fig. 6). Two highest doses of WO3 NPs (5 and 10 mM) were found to impair locomotor activity at statistically significant levels.
Genotoxicity tests (Drosophila wing spot test and Comet assay)
Drosophila SMART assay
We undertook a series of Drosophila SMART assays to determine WO3 mutagenicity and recombinegenic sequences, whose results are presented in Tables 1, 2. In Table 1, we present data relating to trans-heterozygous 3rd instar (3-day-old) larvae exposed to varying doses of WO3 NPs (1 to 10 mM) until they completed larval development. In such test conditions, single mutant spots on the wings are triggered by both somatic mutations and somatic recombination events, whereas twin spots on wing blades occur only because of somatic mutations. WO3 NPs were found to cause a significant rise in the frequency of small single mwh spots, large single spots, and in the total mutant spots in a dose-dependent manner. Genotoxic potential of an agent does not singly determine the presence of small or large wing spots, rather the exposure time needed to allow the compound to reach the target cells appears to be the key factor, so we can assume that WO3 NPs caused genetic damage during the final stages of larval development.
We carried out one experiment on balanced heterozygous Drosophila larvae, a genotype suppressing gene recombination and allowing observation of clones induced by somatic mutation only, in an attempt to gain insight into possible mechanisms through which WO3 NPs could induce mutant wing spots, however, no significant single, large, or total mutant clones were observed; the findings of this experiment are summarized in Table 1. We can therefore suggest that WO3 NPs caused genotoxicity mainly through somatic recombination. We should also note that exposure doses were found to increase incidence of mutant clones depending on doses, yet this was not statistically significant.
Finally, we assessed the genotoxic potential of WO3 MPs, and doses varying between 1 and 10 mM caused no significant genotoxic effects (Table 2); however, high doses of WO3 MPs could have greater genotoxicity than do WO3 NPs; Fig. 7 presents a comparison of WO3 NPs and MPs. Examination of results obtained in negative and positive groups reveals that our findings are mostly in parallel with those reported in our earlier papers [6, 58, 59, 84, 85].
Comet assay
Assessment of genotoxicity is vital in any risk assessment of physical or chemical compounds. Comet assay is a useful and practical technique to detect DNA strand breaks. Our Comet assays revealed that all doses of WO3 NPs significantly elevated % DNA in the tail (1, 2.5, 5, and 10 mM), in a dose-dependent way, as compared to the controls exposed only to sterile distilled water (Fig. 8A). This is especially observed for WO3 NPs (Fig. 8B). The greatest dose of WO3 NPs (10 mM) resulted in more severe DNA damage in the hemocytes, by 8.67%, as compared with control. No DNA damage occurred in the group exposed to WO3 MPs (Fig. 8A). Our findings seem to confirm that the amount of WO3 NPs in the concentration is a primary determinant of genotoxic effects.
Comet assay shows genotoxic effects of WO3 NPs and MPs and viability of Drosophila hemocytes (A). After 24-h exposure to WO3 NPs and MPs, the % of DNA tail in larvae (three replicates were performed and 100 randomly selected cells were examined for each exposure dose). Numerical values represent the mean ± standard error (SE) of the mean. Positive control was EMS (4 mM). ***P ≤ 0.001 as compared to the negative control by Student’s t-test. Representative comet images are shown (B)
Discussion
The field of nanotechnology has been expanding at an accelerating rate, bringing new synthesized NPs into our lives with everyday products. Over the recent years, WO3 NPs have garnered significant attention in various fields owing to their superior properties like thermal stability and conductivity [10]. However, the potential hazards of WO3 NPs to animal and plant life have not been explored in detail. The endpoint biomarkers that we used in this study not only revealed the toxic and genotoxic potential of WO3 NPs and its MPs for the first time but also reinforced, once again, the practicality of D. melanogaster as an in vivo model organism. The current study was conducted to obtain the first report of the toxicity, genotoxicity, ROS induction, locomotor behavior, and phenotypic alterations caused by WO3 NPs and MPs upon exposure via oral route (1, 2.5, 5, and 10 mM) in D. melanogaster. Accordingly, WO3 NPs were found to cause genotoxic effects at all tested doses without any toxicity/mortality in the Comet assay. Our SMART showed induction of mutant clone formations at the two highest doses (5 and 10 mM), which could be associated with somatic mutations. In contrast, WO3 MPs caused no genotoxicity in any doses, as revealed by SMART and Comet assays, and similar results were obtained from tests for ROS level determination and phenotypic alterations. Mouth and wing defects were detected in flies exposed to WO3 NPs (1, 2.5, 5, and 10 mM), while intracellular ROS production elevation was detected in only hemocytes of WO3-exposed flies. We assume that the entrance of NPs into the cells was much easier than microparticles due to their smaller size, wide surface area, and crystal form. In this context, the nanoparticle form of WO3 could be considered a toxic substance in cases of high doses of acute exposure, which appears to be in accordance with previous in vivo studies conducted with mammals [8]. In addition, biobehavior of NPs may be influenced by a wide range of physicochemical properties, which in turn increase their toxic/genotoxic effects as compared to their MPs, especially if exposed via oral route [8, 86,87,88,89].
Genotoxicity (DNA damage) not only causes carcinogenesis but also affects the fertility and health of offspring if a compound penetrates the reproductive cells. Genotoxicological research has been focusing on revealing the genetic damage potentials of different test compounds and risk assessment of potential carcinogens. Because of their small size, NPs can easily pass into the cells and affect the biomolecules including DNA [90, 91]. Both in vivo and in vitro studies have reported that various classes of NPs showed genotoxic effects [5, 36, 57, 89, 92,93,94,95].
We assessed the amount of damage in DNA on Drosophila hemocytes caused by NPs through genotoxicity assays, including Comet assays to detect single and double-strand breaks in DNA, often used in a number of cell targets in this species, for example, hemocytes responsible for immune response [96, 97]. Hemocyctes in fruit flies have functions comparable to those of lymphocytes found in the bloodstream of mammals [72]. Therefore, they could suffer direct exposure to certain compounds and agents running through the hemolymph system, hence a valuable focus of research on the toxic and genotoxic capacity of various nanomaterials [57, 60]. Comet assays have been widely employed in ecotoxicology research efforts involving the testing of substances on various organisms like fruit flies [89]. In this study, Comet assay results revealed that WO3 NPs induced % tail DNA significantly in D. melanogaster hemocytes at all tested doses as compared to the distilled water. Similarly, limited number of genotoxicity studies looking into WO3 NPs with Comet assays have reported DNA damage induction after in vivo testing [8, 20]. The current literature contains rather limited research into in vivo toxicity of WO3 NPs [24, 26]. Chinde and Grover [8] reported that WO3 NPs induced DNA damage in white blood cells and liver cells of Wistar rats upon chronic oral exposure for 28 days at higher doses (1000 mg/kg). Besides, Chinde et al. [20] also showed that oral single administration of WO3 NP (1000 mg/kg) caused DNA damage in white blood cells (leukocytes) and liver cells of Wistar rats. Another in vivo study on the toxic potential of WO3 NPs explored the impacts of exposure via inhalation and its results showed that WO3 NPs were toxic to the cells, caused morphological changes, and lung injury in tested rodents [24]. Most recently, research investigating in vivo effects upon intraperitoneal injection of WO3 nanorods has reported that such exposure induced hepatotoxicity while melatonin administration diminished this toxicity in rats [26]. Researchers note that smaller WO3 nanorods (125–200 nm) caused more severe toxic effects and oxidative stress than did micro-size particles (0.8–2 μm) [26].
Most of the research into WO3 NPs toxicity was carried out as in vitro using different kinds of cell types such as Caco-2 and A549 [18,19,20,21]. Data from in vitro tests should be compared and reinforced with in vivo results to understand true effects within a living organism by using reliable in vivo model organisms in experiments [98]. As Drosophila has shown its potential as a reliable testing model in toxicity/genotoxicity assessment of several nanomaterials, we chose to use this organism in our study into WO3 NPs and MPs.
Furthermore, intracellular ROS levels in hemocytes were accepted as an oxidative stress parameter in this current study. Although the genotoxic mechanism has yet to be fully illuminated, the most prominent source of NP-induced toxicity is thought to be oxidative stress caused by ROS production. Elevated levels of ROS could lead to DNA and cell membrane damage, disrupt the protein-lipid balance, and impair enzyme and hormone mechanisms [99]. A recent in vivo study by Mishra and Panda [39] proposed that ROS accumulation could be associated with the toxic impacts of nanomaterials in D. melanogaster. Ever since the first research to address the toxicity of NPs in Drosophila [100], substantial research efforts have been geared towards characterizing a through toxic profile of many nanomaterials [66, 101]. The toxic effects of certain compounds mediated by cytotoxicity, cellular internalization, and pigmentation may cause alterations in the genetic composition of fruit flies. Despite the knowledge gaps existing in the mechanism by which genotoxicity occurs, oxidative stress triggered by ROS accumulation is often considered as the culprit behind the nanoparticle toxicity [101, 102]. Increased ROS levels are known to trigger inflammation response, as well as protein damage and DNA strand breaks [103, 104]. Our findings suggest that the injurious effects of WO3 NPs responsible for modifications in normal functions of DNA could be attributed to ROS production in cells. However, WO3 MPs did not cause significant elevation in ROS levels. Similar results obtained upon exposure to WO3 NPs and MPs in mice hint that smaller WO3 particles could induce greater hepatoxicity [26]. Different properties of NPs such as particle size, shape, and surface area may determine their toxicity and genotoxicity.
Conclusions
The findings of this study confirm that both nanoparticles and MPs of WO3, if exposed to high doses can cause some hazardous effects on D. melanogaster, underscoring the importance of a thorough risk assessment of WO3 NPs for the first time. This is the first study to use Drosophila in testing in vivo effects including parameters like toxicity, genotoxicity, phenotypic alterations, locomotor behavior impairments, and ROS induction of exposure to WO3 NPs (43.71 ± 1.59 nm) and MPs of WO3 at varying doses. Non-toxic doses of WO3 NPs caused significant % tail DNA damage, elevated ROS production in hemocytes, phenotypic alterations in wing shapes, and mouth parts as well as impaired locomotor behavior in adult flies. Most importantly, evidence from our assays establishes that a considerable portion of toxic effects is linked to somatic mutations (5 and 10 mM doses of WO3 NPs), which may alter key functions in cells and play a role in carcinogenesis. In this context, the use of Drosophila could be further extended to research into cancer and nanoparticles. Our model organism, Drosophila, has shown its potential to reliably detect and quantify potential biological and genetic effects of WO3 NPs and its MPs. Future studies should focus on the underlying mechanisms of various endpoint biomarkers of such widely adopted substances.
References
Hasan S (2015) A review on nanoparticles: their synthesis and types biosynthesis: mechanism. Res J Recent Sci 4:9–11
Bradfield SJ, Kumar P, White JC, Ebbs SD (2017) Zinc, copper, or cerium accumulation from metal oxide nanoparticles or ions in sweet potato: yield effects and projected dietary intake from consumption. Plant Physiol Biochem 110:128–137
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150:5–22
McShan D, Ray PC, Yu H (2014) Molecular toxicity mechanism of nanosilver. J Food Drug Anal 22:116–127
Alaraby M, Demir E, Domenech J, Velázquez A, Hernández A, Marcos R (2020) In vivo evaluation of the toxic and genotoxic effects of exposure to cobalt nanoparticles using Drosophila melanogaster. Environ Sci Nano 7:610–622
Demir E (2021) Adverse biological effects of ingested polystyrene microplastics using Drosophila melanogaster as a model in vivo organism. J Toxicol Environ Health Part A 84:649–660
Ong C, Yung LYL, Cai Y, Bay BH, Baeg GH (2015) Drosophila melanogaster as a model organism to study nanotoxicity. Nanotoxicology 9:396–403
Chinde S, Grover P (2017) Toxicological assessment of nano and micron-sized tungsten oxide after 28 days repeated oral administration to Wistar rats. Mutat Res Genet Toxicol Environ Mutagen 819:1–13
Erik L, Wolf-Dieter S (1999) Tungsten: properties, chemistry, technology of the element, alloys, and chemical compounds. Kluwer Academic, USA
Zhou G, Hou Y, Liu L, Liu H, Liu C, Liu J, Qiao H, Liu W, Fan Y, Shen S, Rong L (2012) Preparation and characterization of NiW-nHA composite catalyst for hydrocracking. Nanoscale 4:7698–7703
Cong S, Geng F, Zhao Z (2016) Tungsten oxide materials for optoelectronic applications. Adv Mater 28:10518–10528
Granqvist CG (2014) Electrochromics for smart windows: oxide based thin films and devices. Thin Solid Films 564:1–38
Hu L, Hu P, Chen Y, Lin Z, Qiu C (2018) Synthesis and gas-sensing property of highly self-assembled tungsten oxide nanosheets. Front Chem 6:452
Chen X, Zhou Y, Liu Q, Li Z, Liu J, Zou Z (2012) Ultrathin, single-crystal wo3 nanosheets by two-dimensional oriented attachment toward enhanced photocatalystic reduction of CO2 into hydrocarbon fuels under visible light. ACS Appl Mater Interfaces 4:3372–3377
Wang P, Huang B, Qin X, Zhang X, Dai Y, Whangbo MH (2009) Ag/AgBr/WO3·H2O: visible-light photocatalyst for bacteria destruction. Inorg Chem 48:10697–10702
Ahmed S, Hassan IAI, Roy H, Marken F (2013) Photoelectrochemical transients for chlorine/hypochlorite formation at “Roll-On” nano-WO3 film electrodes. J Phys Chem C 117:7005–7012
Hasegawa G, Shimonaka M, Ishihara Y (2012) Differential genotoxicity of chemical properties and particle size of rare metal and metal oxide nanoparticles. J Appl Toxicol 32:72–80
Turkez H, Sonmez E, Turkez O, Mokhtar YI, Stefano AD, Turgut G (2014) The risk evaluation of tungsten oxide nanoparticles in cultured rat liver cells for its safe applications in nanotechnology. Braz Arch Biol Technol 57:532–541
Ivask A, Titma T, Visnapuu M, Vija H, Kakinen A, Sihtmae M, Kahru A (2015) Toxicity of 11 metal oxide nanoparticles to three mammalian cell types in vitro. Curr Top Med Chem 5:1914–1929
Chinde S, Dumala N, Rahman MF, Kamal SSK, Kumari SI, Mahboob M, Grover P (2017) Toxicological assessment of tungsten oxide nanoparticles in rats after acute oral exposure. Environ Sci Pollut Res 24:13576–13593
Hassanvand A, Zare MH, Shams A, Nickfarjam A, Shabani M, Rahavi H (2019) Investigation of the effect of radiosensitization of tungsten oxide nanoparticles on AGS cell line of human stomach cancer in megavoltage photons radiation. J Nanostructures 9:563–578
Akbaba BG, Turkez H, Sonmez E, Akbaba U, Aydın E, Tatar A, Turgut G, Cerig S (2016) In vitro genotoxicity evaluation of tungsten (VI) oxide nanopowder using human lymphocytes. Biomed Res 27:229–234
Turkez H, Cakmak B, Celik K (2013) Evaluation of the potential in vivo genotoxicity of tungsten (VI) oxide nanopowder for human health. Key Eng Mater 543:89–92
Prajapati MV, Adebolu OO, Morrow BM, Cerreta JM (2017) Evaluation of pulmonary response to inhaled tungsten (iv) oxide nanoparticles in golden syrian hamsters. Exper Biol Med 242:29–44
Areecheewakul S, Adamcakova-Dodd A, Givens BE, Steines BR, Wang Y, Meyerholz DK, Parizek NJ, Altmaier R, Haque E, O’Shausghnessy PT, Salem AK, Thorne PS (2020) Toxicity assessment of metal oxide nanomaterials using in vitro screening and murine acute inhalation studies. NanoImpact 18:100214
Mao L, Zheng L, You H, Ullah MW, Cheng H, Guo Q, Li R (2021) A comparison of hepatotoxicity induced by different lengths of tungsten trioxide nanorods and the protective effects of melatonin in BALB/c mice. Environ Sci Pollut Res 28:40793–40807
Contreras EQ, Cho M, Zhu H, Puppala HL, Escalera G, Zhong W, Colvin VL (2012) Toxicity of quantum dots and cadmium salt to Caenorhabditis elegans after multigenerational exposure. Environ Sci Technol 47:1148–1154
Hunt PR, Marquis BJ, Tyner KM, Conklin S, Olejnik N, Nelson BC, Sprando RL (2013) Nanosilver suppresses growth and induces oxidative damage to DNA in Caenorhabditis elegans. J Appl Toxicol 33:1131–1142
Chatterjee N, Eom HJ, Choi J (2014) Effects of silver nanoparticles on oxidative DNA damage-repair as a function of p38 MAPK status: a comparative approach using human Jurkat T cells and the nematode Caenorhabditis elegans. Environ Mol Mutagen 55:122–133
Chatterjee N, Yang J, Kim HM (2014) Potential toxicity of differential functionalized multiwalled carbon nanotubes (MWCNT) in human cell line (BEAS2B) and Caenorhabditis elegans. J Toxicol Environ Health Part A 77:1399–1408
Pappus SA, Mishra M (2018) A Drosophila model to decipher the toxicity of nanoparticles taken through oral routes. Adv Exp Med Biol 1048:311–322
Gao M, Zhang Z, Lv M, Song W, Lv Y (2018) Toxic effects of nanomaterial-adsorbed cadmium on Daphnia magna. Ecotoxicol Environ Saf 148:261–268
Shariati F, Poordeljoo T, Zanjanchi P (2020) The acute toxicity of SiO2 and Fe3O4 nano-particles on Daphnia magna. SILICON 12:2941–2946
Pappus SA, Ekka B, Sahu S, Sabat D, Dash P, Mishra M (2017) A toxicity assessment of hydroxyapatite nanoparticles on development and behaviour of Drosophila melanogaster. J Nanoparticle Res 19(4):136
Anand AS, Gahlot U, Prasad DN, Amitabh Kohli E (2019) Aluminum oxide nanoparticles mediated toxicity, loss of appendages in progeny of Drosophila melanogaster on chronic exposure. Nanotoxicology 13:977–989
Demir E (2020) An in vivo study of nanorod, nanosphere, and nanowire forms of titanium dioxide using Drosophila melanogaster: toxicity, cellular uptake, oxidative stress, and DNA damage. J Toxicol Environ Health Part A 83:456–469
Mendoza RP, Brown JM (2019) Engineered nanomaterials and oxidative stress: current understanding and future challenges. Curr Opin Toxicol 13:74–80
Dan P, Sundararajan V, Ganeshkumar H, Gnanabarathi B, Subramanian AK, Venkatasubu GD, Ichihara SS (2019) Evaluation of hydroxyapatite nanoparticles-induced in vivo toxicity in Drosophila melanogaster. Appl Surf Sci 484:568–577
Mishra M, Panda M (2021) Reactive oxygen species: The root cause of nanoparticle-induced toxicity in Drosophila melanogaster. Free Radic Res 55:919–935
Reiter LT, Potocki L, Chien S, Gribskov M, Bier E (2001) A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res 88:1114–1125
Demir E, Turna Demir F, Marcos R (2022) Drosophila as a suitable in vivo model in the safety assessment of nanomaterials. Adv Exp Med Biol 1357:275–301
Wolf MJ, Amrein H, Izatt JA, Choma MA, Reedy MC, Rockman HA (2006) From the cover: Drosophila as a model for the identification of genes causing adult human heart disease. Proc Natl Acad Sci 103:1394–1399
Bier E (2005) Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet 6:9–23
Gonzalez C (2013) Drosophila melanogaster: a model and a tool to investigate malignancy and identify new therapeutics. Nat Rev Cancer 13:172–183
Latouche M, Lasbleiz C, Martin E, Monnier V, Debeir T, Mouatt-Prigent A, Muriel MP, Morel L, Ruberg M, Brice A, Stevanin G, Tricoire H (2007) A conditional pan neuronal drosophila model of spinocerebellar ataxia 7 with a reversible adult phenotype suitable for identifying modifier genes. J Neurosci Res 27:2483–2492
Bilen J, Bonini NM (2005) Drosophila as a model for human neurodegenerative disease. Annu Rev Genet 39:153–171
Moloney A, Sattelle DB, Lomas DA, Crowther DC (2010) Alzheimer’s disease: insightsFrom Drosophila melanogaster models. Trends Biochem Sci 35:228–235
Ng CT, Ong CN, Yu LE, Bay BH, Baeg GH (2019) Toxicity study of zinc oxide nanoparticles in cell culture and in Drosophila melanogaster. J Vis Exp 151:e59510
Flecknell P (2002) Replacement, reduction and refinement. Altex 19:73–78
Jennings BH (2011) Drosophila-a versatile model in biology & medicine. Mater Today 14:190–195
Rand MD (2010) Drosophotoxicology: the growing potential for Drosophila in neurotoxicology. Neurotoxicol Teratol 32:74–83
Rand MD, Vorojeikina D, Peppriell A, Gunderson J, Prince LM (2019) Drosophotoxicology: elucidating kinetic and dynamic pathways of methylmercury toxicity in a Drosophila model. Front Genet 10:666
Chifiriuc MC, Ratiu AC, Popa M, Ecovoiu AA (2016) Drosophotoxicology: an emerging research area for assessing nanoparticles interaction with living organisms. Int J Mol Sci 17:36
Benford DJ, Hanley AB, Bottrill K, Oehlschlager S, Balls M, Branca F, Castegnaro JJ, Descotes J, Hemminiki K, Lindsay D, Schilter B (2000) Biomarkers as predictive tools in toxicity testing. ATLA 28:119–131
Rajak P, Dutta M, Roy S (2015) Altered differential hemocyte count in 3rd instar larvae of Drosophila melanogaster as a response to chronic exposure of acephate. Interdiscip Toxicol 8:84–88
Festing MFW, Baumans V, Combes DR, Hadler M, Hendriksen FM, Howard BR, Lovell DP, Moore GJ, Overend P, Wilson MS (1998) Reducing the use of laboratory animals in biomedical research: problems and possible solutions. Altern Lab Anim 26:283–301
Demir E, Marcos R (2018) Antigenotoxic potential of boron nitride nanotubes. Nanotoxicology 12:868–884
Demir E (2022) Mechanisms and biological impacts of graphene and multi-walled carbon nanotubes on Drosophila melanogaster: oxidative stress, genotoxic damage, phenotypic variations, locomotor behavior, parasitoid resistance, and cellular immune response. J Appl Toxicol 42:450–474
Demir E, Turna F, Aksakal S, Kaya B, Marcos R (2014) Genotoxicity of different sweeteners in Drosophila. Fresenius Environ Bull 23:3426–3432
Demir E, Marcos R (2017) Assessing the genotoxic effects of two lipid peroxidation products (4-oxo-2-nonenal and 4-hydroxy-hexenal) in haemocytes and midgut cells of Drosophila melanogaster larvae. Food Chem Toxicol 105:1–7
Nanogenotox (2011) http://www.nanogenotox.eu/files/PDF/Deliverables/nanogenotox%20deliverable%203_wp4_%20dispersion%20protocol.pdf
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Pendleton RG, Parvez F, Sayed M, Hillman R (2002) Effects of pharmacological agents upon a transgenic model of Parkinson’s disease in Drosophila melanogaster. J Pharmacol Exp Ther 300:91–96
Martinez VG, Javadi CS, Ngo E, Ngo L, Lagow RD, Zhang B (2007) Age-related changes in climbing behavior and neural circuit physiology in Drosophila. Dev Neurobiol 67:778–791
Anand AS, Prasad DN, Singh SB, Kohli E (2017) Chronic exposure of zinc oxide nanoparticles causes deviant phenotype in Drosophila melanogaster. J Hazard Mater 327:180–186
Priyadarsini S, Sahoo SK, Sahu S, Mukherjee S, Hota G, Mishra M (2019) Oral administration of graphene oxide nano-sheets induces oxidative stress, genotoxicity, and behavioral teratogenicity in Drosophila melanogaster. Environ Sci Pollut Res 26:19560–19574
Mishra M, Sabat D, Ekka B, Sahu S, Unnikannan P, Dash P (2017) Oral intake of zirconia nanoparticle alters neuronal development and behaviour of Drosophila melanogaster. J Nanoparticle Res 19:282
Sood K, Kaur J, Singh H, Arya SK, Khatri M (2019) Comparative toxicity evaluation of graphene oxide (GO) and zinc oxide (ZnO) nanoparticles on Drosophila melanogaster. Toxicol Rep 6:768–781
Graf U, Würgler FE, Katz AJ, Frei H, Juan H, Hall CB, Kale PG (1984) Somatic mutation and recombination test in Drosophila melanogaster. Environ Mol Mutagen 6:153–188
Lindsley DL, Zimm GG (1992) The genome of Drosophila melanogaster. Academic Press, San Diego, CA
Turna F, Aksakal S, Demir E, Kaya B (2014) Antigenotoxic effects of Resveratrol in somatic cells of Drosophila melanogaster. Fresenius Environ Bull 23:2116–2125
Irving P, Ubeda JM, Doucet D, Troxler L, Lagueux M, Zachary D, Hoffmann JA, Hetru C, Meister M (2005) New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell Microbiol 7:335–350
Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Ghosh M, Manivannan J, Sinha S, Chakraborty A, Mallick SK, Bandyopadhyay M, Mukherjee A (2012) in vitro and in vivo genotoxicity of silver nanoparticles. Mutat Res 749:60–69
Tice RR, Andrews PW, Singh N (1990) The single cell gel assay. A sensitive technique for evaluating intercellular differences in DNA damage and repair. B.M. Sutherland, A.D. Wordhead (Eds.), DNA damage and repair in human tissues, Plenum, New York, NY (1990), pp. 291–302
Mukhopadhyay I, Chowdhuri DK, Bajpayee M, Dhawan A (2004) Evaluation of in vivo genotoxicity of cypermethrin in Drosophila melanogaster using the alkaline comet assay. Mutagenesis 19:85–90
Siddique HR, Chowdhuri DK, Saxena DK, Dhawan A (2005) Validation of Drosophila melanogaster as an in vivo model for genotoxicity assessment using modified alkaline comet assay. Mutagenesis 20:285–290
Końca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Góźdź S, Koza Z, Wojcik A (2003) A cross-platform public domain pc image-analysis program for the comet assay. Mutat Res-Genet Toxicol Environ Mutagen 534:15–20
Turna Demir F, Yavuz M (2020) Heavy metal accumulation and genotoxic effects in levant vole (Microtus guentheri) collected from contaminated areas due to mining activities. Environ Pollut 256:113378
Kastenbaum MA, Bowman KO (1970) Tables for determining the statistical significance of mutation frequencies. Mutat Res 9:527–549
Frei H, Würgler FE (1995) Optimal experimental design and sample size for the statistical evaluation of data from somatic mutation and recombination tests (SMART) in Drosophila. Mutat Res 334:247–225
Frei H, Würgler FE (1988) Statistical methods to decide whether mutagenicity test data from Drosophila assays indicate a positive, negative, or inconclusive results. Mutat Res 203:297–308
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S (2018) Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10:57
Demir E, Vales G, Kaya B, Creus A, Marcos R (2011) Genotoxic analysis of silver nanoparticles in Drosophila. Nanotoxicology 5:417–424
Turna Demir F (2022) In vivo effects of 1,4-dioxane on genotoxic parameters and behavioral alterations in Drosophila melanogaster. J Toxicol Environ Health Part A 85:414–430
Karlsson HL, Gustafsson J, Cronholm P, Möller L (2009) Size-dependent toxicity of metal oxide particles-a comparison between nano-and micrometer size. Toxicol Lett 188:112–118
Arnold M, Badireddy A, Wiesner M, Di Giulio R, Meyer J (2013) Cerium oxide nanoparticles are more toxic than equimolar bulk cerium oxide in Caenorhabditis elegans. Arch Environ Contam Toxicol 65:224–233
Vales G, Demir E, Kaya B, Creus A, Marcos R (2013) Genotoxicity of cobalt nanoparticles and ions in Drosophila. Nanotoxicology 7:462–468
Demir E, Aksakal S, Turna F, Kaya B, Marcos R (2015) In vivo genotoxic effects of four different nano-sizes forms of silica nanoparticles in Drosophila melanogaster. J Hazard Mater 283:260–266
AshaRani PV, Mun GLK, Hande MP, Valiyaveettil S (2008) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3:279–290
Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, Wright CJ, Doak SH (2009) Nanogenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials 30:3891–3914
Klien K, Godnić-Cvar J (2012) Genotoxicity of metal nanoparticles: focus on in vivo studies. Arh Hig Rada Toksikol 63:133–145
Magdolenova Z, Collins A, Kumar A, Dhawan A, Stone V, Dusinska M (2014) Mechanisms of genotoxicity. a review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 8:233–278
Demir E, Turna F, Burgucu D, Kılıç Z, Burunkaya E, Kesmez Ö, Kaya B (2013) Genotoxicity of different nano-sizes and ions of silica nanoparticles. Fresenius Environ Bull 22:2901–2909
Domenech J, Hernández A, Demir E, Marcos R, Cortés C (2020) Interactions of graphene oxide and graphene nanoplatelets with the in vitro caco-2/ht29 model of intestinal barrier. Sci Rep 10:1–15
Carmona ER, Guecheva TN, Creus A, Marcos R (2011) Proposal of an in vivo comet assay using haemocytes of Drosophila melanogaster. Environ Mol Mutagen 52:165–169
Gaivao I, Sierra LM (2014) Drosophila comet assay: insights, uses, and future perspectives. Front Genet 5:304
Alaraby M, Annangi B, Marcos R, Hernández A (2016) Drosophila melanogaster as a suitable in vivo model to determine potential side effects of nanomaterials: a review. J Toxicol Environ Health B Crit Rev 19:65–104
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L (2016) A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res 23:8244–8259
Strawn ET, Cohen CA, Rzigalinski BA (2006) Cerium oxide nanoparticles increase lifespan and protect against free radical-mediated toxicity. FASEB J 20:A1356–A1356
Baeg E, Sooklert K, Sereemaspun A (2018) Copper oxide nanoparticles cause a dose-dependent toxicity via inducing reactive oxygen species in Drosophila. Nanomaterials 8:824
Paithankar JG, Kushalan S, Nijil S, Hegde S, Kini S, Sharma A (2022) Systematic toxicity assessment of cdte quantum dots in Drosophila melanogaster. Chemosphere 295:133836
Cui Y, Gong X, Duan Y, Li N, Hu R, Liu H, Hong F (2010) Hepatocyte apoptosis and its molecular mechanisms in mice caused by titanium dioxide nanoparticles. J Hazard Mater 183:874–880
Ng CT, Yong LQ, Hande MP, Ong CN, Yu LE, Bay BH, Baeg GH (2017) Zinc Oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int J Nanomed 12:1621
Author information
Authors and Affiliations
Contributions
FTD and ED contributed to the conception, experimental design, experimental performance, data analysis, and writing original manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turna Demir, F., Demir, E. Novel insights into acute/chronic genotoxic impact of exposure to tungsten oxide nanoparticles on Drosophila melanogaster. J Nanopart Res 24, 215 (2022). https://doi.org/10.1007/s11051-022-05593-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-022-05593-2